ABSTRACT
Highly active antiretroviral therapy (HAART) is considered toxic and has other life-threatening side effects including dyslipidemia. There is no research report on the health effects of Ethiopian highland green tea. Previous work data from elsewhere suggest that lipid abnormalities are associated with cardiovascular morbidity and mortality. The aim of this study was to investigate the possible protective effect of green tea (Ocimum gratissimum) hydro-ethanolic leaf extract on highly active antiretroviral therapy induced dyslipidemia in albino Wistar rats. Thirty rats of age 10 to 12 weeks and similar weights were selected and divided in to 5 groups of six rats each. Group-I (normal control group) were given distilled water, Group II were given HAART only, Groups III, IV and V were given antiretroviral therapy and 100, 200 and 400 mg/kg of extract, respectively for sixty days. The dissolved crude extracts of different doses were given to rats using oral gavage. On experiment day, the rats were fasted overnight, sacrificed by cervical dislocation and blood was taken by cardiac puncture for lipid profile investigation. Lipid profile was measured spectrophotometrically using standard kits and procedures. Elevated levels of serum total cholesterol, triacylglycerol, low density lipoprotein cholesterol and high density lipoprotein cholesterol were observed in highly active antiretroviral therapy treated group. The rats that received HAART+400 mg of O. gratissimum showed a significant decrement of serum total cholesterol, triglycerides, and low density lipoprotein cholesterol (p<0.05) with no alteration of high density lipoprotein cholesterol. The green tea leaf extract with a dose of 400 mg/kg has a good protective effect against HAART induced dyslipidemia which might be due to its antioxidant property.
Key words: Highly active anti-retroviral therapy, green tea leaf extract, dyslipidemia, rats.
Highly active antiretroviral therapy (HAART) regimens are currently in use and have been found effective in increasing life expectancy and immune status of HIV positive patients in the world since 1996 (Rouleau et al.,2011). These HAART regimens typically include a combination of at least three drugs, such as different association of protease inhibitors (PIs), non-nucleoside reverse transcriptase inhibitors and nucleoside/tide reverse transcriptase inhibitors (Gsponer et al., 2012). Thus, the introduction of HAART has led to a marked reduction in AIDS-related morbidity and mortality, because of the diversity of these drugs and their interference at different sites in the life cycle of the virus and disruption of the progress of viral proliferation in the body of the patient aggressively (Bhaskaran et al., 2008). However, besides the substantial benefits that result from the use of HAART regimens, laboratory and clinical experience have shown that they can induce considerable side effects on lipid metabolism which is characterized by lipodystrophy, central adiposity, dyslipidemia, increased risk of cardiovascular diseases and atherosclerosis (Sprinz et al., 2010). HAART associated dyslipidemia which is characterized by hypertriglyceridemia, hypercholesterolemia, and decreased serum levels of HDL-c either accompanied or not by increased levels of LDL-c that also involves hormonal and genetic predisposition is one of the complex lipid metabolism derangements (Fisher et al., 2006).
HAART also affects the hydrolysis of triacylglycerol rich lipoproteins and tissue lipase, which disrupts normal postprandial free fatty acid and lipoprotein catabolism and interferes with peripheral fatty acid trapping. The NRTI-based HAART, zidovudine, stavudine or lamivudine has become associated with the occurrence of dyslipidemia; however, lipid metabolism disorders are most evident in individuals who are using PIs (Abebe et al., 2014). Protease inhibitors promote dyslipidemia (hypertriglyceridemia, increase in total cholesterol and LDL-c and decrease in HDL-c) even in short-term studies (≤4 weeks) with RTV (Purnell et al., 2000) and LPV/RTV (Lee et al., 2005) in HIV-seronegative men. Protease inhibitors stimulate hepatic lipid synthesis by maintaining the nuclear activity of the sterol regulatory element binding protein (SREBP) (Tran et al., 2003). PIs bind to the glucose transporter (GLUT 4) and prevent glucose transport to the adipocytes and muscle cells but have no effect on liver (Flint et al., 2005). There is also no concrete evidence that PIs affect the transport of fatty acids by fatty acid transporter proteins. Instead, PIs affect hepatocyte metabolism by extending the activity of transcription factors involved in regulating lipid synthesis (Parker et al., 2005). Camelia genus is an evergreen shrub of the Theaceae family native to Japan, China, India, Southeastern Asia, with dark green shiny leaves and white flowers that is used for preparation of the second largely consumed drink in the world. Camelia genus contains caffeine, theophylline, and theobromine, glutamide derivative theanine and also contains many nutritional components, such as vitamin E, vitamin C, fluoride, and potassium.
