ABSTRACT
The genus Swietenia (Meliaceae) has a wide variety of secondary metabolites with reported antioxidant activity, such as flavonoids and limonoids. In the present study, the antioxidant capacity, along with the phenol and flavonoid contents of the leaf extracts of three species of this genus: Swietenia mahagoni, Swietenia macrophylla, and Swietenia humilis were evaluated. The antioxidant activity was determined by the 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2'-azino-bis-(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS), ferric reducing/antioxidant power (FRAP), and oxygen radical absorbance capacity (ORAC) methods. The results showed that the three species had significant antioxidant activity and substantial contents of phenolic compounds and flavonoids. The species S. macrophylla was the most effective, and compounds with recognized antioxidant capability were detected by gas chromatography coupled with mass spectrometry (GC-MS). Catechin was the most abundant constituent in the active fractions, and was confirmed and quantified by high performance liquid chromatography (HPLC).
Key words: Swietenia macrophylla, antioxidant, (+)-catechin, flavonoids, phenols.
Free radicals and other reactive oxygen species (ROS) are produced constantly in metabolic reactions of aerobic organisms. When there is an excess of oxygen in cells, or its reduction is not enough, ROS such as the superoxide anion (O2-), hydroxyl radical (·OH), and hydrogen peroxide (H2O2), are generated (Cotgreave et al., 1988). Aerobic organisms have a natural defensive system of enzymatic and non-enzymatic mechanisms of detoxification of these radicals. However, when this endogenous system fails, the cell goes into a stage of oxidative stress responsible for cellular degeneration. In this way, free radicals and other ROS can react with proteins, lipids, and DNA, causing irreversible damages (Donaldson et al., 1996). Furthermore, oxidative stress in cells cause a wide range of diseases such as cancer, atherosclerosis, cataracts, neurodegenerative disorders, and inflammation (Aruoma, 1998). Antioxidant molecules that are able to donate electrons to stabilize free radicals and neutralize their effects are relevant to prevent oxidative stress. Thus, there is an increasing interest in antioxidant and free radical scavenging properties of compounds derived from plants, with numerous reports of plant products with a range of antioxidant properties. Some groups of metabolites such as terpenes, flavonoids (flavones, isoflavones, anthocyanins, flavanones), and other polyphenols (ellagic acid, gallic acid, and tannins) have shown promising antioxidant properties (Prat, 1999).
The genus Swietenia (Meliaceae) includes about 145 species distributed in the Neotropics. The species Swietenia mahagoni Jacq, Swietenia macrophylla King and Swietenia humilis Zucc are timber species widely used in traditional medicine. The three species have previous reports validating some of the traditional uses or of promising bioactivities in the laboratory. In the case of S. mahagoni the antagonist activity of the platelet-activating factor was determined (Kadota et al., 1989). In addition, methyl esters of the cholinergic acid with a high antioxidant activity were reported for this species (Matsuse et al., 1997). Similarly, the in vitro antioxidant potential of the methanol extract of seeds of S. mahagoni was determined using different techniques, with a positive effect to scavenge free radicals and inhibit the enzyme xanthine oxidase, responsible for generating ROS (Sahgal et al., 2009). Antioxidant properties of different organic extracts of flowers and bark were also reported for this species (Rahman et al., 2014). For S. macrophylla, the antioxidant potential of several limonoids was demonstrated through inhibition of superoxide anion generation in human neutrophils as a response to formyl-L-methionyl-L-leucyl-L-phenylalanine (fMLP) (Chen et al., 2010). In addition, from the bark of S. macrophylla a new compound, swietemacrophyllanin-catechin-8,7,7,2-epoxy-(methyl-4,5-dihidroxifenil propanoate) was isolated (Falah et al., 2008). This metabolite, in scavenging the DPPH radical, showed an IC50 of 56 μg/ml using Trolox as a standard. This was a remarkable result when compared to catechin (IC50 70 μg/ml) and epicatechin (IC50 59 μg/ml) (Falah et al., 2008). Previous studies on S. humilis were mainly on its antibacterial (López et al., 2007), antifungal (Angulo et al., 2009), and insecticidal activities (Jiménez et al., 1997).
Based on the antioxidant properties reported for S. mahagoni and S. macrophylla, the purpose of this study was to focus on the comparative evaluation of the three local species within the genus Swietenia. The aim of this research was to evaluate the antioxidant potential of the ethanol extract of the leaves of S. mahagoni, S. macrophylla, and S. humilis using different assessment methods, including the 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2'-azino-bis-(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS), oxygen radical absorbance capacity (ORAC), and ferric reducing/antioxidant power (FRAP) assays.
All of the reagents used for the antioxidant assays and organic solvents for extraction and fractionation, along with HPLC grade water were obtained from Sigma Aldrich® (St. Louis, MO, USA). For thin layer chromatography aluminum, silica gel (G-60) plates of 0.25 mm (F254) were used (Merck, Darmstadt, Germany). The derivatizing agent used (BSTFA+TMCS) was purchased from Supelco (Bellefonte, PA, USA).
Plant
For the extraction and biological evaluation, dried leaves (2 kg) of S. humilis, S. macrophylla and S. mahagoni were collected in the tropical and pre-mountain forests of Medellin, Colombia, at 1600 m.s.l (6° 15’41” N, 75° 34’35.5” W). A voucher sample of each species remains at the Herbarium “Gabriel Gutiérrez Villegas” (MEDEL) (Universidad Nacional de Colombia, Medellin branch) under accession numbers TL-102, TL-103 y TL-104, respectively. Samples were identified by Leon Morales (Universidad Nacional de Colombia).
