ABSTRACT
Resistance of malaria parasites to several antimalarial drugs mandates the need for new compounds from affordable sources. Thus the current study was conducted to justify the traditional usage of Lantana trifolia roots and Premna oligotricha leaves to combat malaria. The powdered roots of Lantana trifolia and leaves of Premna oligotricha were macerated using 70% ethanol. Secondary metabolites present in the extracts were determined using the standard chemical method. The in vivo antiplasmodial effect of the crude extracts were evaluated using the four-day suppressive test in chloroquine (CQ) sensitive strain of Plasmodium berghei infected mice. Parameters such as parasitaemia, packed cell volume, body weight change and survival time of mice were then determined as using standard tests. The ethanol extracts showed a dose-dependent chemosuppression when compared to the negative control in this study. The chemosuppressive effect produced by all doses was very significant (P <0.001). Concentrations at 350, 500 mg/kg of leaf extract and 200 mg/kg from root extract have demonstrated prevention effect on weight loss of mice (P <0.001). All the test extracts employed in this study have no significant prevention effect on PCV loss of mice at all dose levels. The present study has demonstrated the efficacy of the extracts of Premna oligotricha leaf and Lantana trifolia root traditionally used in chemotherapy of malaria infection. These observations provide the basis for the traditional use of these plants in treatments of malaria and potential for development of novel therapeutics in the conventional medicine.
Key words: Antimalarial, In vivo, Lantana trifolia, parasitaemia, Plasmodium berghei, Premna oligotricha.
Malaria is a life-threatening infectious disease caused by plasmodium parasite. It affects more than 200 million people worldwide with an estimated death of nearly 1 million people, mostly children. The disease is also responsible for causing significant economic loss, especially in developing countries (WHO, 2015). In Ethiopia, for example, malaria adversely affects the health of the public and the country’s economy as a result of significant expansion to previously non-affected areas with endemicity of about 75% (WHO, 2004; Adhanom et al., 2006; Gebreyohannes et al., 2017). Subsequently, it attracts significant attention from various stakeholders in designing a strategy to prevent and control the disease.
Treatment of malaria infection with conventionally used anti-malarial drugs are puzzled by the emergence of resistant parasites, which reduces the importance of these drugs in controlling malaria infection (Kilama, 2005; Birru et al., 2017). Various strategies are designed and undertaken to enhance the treatment outcomes of anti-malarial drugs, including combination of drugs and the discovery of new compounds from plants (Tchoumbougnang et al., 2004; Okafor et al., 2013). Most of the currently used antimalarial drugs including chloroquine, primaquine and artimisinin derivatives are derived from plants which have been traditionally used against malaria.
In addition, the society relies on traditional medicine practice; and plants having antimalarial properties is commonly used as an alternative therapy to combat malaria (Waako et al., 2007; Builders et al., 2011). These necessitates the importance of further studies on medicinal plants which are reported to have antimalarial activity. In Ethiopia, despite the presence of a huge number of plants which are claimed to have anti-malarial activity, only few studies have been conducted to evaluate the anti-malarial activity and safety margins of these plants (Tadesse and Wubneh, 2017). The roots of Lanata trifolia are traditionally used for the treatment of various human ailments such as malaria, fever, dermatitis, wound, bleeding, asthma, cough, colds, diarrhea, and bronchitis (Atkin and Kadercit, 2004).
The plant is also used in East Africa to treat fever, epilepsy and cerebral malaria (Mukungu et al., 2016; Nalubega et al., 2013). Consumption of L. trifolia fruit has also been reported in Konso, Ethiopia (Ocho, 2012). Previous study demonstrated that the plant has shown anti-inflammatory and anti-nociceptive activity (Silva et al., 2005; Johnson et al., 2017). Based on ethnobotanical survey conducted in South Omo, the leaves of Premna oligotricha is used for the treatment of malaria (Waako, 2007). P. oligotricha, is distributed in Ethiopia, Kenya and Somalia. The plant is locally known as ‘yeweba medhanit’ or ‘drug of malaria’ by the society in South Omo, Southern Ethiopia.