Chacko et al. (2010) revealed the health benefits of consuming green tea which includes the prevention of cancer and cardiovascular diseases, the anti-inflammatory, antiarthritic, antibacterial, antiangiogenic, antioxidative, antiviral, neuroprotective, and cholesterol-lowering effects associated mainly with its antioxidant properties that is attributed to its high content of polyphenols. Most of these polyphenols in tea are flavanols commonly called catechins (Balentine et al., 1997). The main catechins in green tea are epicatechin (EC), epicatechin gallate (ECG), epigallocatechin (EGC) and epigallocatechin gallate (EGCG). These catechins account for 30 to 42% of the dry weight of green tea and EGCG accounts for 50 to 80% of the total catechins (Arshad et al., 2015). It was reported that radical-scavenging ability of EGCG was higher than that of other catechins due to the presence of a gallate moiety in the C ring (Xu et al., 2004). The potency of this property varies from country to country and even place to place in the same country because the content of its active constituents are affected by the type of soil, climate, and season of collection and age of the plant (Rani et al., 2014; Govarthanan et al., 2015). The aim of the present study was to explore antilipidemic roles of hydroethanolic extract of Camelia genus in HAART-induced dyslipidemia. To date no such work has been done on the Ethiopian highland green tea and to our knowledge there is no any report on science journals.
Plant and extraction
The packed green tea was purchased from Ethio Agri-CEFT private limited company in Ethiopia. The coarse powder of 918 g of green tea (Camellia sinensis) was macerated in 80% methanol (W/V) with a ratio of 1:10 for three days (72 h) by mechanical shaking at room temperature. The extract was first filtered using filtering cloth followed by Whatman filter paper No. 1 and then the filtrate was dried by rotary evaporator. Then the filtrate was evaporated in thermostatic oven at 40°C to remove the remaining methanol. The final gummy green tea leaf extract (GTE) was lyophilized, weighed, put in tight glass containers and kept in a refrigerator.
In-vitro antioxidant activity determination
Rapid screening of antioxidant activity of green leaf extract was done by dot-blot staining using 2, 2-diphenyl-1-picrylhydrazyl (DPPH) scavenging activity following the method of Soler-Rivas et al. (2000).
Photometric assay of DPPH scavenging activity
The DPPH scavenging activity of the extract of green tea was measured according to the method of Gyamfi et al. (1999) photometrically.
Experimental animals
Thirty adult female albino rats weighing about 200 to 250 g of age 10 to 12 weeks were obtained from Pharmacology Department, School of Medicine, College of Health Sciences, Addis Ababa University and housed in polypropylene cages and maintained at standard laboratory condition. They were provided with drinking water and standard pellet rat diet supplied by Kality Animal Nutrition Production Ltd., Addis Ababa Ethiopia ad libitum.
HAART dose extrapolation to experimental animals
The human dose of HAART drug was extrapolated to rats by the following formula as developed by Chen (2016): Dose for rats = (X mg/kg × 70 kg × 0.018) / 0.2 kg = 6.3X mg/kg, where X = the effective dose for man; 70 kg = the average standard weight of adult human; 0.018 = ratio of the equivalent dose between man and rat based on body surface area; 0.2 kg = the standard weight of a rat. The extrapolated HAART dose was given for rats for 60 days. The HAART given during the study period were in combination of ZDV/3TC+LPV/RTV with adult dose of 300/150 mg Po bid and 200/50 mg 2 tablets Po bid respectively. Then dose for rats were extrapolated as follows: ZDV/3TC (6.3 × 900/70 = 81 mg/kg) and LPV/RTV (250 × 4= 1000 mg which is normal adult dose/day). Hence, dose for rats was calculated as 6.3 × 1000/70 = 90 mg/kg per day.
Ethical approval
This study was conducted after experimental protocols approved by the departmental research and ethical review committee (DRERC), meeting number DRERC 03/15, and by protocol number MSc Thesis 05/15, on 04 September, 2015. All rules applying to animal handling, safety and care were properly followed.
Animal grouping and drug dose
The following are the groups and drug dose: Group-I normal control, given distilled water only; Group-II positive control, given HAART drugs only; Group-III, given HAART drugs + 100 mg/kg of green tea extract/per day/60 days; Group-IV, given HAART drugs + 200 mg/kg of green tea extract/per day/60 days; Group-V, given HAART drugs + 400 mg/kg of green tea extract/per day/60 days.
Blood sample collection and analyses
At the end of the experimental day the rats were fasted overnight and sacrificed by cervical dislocation after anesthesia using diethyl ether. Then blood was collected from each rat by cardiac puncture. Serum lipid profiles, that is, total cholesterol (TC), triglyceride (TG), high density lipoprotein (HDL), and low density lipoprotein (LDL) were measured by using autoanalyzer machine (Humastar300SR, Germany). The protocols given by the manufacturer were used during sample analyses.