Extraction of plant material
The leaf plant material of the three species (500 g each) was air-dried, milled, and extracted by percolation at room temperature overnight with 90% ethanol (1 L × 100 g). The crude ethanol extract was filtered and concentrated under reduced pressure, using a rotary evaporator (Büchi R-144) at a temperature below 40°C. The resultant ethanol extract was mixed with distilled water to 10%, and then defatted with hexane. Subsequently, the ethanol-aqueous extract was evaporated and lyophilized.
Fractionation of the most active species
The most active species in the antioxidant panel of bioassays was selected for further fractionation. Thus, the ethanolic extract of S. macrophylla leaf material was fractionated using a silica gel 60 F254 open column, eluting with a gradient of dichloromethane-acetone-methanol, beginning with the less polar solvent and ending with methanol. According to the chromatographic profile obtained by thin layer chromatography (TLC), twenty-five initial fractions were reduced to eight final fractions that were evaluated in the bioassays. After testing, the active fractions F4 and F5 were submitted to GC-MS and HPLC analysis.
Characterization of compounds using gas chromatography-mass spectrometry (GC-MS)
The chemical profiles of the ethanolic extracts of the three species of Swietenia and the most promising fractions of S. macrophylla were analyzed using a gas chromatograph (Agilent 6890), coupled with a mass spectrometer (Agilent 5973), employing a capillary column of fused silica (Agilent HP-5, 0.25 mm × 30 m × 0.25 µm) covered with 5% phenyl methyl siloxane. All of the samples (extract and fractions) were derivatized before injection according to the method described by Silici and Kutluca (2005). One mg of each sample was diluted in 50 μl of pyridine and a mixture of 100 μl of BSTFA (N,O-bis(trimethylsilyl) trifluoroacetamide) with 1% of trimethylchlorosilane (TMCS) was added. The mixture was heated for 30 min at 100°C. For each sample, 5.0 µl was injected, using helium gas grade 5 (AGA Fano S.A., UAP 99.999%) at a flux of 1.0 ml/min (lineal velocity 37 cm/s). The injection used the split-less mode with an initial temperature of 200°C for 3 min, which was raised to 250°C and maintained for 1 min. Finally, the temperature was raised by 2°C per minute to a maximum of 350°C for 60 min. To obtain the mass data, the detector was fixed at 350°C. The run was made using the SCAN mode between m/z 30 to 800. The chromatograms were analyzed with Automated Mass Spectral Deconvolution and Identification System (AMDIS) software, and the spectral database NIST 98 (2001). The identification of compounds was done though comparison of the mass spectral fragmentation patterns of each compound with the databases mentioned.
Determination of (+)-catechin and (-)-epicatechin in the most active species
The confirmation and quantification of (+)-catechin and (-)-epicatechin in the ethanolic extract and active fractions of S. macrophylla was performed through HPLC analysis. The separation of both compounds was done using an ultra-aqueous C18 column with a particle size of 5 µm (250 mm × 4.6 mm, Merck). As a mobile phase, methanol (A), and formic acid (0.1%) were used in a gradient system of elution of 0.01 min 60% of A; 5 to 12 min 80% of A; 13 to 14 min 60% of A. The mobile phase flux was 1.0 ml/min. The identification of the compounds in the active fractions of S. macrophylla was done by comparison with standard samples of (+)-catechin and (-)-epicatechin (Sigma).
Antioxidant determination
The leaf ethanolic extracts of S. humillis (Sh), S. macrophylla (Smc), and S. mahagony (Smh), and the fractions of S. macrophylla were ran in an antioxidant panel of assays. All tests were done in triplicate and expressed in internationally accepted units, such as Trolox equivalent antioxidant capacity values (TEAC) through the construction of a standard curve using Trolox®. For the FRAP assay, the antioxidant potential was expressed in vitamin C equivalents (VCEAC). All of the spectrophotometric experiments were carried out in a Multiskan Spectrum UV-Vis plate reader (Thermo Scientific, Finland).
DPPH assay
To quantify the free radical scavenging capacity of the leaf ethanolic extracts of the three species of Swietenia and fractions of S. macrophylla, the DPPH assay was done according to the method of Brand-Williams et al. (1995) with some modifications (Peyrat-Maillard et al., 2000). The stock solution was 20 mg/L of DPPH dissolved in methanol. The working solution was 990 μl of the stock solution with 10 μl of the extract or fractions at different concentrations. A standard sample was 990 μl of methanol with 10 μl of the sample, and a simple standard of 990 μl DPPH with 10 μl of solvent. Samples reacted for 30 min at room temperature, in the dark. The absorbance was measured at 517 nm. Results were expressed in μM of Trolox equivalent per g of extract.
ABTS•+ assay
This technique was performed following the procedure by Re et al. (1999). For the biological evaluation, 10 μl of the extracts or fractions, and 990 μl of the ABTS solution were mixed. Samples reacted for 30 min at room temperature, in the dark. The reference sample was an ABTS solution and the solvent used to dissolve the sample. The absorbance of all samples was taken at 734 nm. Results were expressed in μM of Trolox equivalent per g of extract.