As a result of pleasant smelling, the plant is also used to fumigate and cleanse gourds in Marsabit districts of Ethiopia and Kenyas (Ketemma et al., 2013). Studies also revealed the presence of two antibacterial diterpenes, a clerodane and labdane (WHO, 2005; Habtemariam et al., 1990). Although L. trifolia and P. oligotricha are traditionally used for the treatment of malaria in Ethiopia, there is no study which evaluates the anti-malarial activities and safety of these plants. Therefore, this study is designed to evaluate the in vivo antimalarial activities of crude extract of L. trifolia and P. oligotricha leave in Plasmodium berghei infected mice. The study also examined the safety profiles and phytochemical constituents of both plants.
Collections and preparation of plant materials
The roots of L. trifolia were collected from the premises of Hawassa city, South Ethiopia. Whereas, the leaves of P. oligotricha were collected from the location 50 km from Bulle Hora on the highway to Yabello, Oromiya regional state, Ethiopia. After collection, the plant materials were confirmed by a taxonomist and a voucher specimen (AE-001/08) was deposited at the herbarium of Addis Ababa University (AAU), Ethiopia.
Extraction
Prior to extraction, the plant parts were washed with distilled water and dried under shade just after specimen collection. The powdered roots and leaves were macerated using 70% ethanol. Then, the extracts of both plants were filtered using Whatmann filter paper No. 1 and the ethanol was removed using rotary evaporator at average of 40 RPM under reduced pressure at a temperature of 40 to 45oC. The crude extracts obtained were kept in a refrigerator at 4°C and fresh solutions using 3% tween 80 were prepared for each extract immediately before the test.
Laboratory animal
Swiss albino mice of 6 to 8 weeks of age, weighing 25 to 32g, were maintained in a standard room and acclimatized to the laboratory condition for 14 days in 12 h light and dark cycle. Animals were provided with a standard pellet diet and water on ad libitum. Mice were handled according to the international guidelines for the care and use of animals in the experiments (European Community Guidelines, 1986). The study was also approved by the insttuional review board (IRB/026/08) of Hawassa University, Ethiopia.
The parasite strain
The anti-malarial activity of L. trifolia roots and P. oligotricha leaves were tested on mice infected with chloroquine-sensitive P. berghei strain ANKA which was obtained from the Department of Biomedical Sciences, Addis Ababa University. Viable strains of P. berghei was maintained by a weekly passage of blood from an infected donor mouse (with a rising parasitemia of 30%) to non-infected mice. Blood from infected mouse was collected through cardiac puncture. Taking into account the level of parasitemia of infected mouse and the erythrocyte count of non-infected mouse, the blood was diluted to get 1 × 107 P. berghei- infected RBCs in 0.2 ml normal saline diluted blood. On the first day (D0), mice were injected intraperitoneally with the 0.2 ml of infected and diluted blood (Knight and Peters, 1980).
Grouping of animals
Then, infected mice were allocated randomly into 8 groups of 5 mice each. The first 6 groups received an oral dose of 200, 350 and 500mg/kg b. wt root and leave extracts of L. trifolia and P. oligotricha, respectively. The other two groups were served as positive and negative control groups, and received an equal volume of 3% of Tween 80 (vehicle) and chloroquine (25 mg/kg/day; orally)
(Ethiopian Pharmaceutical Manufacturing, Ethiopia), respectively.
In vivo anti-malarial test
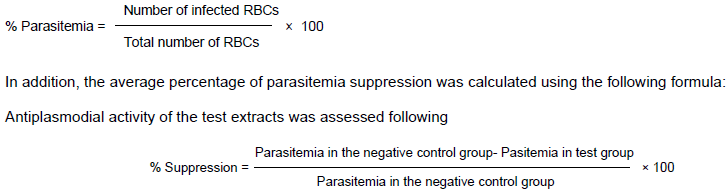
The standard four-day suppressive test protocol (Waako et al., 2005). The mice were divided randomly into five groups consisting of five mice each. 3 h after infection, 200, 350 and 500 mg/kg/day oral doses of L.trifolia and P.oligotricha crude extracts were given to the test groups. The control groups were treated with chloroquine (25 mg/kg/day, orally) and an equal volume of 3% Tween 80. Treatment of the test and control groups was conducted for four consecutive days (day 0 to day 3). On day 4, blood samples were collected from the tail of each mouse. Thin blood smears were made and stained with 10% Giemsa stain. The numbers of parasitized RBCs out of 100 RBCs in random fields were used to determine the parasitemia level. Then, average percentage parasitemia was determined using the following formula:
Body weight changes and packed cell volume (PCV)
The body weights and PCV at D0 and D4 of mice were taken to observe whether the test extracts prevented the weight loss and reduction in PCV that are commonly observed with increasing parasitaemia in P. berghei infected mice. The PCV was determined by the following equation:

Determination of the percentage survival
The curative potential of the fractions was assessed by monitoring the mortality starting from the day of treatment in infected mice, daily. The number of animals survived from the time injecting blood containing the parasite up to 10th day was determined for each group. The comparison was made based on the percentage of animals that survived on the 10th day after treatment.