The amount of crude extract which was obtained from 918 g of C. sinensis leaf coarse powder was 146 g. Therefore, the percentage yield of this extraction by using 80% methanol was calculated and given as:
%Yield = (146/918) × 100 = 15.9% (w/w)
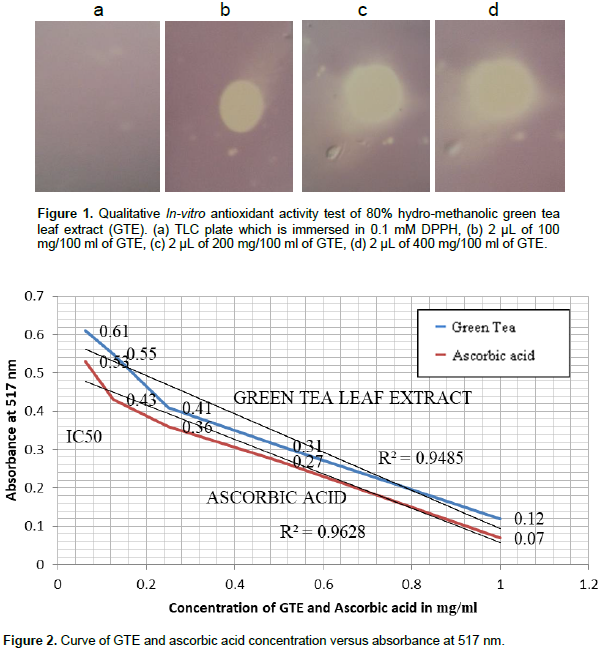
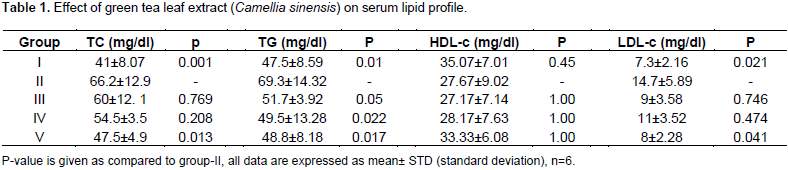
To observe the effect of GTE (C. sinensis) against HAART induced dyslipidemia in albino Wistar rats, the in-vitro antioxidant activity of GTE was studied qualitatively and quantitatively and lipid derangements were biochemically investigated in all experimental groups and presented in table and figures. As shown in Figure 1, the diameter of reduced zone increased in direct proportion to increase in extract concentration used. The yellowish pale color shows a reduced zone that masks the color of DPPH and the unreduced region or the region where the antioxidant cannot reach remains violet, which is the color of DPPH. The development of this pale yellowish color shows a reduction of DPPH (violet color) to DPPH-H, consistent with the postulate of Muthusamy et al. (2015) and work of Ebrahimzadeh et al. (2009) which is believed to be due to the transfer of electrons from the reducing agent (antioxidant) to DPPH. Quantitatively, the IC50 (inhibitory concentration 50, the concentration of the extract that decrease the absorbance of DPPH by half) of crude GTE was estimated as shown in Figure 2 which was ~0.16 mg/ml, whereas that of ascorbic acid is ~0.08 mg/ml. This result shows that the crude green tea extract has a closer antioxidant activity with ascorbic acid and also both ascorbic acid and GTE showed a decrease in the absorbance of DPPH in dose dependent manner, that is, their inhibitory effect increased as their concentration increased.
The absorbance of 0.008% DPPH was 0.98 which was used as a control and the absorbance of methanol (reference blank) was 0.0017 at 517 nm. As indicated in Table 1, the HAART-treated rats showed a significant increase in their serum level of TC, TG and LDL-c. However, the serum level of HDL-c showed numerical decrement with no statistical significance. In the GTE treated groups, the serum level of TC, TG and LDL-c decreased but statistically significant reduction was observed in group-V rats which were treated with the highest dose, that is, 400 mg/kg of GTE. However, serum level of TG was significantly reduced for all doses of GTE. HDL-c also showed a slight increment in all GTE treated groups but it was not statistically significant. Though dyslipidemia is seen sometimes in individuals who are not taking HAART, it is usually associated with HAART intake (Shafran et al., 2005; Chantry et al., 2008; Tassiopoulos et al., 2008). This study showed that, HAART treated group and HAART plus the lowest dose (100 mg/kg) of GTE treated group (Group-III) showed a significant increase (p<0.05) in the serum level of TC as compared to normal control group. But, group-V rats which were treated with 400 mg/kg and HAART showed a significant decrease in the serum level of TC as compared to group-II. Group-II also showed a significant increment (p<0.05) in the serum level of TG and LDL-c as compared to normal control group (G-I). Group-V rats showed a significantly low (p<0.05) serum level of TG and LDL-c.