Ferric reducing/antioxidant power assay (FRAP)
This method evaluated the antioxidant potential of the samples according to its capability to reduce Fe+3 to Fe+2 in a complex with the ferrous tripyridyl triazine complex reagent (TPTZ) (Benzie and Strain, 1996). The stock solution included acetic acid-sodium acetate (pH 3.4), with TPTZ and FeCl3. The working solution was 900 μl of the stock solution, 50 μl of the extracts or fractions, and 50 μl of distilled water. After 60 min of reaction, the absorbance was determined at a wavelength of 593 nm. The standard curve was done using ascorbic acid as a standard reference and results expressed as mg of ascorbic acid per g of extract.
Oxygen radical absorbance capacity assay (ORAC)
This method evaluated the ability of the extracts and fractions to trap peroxil radicals (ROO∙) responsible for the discoloration of the fluorescent probe fluorescein. The procedure was performed following the literature (Ou et al., 2001; Atala et al., 2009), using Trolox as standard and at controlled conditions of temperature at 37°C and pH at 7.4. Solutions of fluorescein 1 × 10-2 M in PBS (75 mM) and AAPH 0.6 M in OPBS (75 mM), were used. The working solution consisted of 21 μl of fluorescein, 3 μl of PBS, 30 μl of the extracts or fractions, and 50 μl of AAPH. Readings were done at a ÊŽ of excitation spectrum of 493 nm and slit of excitation 5, ÊŽ of emission of 515 nm and slit of emission 13, with attenuator of 1% and without attenuator plate. The protective effect was calculated using the differences of the areas under the curve (AUC) of the decrease of fluorescein, between the standard and the sample (extracts or fractions). This was compared with the Trolox curve and results were expressed in micromoles equivalents of Trolox per gram of extract. The area under the fluorescence decay curve (AUC) was calculated as:
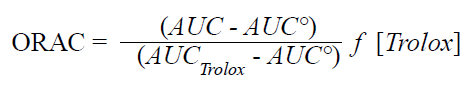
Where AUC is the area under the curve of the extracts or fractions, AUC° is the area under the curve for the standard sample, AUCTrolox is the area under the curve for Trolox, and f is the factor of dilution of the extracts.
Total phenols assay