Test for acute oral toxicity studies
In order to assess the safety profile of crude extracts of L. trifolia and P. oligotricha, mice were divided randomly into two groups of six animals each (three male and three female). Mice were fasted for one night prior to administration of the extracts and provided with only water. Then, the extracts were given with an increasing doses of 2000, 3500, 5000 mg/kg using oral gavage following the standard guidelines (WHO, 2001). Animals were observed for any signs of toxicity for 14 days. Observation was carried out for any signs of overt toxicity such as hair erection, salivation, lacrimation, diarrhea, tremor, convulsion, and mortality.
Phytochemical screening
Crude extracts of L. trifolia and P. oligotricha were evaluated for the presence of secondary metabolites such as flavonoids, alkaloids, triterpenoids, resine, tannins, saponins and steroids, according to the previously established protocols (Debella, 2002; Jones and Kinghorn, 2006).
Statistical analysis
Data were analyzed using Windows, statisitical package for social science (SPSS) Version 22.0. Comparison of percentage suppression, PCV, weight changes and percentage survival with the negative control group was made using One-way Analysis of Variance (ANOVA) followed by Tukey’s honest significant difference (HSD) test. P-values less than 0.05 was considered statistically significant.
Acute toxicity study
Oral acute toxicity studies on the extracts of L. trifolia roots and P. oligotricha leaves showed that the extracts were found to be safe up to a maximum dose of 5000 mg/kg. Administration of either of the extracts did not produce mortality, signs of morbidity and a significant behavioral and physical changes in graded doses given to experimental animals. Therefore, the results reveals that the medium lethal doses (LD50) of the extracts are greater than 5000 mg/kg body weight.
Effect of the extracts on percentage parasitemia and survival of mice
The effects of treatment with crude extracts of P. oligotricha leaf and L. trifolia root on parasitemia suppression and survival of P. berghei infected mice are shown in Table 1. The study exhibited that crude extracts of both plants exhibited a significant (P<0.05) and dose-dependent chemosuppression compared with the negative control. The chemosuppression was maximum at higher doses for both extracts (54 and 46% for P. oligotricha and L.trifolia, respectively at 500 mg/kg dose) compared with the negative control. In addition, 10th day assessment of infected and extract treated mice demonstrated that the extracts prevented mortality in 80 to 90% of mice. Accordingly, mice treated with all doses of extract lived longer than the negative control (Table 1).

Effect of the extracts on body weight loss
Doses of 350 and 500 mg/kg P. oligotricha leaf extract and 200 mg/kg L. trifolia root extract demonstrated a significant (P <0.05) weight gain in mice compared with the negative control group (Table 2). However, the 350 mg/kg dose of P. oligotricha leaf extract caused significant body weight loss (P <0.001) compared to the negative control group (Table 2).

Effect of the extracts on PCV
The extracts employed in this study failed to prevent PCV loss at any dose (P >0.05), compared with the negative control group (Table 3).
Phytochemical screening
Phytochemical evaluation of the root and leaf extracts of L. trifolia and P. oligotricha, respectively was carried out following the standard protocols. Steroids, terpenoids, steroidal glycosides, saponins, and glycosides were found in the extracts of both plants (Table 4).

The antimalarial activity of a compound is commonly assessed using the P. berghei infected rodent model because of the similarity with human malaria infection, and the sensitivity of P. berghei to chloroquine. The model takes into account the contribution of the immune system in the elimination of infection (Waako et al., 2007). The study demonstrated the antimalarial activity of crude ethanol extracts of P. oligotricha leaves and L. trifolia roots which are traditionally used in Ethiopia and elsewhere, against malaria infection. The four-day suppressive test was used to evaluate the impact of treatment with extracts on parasitemia suppression, survival time, PCV and body weight changes. Moreover, the acute toxicity profile and the phytochemical constituents of both plants were determined.