The determination of total phenols in the extracts was performed by the Folin-Ciocalteu colorimetric method (Singleton and Rossi, 1965). An amount of 50 μl of the extracts was mixed with 125 μl of the Folin reagent, and 400 μl of sodium carbonate 7.1% (w/v), adding distilled water up to 1000 μl. The reading was done at 760 nm and a comparison was established with the standard curve using gallic acid as the phenolic standard. Results were expressed as mg of equivalent of gallic acid per grams of extract.
Flavonoids total assay
The determination of flavonoids was done following the colorimetric method described my Marinova et al. (2005) with some modifications. An amount of 100 μl of extracts was mixed with 30 μl of NaNO2 5% (w/v), 30 μl of AlCl3 10% (w/v), and 200 μl of NaOH 1 M, adding distilled water up to 1000 μl. The reading was done at 510 nm and a comparison was established with the standard curve using (+)-catechin as the standard flavonoid. Results were expressed as mg of equivalent of (+)-catechin per grams of extract.
Statistical analysis
To identify the variation in the biological activity, a one-way analysis of variance (ANOVA) was carried out. When significant differences were detected (α ≤ 0.05), a Turkey’s range test, with a confidence level of 95%, was done to establish the differences in each level of activity. Additionally, correlations among data obtained for the antioxidant activity of the fractions of S. macrophylla were calculated using Pearson’s correlation coefficient. All tests were done in triplicate and expressed as the median and the standard deviation using R, version 2.15 (R Development Core Team, 2012).
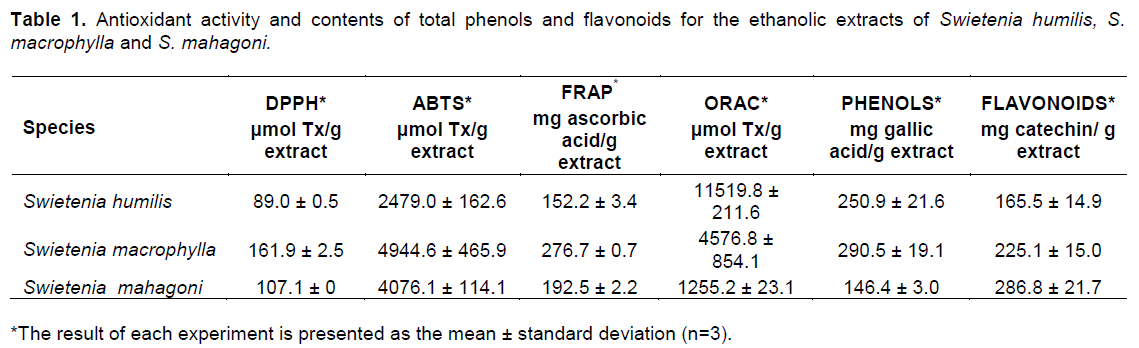
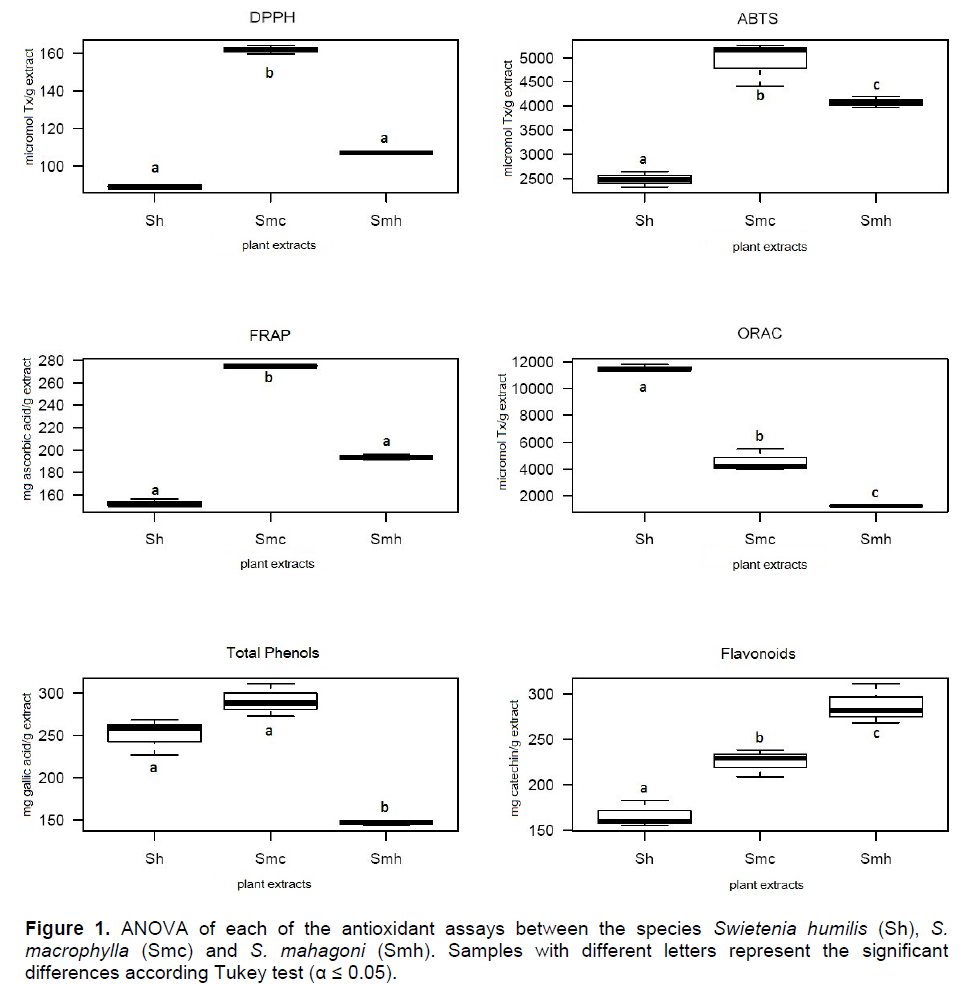
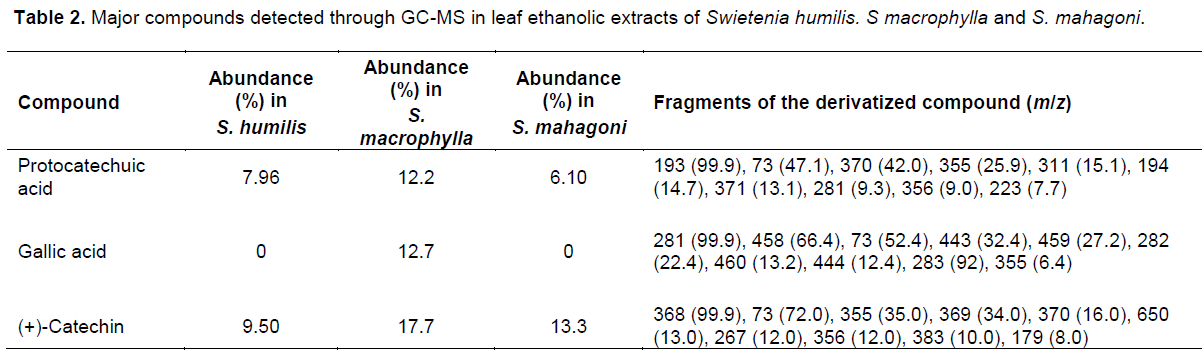
The three species of Swietenia showed antioxidant properties in the different assays, and considerable phenol and flavonoid contents in the different assays (Table 1). These results were supported by the ANOVA of the antioxidant potential of the leaf extracts (Figure 1). In the mass spectra of the ethanolic extract of S. humillis, S macrophylla, and S. mahagony, fragmentation patterns of phenolic compounds were detected. The most abundant metabolites in each species are shown in Table 2.
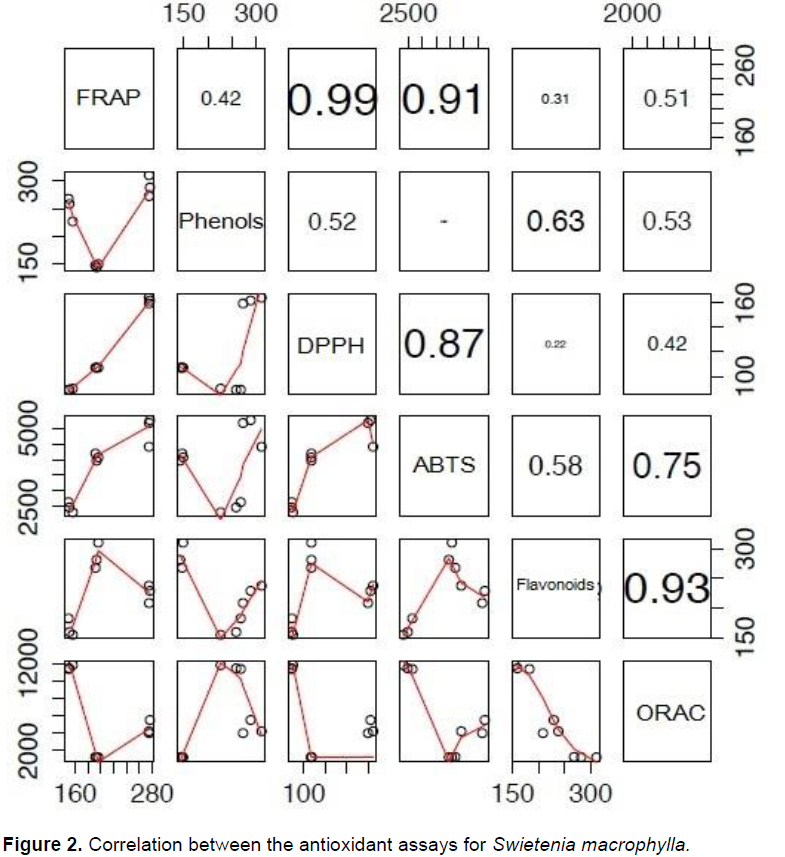
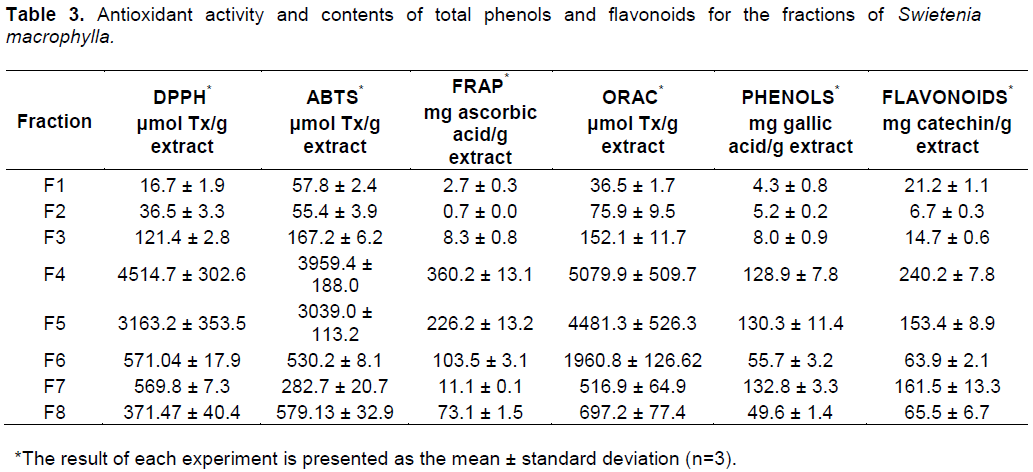
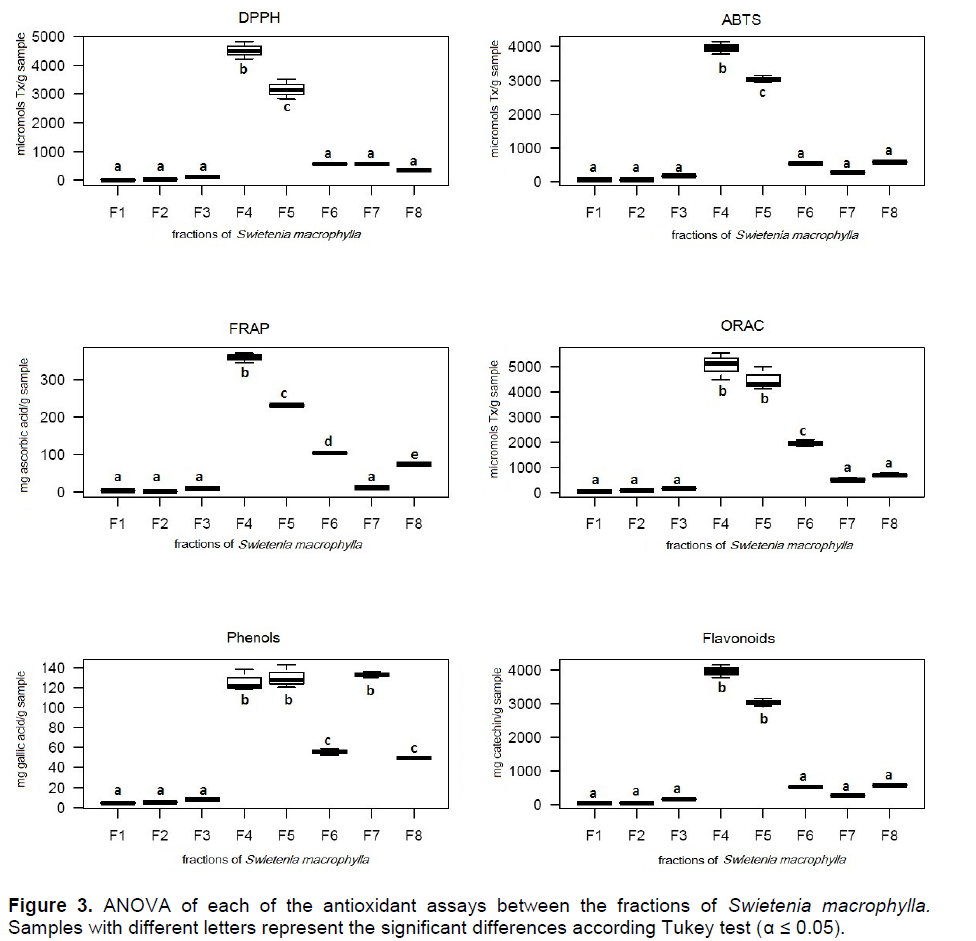
Due to the highest antioxidant potential and the abundance of phenolic compounds, S. macrophylla was selected as the most promising lead from the three species evaluated. In order to elucidate the capability of this particular species to trap different free radicals in diverse systems, statistical correlations between each of the antioxidant results were performed (Figure 2). To support the aforementioned results, fractionation of the ethanolic extract of S. macrophylla and further bioassays were performed for each fraction. There was differential in the antioxidant activity between the eight fractions derived from the leaf extract (Table 3), with the most significant activity found in fractions F4 and F5 (Figure 3).