A compound which produces a minimum of 30% parasitemia suppression or greater percent survival compared to infected and non-treated mice is commonly considered to be an active antimalarial agent (David et al., 2004; Fentahun et al., 2017). The four-day suppressive test showed that the crude extracts of P. oligotricha and L. trifolia demonstrated a significant and dose-dependent parasitemia suppression. The suppression caused by both plants was greater than 30% at 350 and 500 mg/kg doses. The results indicates that the crude extracts of both plants are endowed with antimalarial activity with an optimal minimal dose of 350 mg/kg body weight. On the other hand, treatment with the crude extracts of both plants significantly enhance the percent survival of mice on the 10th day relative to the negative control group. The enhanced survival of mice in the extract treated group could be attributed to the suppressive effect of the extracts on parasitemia.
The activity of the extracts, in suppressing parasitemia and enhancing survival time of infected mice, was in agreement with previous studies on other plant extracts such as Echnopis kebericho (Toma et al., 2015), Croton Machrostachyus, Dodonaea angustifolia (Mengiste et al., 2012) and Nigella sativa (Dikasso et al, 2006), at a relatively comparable doses.
Phytoconstituents are frequently reported for the therapeutic benefits of herbal preparations (Habtemariam et al., 1991; Ayoola et al., 2008). Secondary metabolites such as alkaloids, triterpenoids, quassinoids, xanthones, sesquiterpenes, flavonoids, quinines and phenolic compounds have shown significant antimalarial activities (Habtemariam et al., 1991; Nalubega et al., 2013). Therefore, the antimalarial activity of P. oligotricha and L. trifolia could be as a result of the individual or combined effects of the phytoconstituents present.
Herbal remedies with immunomodulatory and antioxidant (Silva et al., 2005; Okokon et al., 2006) activities are shown to posses antimalarial activity. Moreover, inhibition of protein synthesis and prevention of invasion of new red blood cells (RBCs) by plasmodium parasites (Mukungu et al., 2016; Okokon et al., 2013) could also be considered as a possible mechanism of antimalarial activity. Prevention of anemia and body weight loss in P. berghei infected mice is also a crucial criteria to consider as the best compound for antimalarial agent (Okokon et al., 2013). In this study, except chloroquine treated mice, the significant body weight gain was recorded only in 500 and 200 mg/kg doses of P. oligotricha and L. trifolia, respectively. On the contrary, treatment with all other doses demonstrated variable degrees of body weight loss. This might be due to the presence of appetite-suppressant metabolites in the extracts, which is supported by earlier studies on other plant extracts (Mukungu et al., 2016; Toma et al., 2015; Gebretsadik and Mekonnen, 2016; Verman et al., 2006).
Erythrocyte fragility, reduced PCV, and life-threatening anemia is commonly observed in P. berghei infected mice. The 500 mg/kg dose L. trifolia enhances the PCV of mice, which was comparable to the effects of chloroquine. However, P. oligotricha and the other doses of L. trifolia failed to prevent PCV reduction. The reduction of PCV might be associated with the presence of saponins, which are responsible to cause hemolysis of erythrocytes (Yang et al., 2005). Mice treated with the ethanolic extract of P. oligotricha and L. trifolia were found to be safe at an increasing dose of up to 5 000 mg/kg b. wt. Any orally administered test substance with LD50 greater than 1000 mg/kg or higher than three times the minimum effective dose can be considered as nontoxic, and can be considered for further studies (Toma et al., 2015). Therefore, acute oral exposure to P. oligotricha and L. trifolia, at 5 g/kg can be considered non-toxic.a
The crude ethanol extracts P. oligotricha and L. trifolia exhibited significant and dose-dependent antimalarial activity in P. berghei infected mice, which may justify the traditional uses of the plants against malaria. Further studies are required to isolate and identify the active compound(s) responsible for the antimalarial activity .
The authors have not declared any conflict of interests.