In an attempt to verify if the compounds detected initially by GC-MS in the leaf extracts were present in fractions F4 and F5, the same method was used for each fraction. As a result, gallic acid was not found in either fraction, while protocatechuic acid was detected only for F5 in a 14.3% level of abundance. Catechin was present in both fractions, in a 7.8% level of abundance for F4 and 17.9% for F5. To support these findings, the determination of catechin and epicatechin in the ethanolic extract of S. macrophylla and in fractions F4 and F5 was carried out by HPLC. The ethanolic extract, (+)-catechin was quantified as 2.096 mg/g and (-)-epicatechin as 0.869 mg/g. In fraction F4, (+)-catechin was 1.009 mg/g and (-)-epicatechin as 1.396 mg/g, while in fraction F5 (+)-catechin, 0.184 mg/g and (-)-epicatechin 0.097mg/g. These metabolites were not detected in the other fractions by HPLC.
Even though in the three species of Swietenia there was an evident antioxidant property and substantial phenol and flavonoid contents, the best results were observed for S. macrophylla (Table 1). The results obtained by the different techniques were significantly variable, offering diverse potential according to the types of metabolites present in each plant species (Figure 1). For the DPPH, ABTS and FRAP assays, the species S. macrophylla had the highest activity followed by S. mahagoni, which was in accordance with previous reports (Falah et al., 2008; Matsuse et al., 1997; Rahman et al., 2014; Sahgal et al., 2009). In particular, for the DPPH and ABTS assays for both species, the latest assay showed higher values, which could be due to the low selectivity of the radical ABTS, which reacted with hydroxylated compounds independently of its antioxidant potential (Roginsky and Lissi, 2005). In contrast, the radical DPPH, has a steric inaccessibility that causes a slow reaction of some compounds that could even make them seem inert to this radical (Prior et al., 2005). In addition, if the antioxidant potential of the extracts is due to the presence of flavonoids or other phenols (Es-Safi et al., 2007), it has to be considered that the radical DPPH does not react with flavonoids that do not have hydroxyl groups in the B ring, or phenols that have only one hydroxyl group (Roginsky and Lissi, 2005). This could be another reason for the differences in the values between the DPPH and the ABTS assays, apart from the low selectivity of the ABTS radical or the steric inaccessibility of the DPPH radical.
In the particular case of the FRAP assay, the presence of metabolites with the ability to stabilize a free radical molecule through a single-electron transfer mechanism (SET) was also evidenced for S. macrophylla. This could point to the presence of metabolites with a high reducing potential that is related to molecules with a high degree of hydroxylation or highly conjugated polyphenols (Pulido et al., 2000). This is supported by the significant total phenolics content for this species (Table 1). In contrast, in the ORAC evaluation S. humilis presented the highest number of TEAC equivalents, with interesting results, since no reports of antioxidant activity were found for this species. In this case, we suggest that S. humilis leaves could have a higher content of antioxidant hydrophilic metabolites with a capability to trap peroxide radicals ROO. through a hydrogen atom transfer (HAT) mechanism. The phenolic metabolites protocatechuic acid and catechin were detected differentially in each sample, as mayor compounds while gallic acid was detected only in S. macrophylla (Table 2). The fragmentation pattern for the derivatized samples acquired through MS was in accordance with the literature (Proestos and Komaitis, 2013). The identification of catechin was based on the spectral data of the derivatized sample, which presented a molecular ion of m/z 650 and a characteristic base peak (m/z 368) originated from the excision of the heterocyclic ring through a retro diels alder fragmentation pathway (Zeeb et al., 2001). The TMS derivative of gallic acid presented a molecular ion of m/z 458 and a characteristic base peak of m/z 28, while the TMS derivative of protocacheuic acid evidenced a molecular ion of m/z 370 and a base peak of m/z 193. These metabolites are recognized in the literature for its high free radical scavenging capability that is determined by their ability to act as donors of electrons or hydrogen atoms, the stability of the antioxidant molecule in its radical form their reactivity with other antioxidants, and the capacity to chelate metals (Rice-Evans et al., 1997).Particularly, the detection of catechin in the three plant extracts was relevant, since this flavonoid is known to be an excellent free radical scavenger (Pedrielli et al., 2001). It has been demonstrated that catechin isomers have a lower reduction potential than vitamin E, suggesting that their electron donor capability is higher (Jovanovic et al., 1996).