REFERENCES
|
Adhanom TDW, Witten HK, Getachew A, Seboxa T (2006). Malaria in the epidemiology and ecology of health and disease in Ethiopia 1st edition. Ababa Addis, Ethiopia: Shama PLC. pp. 556-576.
|
|
|
|
Ayoola GA, Coker HB, Adesegun SA, Adepoju-Bello AA, Obaweya K, Ezennia EC, Atangbayila TO (2008). Phytochemical Screening and Antioxidant activities of Some Selected Medicinal Plants Used for Malaria Therapy in Southwestern Nigeria. Trop. J. Pharm. Res. 7(3):1019-1024.
|
|
|
|
|
Birru M, Geta M, Gurmu E (2017). Antiplasmodial activity of Indigofera spicata root extract against Plasmodium berghei infection in mice. Malar. J. 16:198.
Crossref
|
|
|
|
|
Builders M, Wannang N, Aguiyi J (2011). Antiplasmodial activities of parkia biglobosa: in vivo and in vitro studies. Ann Biol Res. 2:8-20.
|
|
|
|
|
Debella A (2002). Manual for phytochemical screening of medicinal plants. Ethiopian Health and Nutrition Research Institute, Addis Ababa, Ethiopia. pp. 35-47.
|
|
|
|
|
Dikasso D, Makonnen E, Debella A, Abebe D, Urga K, Menonnen W, Melaku D, Assefa A, Meknonnen Y (2006). In vivo antimalarial activity of hydroalcoholic extracts from Asparagus africanus Lam. in mice infected with Plasmodium berghei. Ethiop. J. Health Dev. 20:112-118.
|
|
|
|
|
European Community Guidelines (1986). Council directive of 24 November 1986 on the approximation of laws, regulations and administrative provisions of the Member States regarding the protection of animals used for experimental and other scientific purposes (86/609/EEC). Off. J. Eur. Commun. 358:1-29. Available at:
View
|
|
|
|
|
Fentahun S, Makonnen E, Awas T, Giday M (2017). In vivo antimalarial activity of crude extracts and solvent fractions of leaves of Strychnos mitis in Plasmodium berghei infected mice. BMC Complement. Altern. Med. 17:13.
Crossref
|
|
|
|
|
Gebretsadik G, Mekonnen Y (2016). In vivo Antiplasmodial Activity of Fractions and Chromatographic Sub Fractionions of Ethanol Extract of Clerodendrum Myricoides Leaves. J. Drug Design Med. Chem. 2(6):60-64.
Crossref
|
|
|
|
|
Gebreyohannes AE, Bhagavathula AS, Seid SA, Tegegn GH (2017). Antimalarial treatment outcomes in Ethiopia: a systematic review and meta-analysis. Malar J. 16:269.
Crossref
|
|
|
|
|
Habtemariam S, Gray A, Waterman P (1991). Flavonoids from three Ethiopian species of Premna. Zeitschrift für Naturforschung 47b(1):144-147.
|
|
|
|
|
Johnson IS, Ettebong EO, Okokon JE (2017). In vivo antiplasmodial activities of ethanolic leaf extract and fractions of Hilleria latifolia. J. Med. Plants Stud. 5(4):118-122.
|
|
|
|
|
Jones P, Kinghorn D (2006). Extraction of plant secondary metabolites. In Methods in Biotechnology Natural Products Isolation. 2nd edition. Edited by Sarker D, Latif Z, Gray A. Nerw Jersey: Human Press. pp. 323-351.
Crossref
|
|
|
|
|
Ketemma T, Etana D, Athanasiadou S, Adugna T, Gebeyehu G, Hoduijk G (2013). Ethno-medicinal study of plants used for treatment of human and livestock aliments by traditional healers in South Omo, Southern Ethiopia. J. Ethnobiol. Ethnomed. 9(32):1-15.
|
|
|
|
|
Kilama WL (2005). Ethical perspective on malaria research for Africa. Acta Trop. 95:276-284.
Crossref
|
|
|
|
|
Knight DJ, Peters W (1980). The antimalarial activity of N-benzyloxydihydrotriazines: I. The activity of clociguanil (BRL 50216) against rodent malaria, and studies on its mode of action. Ann. Trop. Med. Parasitol. 74(4):393-404.