Based on the overall results, S. macrophylla was the most promising lead, which was in accordance with previous studies (Tan et al., 2009). In the statistical correlations between each of the antioxidant results of this particular species, the high correlation between FRAP and DPPH (R2= 0.99) and FRAP with ABTS (R2= 0.91) (Figure 2) suggests that the reducing agents present in S. macrophylla react through a single electron transfer mechanism (SET). The correlation between ABTS and DPPH was also observed in sorghum and its products, evidencing a similar mode of action (Awika et al., 2003). In contrast, the high correlation among the flavonoid contents and the ORAC assay (R2=0.93) could evidence that the flavonoids in this extract have a high capability to trap peroxil radicals, which is the case for catechin (Tan et al., 2009). As a group, flavonoids are the most diverse phenols, with a high potential as free radical scavengers, and other biological activities (Middleton et al., 2000).As seen in Table 3, the fractions of S. macrophylla exhibited a differential antioxidant activity. The most promising fractions were F4 and F5, with significant differences when compared statistically with the other fractions (Figure 3). In this way, in the GC-MS analysis of these fractions (F4 and F5), protocacheuic acid was present only in fraction F5, while catechin was present in both fractions. In accordance with previous results, catechin as a major compound detected in both fractions could be one of the compounds implicated in the bioactivity, as evidenced in the significantly high activity in the biological tests (Figure 3). It is relevant to point out that both fractions (F4 and F5) had no statistical difference in the bioactivity in the ORAC assay, supporting the presence of hydrophilic antioxidants specific to trap peroxil radicals, such as catechin (Tan et al., 2009). In the determination of catechin and epicatechin in the ethanolic extract of S. macrophylla and in fractions F4 and F5 by HPLC it supports the aforementioned results and strongly suggests the involvement of these metabolites in the observed bioactivity.
Finally, it is important to point out that many assays have been frequently used to estimate antioxidant potentials in natural products, in conducting other studies regarding the isolation or standardization of biological extracts. These techniques have shown different results among crop species and across laboratories. Some authors pointed out that the ORAC assay was the most relevant because it utilized a biologically relevant radical source (Prior et al., 2003). Nevertheless, all the different methods to determine the antioxidant potential have been validated in different studies, and the potential of the three species evaluated in this research was demonstrated as sources of potential antioxidant molecules, with S. macrophylla as the most promising lead.
The Swietenia species evaluated in this study exhibited a high free radical scavenging activity, in which correlations among the different assays that contributed to the inference of the type of metabolites and conditions of the mode of action. The additional results obtained with S. macrophylla support the correlations of the bioactivity and the implication of flavonoids, such as catechin in the results. It would be relevant to conduct further studies of isolation and identification of other compounds in fractions F4 and F5 to explore their antioxidant potential.
The authors have not declared any conflict of interests.
Authors acknowledge the research office “Dirección de Investigaciones DIME”, Universidad Nacional de Colombia sede Medellín, for financial support (code: Hermes 18870). J.A. Pereañez is grateful with the Universidad de Antioquia for additional financial support (Sostenibilidad 2014-2015). We also thank Professor Geoffrey Cordell, University of Florida for his assistance in the preparation of the manuscript and Professor Alvaro Duque, Universidad Nacional de Colombia, for his guidance in the statistical analysis
REFERENCES
|
Angulo MA, Armenta E, García RS, Carrillo JA, Salazar E, Valdéz JB (2009). Extracts of the seed of Swietenia humilis Zucc. With antifungal activity in Rhizopus stolonifer. Rev. Mex. Fitopatol. 27(2):84-92.
|
|
|
|
Aruoma OI (1998). Free radicals, oxidative stress, and antioxidants in human health and disease. J. Am. Oil Chem. Soc. 75:199-212.
Crossref
|
|
|
|
|
Atala E, Vásquez L, Speisky H, Lissi E, López-Alarcón C (2009). Ascorbic acid contribution to ORAC values in berry extracts: an evaluation by the ORAC-pyrogallol red methodology. Food Chem. 113(1):331-335.
Crossref
|
|
|
|
|
Awika JM, Rooney LW, Wu X, Prior RL, Cisneros-Zevallos L (2003). Screening methods to measure antioxidant activity of sorghum (Sorghum bicolor) and sorghum products. J. Agric. Food Chem. 51:6657-6662.
Crossref
|
|
|
|
|
Benzie IF, Strain JJ (1996). The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP assay. Anal. Biochem. 239(1):70-76.
Crossref
|
|
|
|
|
Brand-Williams W, Cuvelier ME, Berset C (1995). Use a of free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 28(1):25-30.
Crossref
|
|
|
|
|
Chen JJ, Huang SS, Chang HL, Dau CW, Ping JS, Tai CW, Ming JC (2010). A new phragmalin-type limonoid and anti-inflammatory constituents from the fruits of Swietenia macrophylla. Food Chem. 120:379-384.
Crossref
|
|
|
|
|
Cotgreave IA, Moldeus P, Orrenius S (1988). Host biochemical defense mechanisms against pro-oxidants. Annu. Rev. Pharmacol. Toxicol. 28:189-212.
Crossref
|
|
|
|
|
Donaldson K, Beswick PH, Gilmour PS (1996). Free radical activity associated with the surface of particles: a unifying factor in determining biological activity. Toxicol. Lett. 88(1-3):293-298.
Crossref
|
|
|
|
|
Es-Safi NE, Ghidouche S, Ducrot PH (2007). Flavonoids: hemisynthesis, reactivity, characterization and free radical scavenging activity. Molecules 12:2228-2258.
Crossref
|
|
|
|
|
Falah S, Suzuki T, Katayama T (2008). Chemical constituents from Swietenia macrophylla bark and their antioxidant activity. Pak. J. Biol. Sci. 11:2007-2012.
Crossref
|
|
|
|
|
Jiménez A, Mata R, Pereda-Miranda R, Calderon J, Isman MB, Nicol R, Arnason JT (1997). Insecticidal limonoids from Swietenia humilis and Cedrela salvadorensis. J. Chem. Ecol. 23(5):1225-1234.
Crossref
|
|
|
|
|
Jovanovic SV, Steenken S, Simic MG (1996). Reduction potentials of flavonoid and model phenoxyl radicals. Which ring in flavonoids is responsible for antioxidant activity? J. Chem. Soc. Perkin Trans. 1(2):2497-2504.
Crossref
|
|
|
|
|
Kadota S, Marpaung L, Kikuchi T, Ekimotob H (1989). Antagonists of platelet activating factor from Swietenia mahogani (L.) Jacq. Tetrahedron Lett. 30(9):1111-1114.
Crossref
|
|
|
|
|
López Y, Angulo M, Martínez C, Soto J, Chaidez C. 2007. Antimicrobial effects of crude extracts of neem (Azadirachta indica A. Juss) and venadillo (Swietenia humilis Zucc) against E. coli, S. aureus and the bacteriophage P22. Bioquimia 32(4):117-125.
|
|
|
|
|
Marinova D, Ribarova F, Atanassova M. 2005. Total phenolics and total flavonoids in bulgarian fruits and vegetables. J. Chem. Technol. Metall. 40(3):255-260.
|
|
|
|
|
Matsuse IT, Nakabayashi T, Lim YA, Hussein GME, Miyashiro H, Kakiuchi N, Hattori M, Stardjo S, Shimotohno K (1997). A human immunodeficiency virus protease inhibitory substance from Swietenia mahagoni. Phytother. Res. 1(6):433-436.
Crossref
|
|
|
|
|
Middleton E, Kandaswami C, Theoharides TC (2000). The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 52(4):673-751.
PMid:11121513
|
|
|
|
|
Ou B, Hampsch-Woodill M, Prior R (2001). Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food. Chem. 49(10):4619-4626.
Crossref
|
|
|
|
|
Pedrielli P, Holkeri LM, Skibsted LH (2001). Antioxidant activity of (+)-catechin. Rate constant for hydrogen-atom transfer to peroxyl radicals. Eur. Food Res. Technol. 213:405-408.
Crossref
|
|
|
|
|
Peyrat-Maillard MN, Bonnely S, Berset C (2000). Determination of the antioxidant activity of phenolic compounds by colometric detection. Talanta 51(4):709-716.
Crossref
|
|
|
|
|
Prat DE (1999). Natural antioxidants from plant material. Phenolic compounds in food and their effects on health II. Antioxidants and Cancer Prevention. Ed American Chemical Society, Washington D.C. pp. 54-71.
|
|
|
|
|
Prior RL, Hoang H, Gu L, Wu X, Bacchiocca M, Howard L, Hampsch-Woodill M, Huang D, Ou B, Jacob R (2003). Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORAC FL)) of plasma and other biological and food samples. J. Agric. Food Chem. 51:3273-3279.
Crossref
|
|
|
|
|
Prior RL, Wu X, Schaich K (2005). Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 53:4290-4302.
Crossref
|
|
|
|
|
Proestos C, Komaitis M (2013). Analysis of naturally occurring phenolic compounds in aromatic plants by RP-HPLC coupled to diode array detector (DAD) and GC-MS after silylation. Foods 2:90-99.
Crossref
|
|
|
|
|
Pulido R, Bravo L, Saura-Calixto F (2000). Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/ antioxidant power assay. J. Agric. Food Chem. 48:3396-3402.
Crossref
|
|
|
|
|
Rahman SM, Akter M, Easmin-Hira T, Yeunus MD, Ahmed I, Rahman MM (2014). Antioxidant and antimicrobial activities of flower and bark extract of Swietenia mahagoni (L.) Jacq. J. Pharmacogn. Phytochem. 2(6):185-188.
|
|
|
|
|
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 26(9-10):1231-1237.
Crossref
|
|
|
|
|
Rice-Evans CA, Miller NJ, Paganga G (1997). Antioxidant properties of phenolic compounds. Trends Plant Sci. 2:152-159.
Crossref
|
|
|
|
|
Roginsky V, Lissi EA (2005). Review of methods to determine chain-breaking antioxidant activity in food. Food Chem. 92:235-254.
Crossref
|
|
|
|
|
Sahgal G, Ramanathan S, Sasidharan S, Mordi MN, Ismail S, Mansor SM (2009). In Vitro antioxidant and xanthine oxidase inhibitory activities of methanolic Swietenia mahagoni seed extracts. Molecules 14(11):4476-4485.
Crossref
|
|
|
|
|
Silici S, Kutluca S (2005). Chemical composition and antibacterial activity of propolis collected by three different races of honeybees in the same region. J. Ethnopharmacol. 99:69-73.
Crossref
|
|
|
|
|
Singleton VL, Rossi JA (1965). Colorimetry of total phenolics with phosphomolybdic–phosphotungstic acid reagents. Am. J. Enol. Vitic. 16(3):144-158.
|
|
|
|
|
Tan SK, Hasnah O, Keng-Chong W, Peng-Lim B, Padzilah I (2009). Antimicrobial and antioxidant activities of Swietenia macrophylla leaf extracts. As. J. Food Ag-Ind. 2(02):181-188.
|
|
|
|
|
Zeeb DJ, Nelson BC, Albert K, Dalluge JJ (2001). Separation and identification of twelve catechins in tea using liquid chromatography/atmospheric pressure chemical ionization-mass spectrometry. Anal. Chem. 72:5020-5026.
Crossref
|
|