Crossref
|
|
|
|
|
Mengiste B, Makonnen E, Urga K (2012). In vivo antimalarial activity of Dodonaea Angustifolia seed extracts against Plasmodium berghei in mice model. Momona Ethiop. J. Sci. 4(1):47-63.
|
|
|
|
|
Mukungu N, Abuga K, Okalebo F, Ingwela R, Mwangi J (2016). Medicinal plants used for management of malaria among the Luhya community of Kakamega East sub-County, Kenya. J. Ethnopharmacol. 194:98-107.
Crossref
|
|
|
|
|
Nalubega R, Nyanzi SA, Nakavuma JL, Kamatenesi-Mugisha M (2013). Ethnobotanical uses of Lantana trifolia L. and Sida cuneifolia Roxb. in Mukungwe and Wabinyonyi Sub-counties of Central Uganda. J. Intercult. Ethnopharmacol. 2(3):155-164.
Crossref
|
|
|
|
|
Ocho D, Stuik P, Price L, Kelbessa E, Kolo K (2012). Assessing the levels of food shortage using the traffic light metaphor by analysing the gathering and crop residues in Konso, Ethiopia. J. Ethnobiol. Ethnomed. 8(1):30.
Crossref
|
|
|
|
|
Okafor A, Nok A, Inuwa H (2013). Antiplasmodial Activity of Aqueous leaf Extract of Mucuna Pruriens Linn in Mice Infected with Plasmodium Berghei (Nk-65 Strain). J. Appl. Pharm. Sci. 3(4 Suppl 1):S52-S55.
|
|
|
|
|
Okokon JE, Ita BN, Udokpoh AE (2006). The in-vivo antimalarial activities of Uvaria chamae and Hippocratea africana. Ann. Trop. Med. Parasitol. 100(7):585-590.
Crossref
|
|
|
|
|
Silva GN, Martins FR, Matheus ME, Leitao SG, Fernandes PD (2005). Investigation of Anti-inflammatory and antinoceptive activities of Lantana trifolia. J. Ethnopharmacol. 100:254-259.
Crossref
|
|
|
|
|
Tadesse SA, Wubneh ZB (2017). Antimalarial activity of Syzygium guineense during early and established Plasmodium infection in rodent models. BMC Complement. Altern. Med. 17:21.
Crossref
|
|
|
|
|
Tchoumbougnang F, Amvam Zollo P, Dagne E, Mekonnen Y (2004). In vivo antimalarial activity of essential oils from Cymbopogon citratus and Ocimum gratissimum on mice infected with Plasmodium berghei. Planta Med. 70:1-4.
|
|
|
|
|
Toma A, Deyno S, Fikru A, Eyado A, Beale A (2015). In vivo antiplasmodial and toxicological effect of crude ethanol extract of Echinops kebericho traditionally used in treatment of malaria in Ethiopia. Malar. J. 14:196.
Crossref
|
|
|
|
|
Verma RK,Verma SK (2006). Phytochemical and termiticidal studies of Lantana camara var aculeata leaves. Fitoterapia 77:466-468.
Crossref
|
|
|
|
|
Waako P, Gumede B, Smith P, Folb P (2005). The in vitro and in vivo antimalarial activity of Cardiospermum halicacabum and Momordica foetida. J. Ethnopharmacol. 99:137-143.
Crossref
|
|
|
|
|
Waako P, Katuura E, Smith P, Folb P (2007). East African medicinal plants as a source of lead compounds for development of new antimalarial drugs. Afr. J. Ecol. 45:1-5.
Crossref
|
|
|
|
|
World Health Organisation (WHO) (2001). OECD guideline for testing of chemicals - Acute Oral Toxicity – Acute Toxic Class. Geneva: World Health Organization. Available at:
View.
|
|
|
|
|
World Health Organisation (WHO) (2004). Communicable disease surveillance and response.
|
|
|
|
|
World Health Organisation (WHO) (2005). The leishmaniasis and leishmania/HIV co-infection: Fact Sheets.
|
|
|
|
|
World Health Organisation (WHO) (2015). World Health Organization Malaria Report: Prospects for malaria elimination. Tecn. Rep. Serv. 1667:1-8.
|
|
|
|
|
Yang ZG, Sun HX, Fang WH (2005). Hemolytic activities and adjuvant effect of Astragalus membranaceus saponins on the immune responses to ovalbumin in mice. Vaccine 23(44):5196-5203.
Crossref
|
|