HAART leads to lipodystrophy which is one or more of several metabolic abnormalities typically associated with dyslipidemia; usually due to elevated TC, TGs, LDL-C and low levels of HDL-C (Feeney and Mallon, 2011). It has been reported, that protease inhibitors treatment did not alter sterol regulatory element binding protein (SREBP) mRNA levels in lipidemic tissues, but promotes the accumulation of more activated SREBP in the nucleus which results in the constitutive induction of lipid biosynthesis through increased expression of lipogenic enzymes such as fatty acid synthase, 4-hydroxymethylglutaryl CoA reductase, acetyl CoA carboxylase and ATP-citrate lyase that cause increased lipogensis in liver. In addition, it also slows down the intracellular degradation of APo-B-100, prompting an overproduction of very low density lipoprotein (VLDL) particles. Antiretroviral drugs may also inhibit the LDL receptor-related protein reducing the clearance of VLDL from circulation (Riddle et al., 2001). Lipoatrophy caused by nucleoside analogues has been proposed to result from inhibition of mitochondrial DNA polymerase gamma within subcutaneous adipocytes. Dideoxinucleosides have a high potential for mitochondrial toxicity, which causes defective beta-oxidation of free fatty acids. Andrea et al. (2010) investigated the effects of HAART on mice and reported that these drugs caused dyslipidemia which contributes to the development of cardiovascular diseases. Findings of the present investigation were in line with those observation, which showed that HAART administration in rats resulted in elevated levels of TC, TG and LDL-C and drastic decrease in HDL-C as compared to normal control.
Green tea as a beverage and as traditional medicine originated and is widely used in Far East countries like China and Japan, and Western countries. It is believed to have healing effect for different health ailments like obesity, diabetes and cancer. This plant is becoming the front line traditional medicinal plant because of its high concentration of polyphenols known as catechins which are unaltered and well preserved in green tea. Catechins in green tea are one of the strongest antioxidants. This antioxidant effect of green tea in turn renders it to prevent a number of metabolic derangements (Zaveri, 2006). The catechins, particularly, EGCG of the green tea inhibits the intestinal absorption of dietary lipids; by interfering with emulsification, digestion, and micellar solubilization of lipids, which results in decreased absorption of TG, cholesterol, and other lipophilic compounds such as a-tocopherol (Loest et al., 2002; Wang et al., 2006a, b; Koo and Noh, 2007). A possible mechanism for the decreased cholesterol may be due to the up regulating potential of GTE on LDL receptor gene which in turn increases the uptake of LDL-C from blood circulation (Bursill et al., 2007). The decrease in serum TG might be due to the suppressing effect of green tea on expression of stearoyl-CoA desaturase (SCD-1) gene which determine hepatic triglycerides synthesis by involving in the biosynthesis of oleate and palmitoleate which are the main monounsaturated fatty acids of triacylglycerol (Rabia et al., 2015). The serum level of HDLc, which is also known as good cholesterol, in HAART only treated group showed a minimal and statistically insignificant decrease as compared to group-I. But, GTE treated groups showed a slight increment though it was insignificant change as compared to group-II.
The slight HDL increase is beneficial in that it promotes the reverse cholesterol transport, which avoids cholesterol accumulation and its pathologies. When the counter effect of various doses of GTE on the serum level of HDL-c of experimental rats is seen, the minimum dose of GTE (100 mg/kg) did not show any change in their serum level of HDL-c in group-III, but groups-IV and V rats showed a relative increment than group-II though not statistically significant. This finding is in agreement with a previous study done by Muramatso et al. (1986) on rats given catechins, where they observed normalization of serum level of TC without significant change in the serum level of HDL-c. The administration of GTE in ovariectomized rats considerably down regulates the hepatic expression of SREBP-1c and its target genes such as FAS and SCD1, and the genes that regulate hepatic cholesterol synthesis (HMGR) and efflux (ABCA1) (Shrestha et al., 2009). This finding suggests that GTE may not alter the expression of genes involved in intestinal lipid uptake and chylomicron assembly. Thus, based on the information available, the TG-lowering effect of GT in plasma and liver may be mediated partly via the suppression of lipogenesis and inhibition of luminal hydrolysis and micellar transfer of lipids to the enterocytes. All these biochemical mechanisms take into account the antidyslipidemic properties of green tea.
Green tea leaf extract has anti-dyslipidemic effect and preventive effect on development of nonalcoholic fatty liver disease satisfactorily and the response is profoundly effective with increasing dosage of GTE administration to rats. It also reverses the dyslipidemia induced by HAART. This indicates that HIV patients who are on HAART treatment will be beneficiary if they take green tea parallel with their antiretroviral treatment. A weight decrement was observed (data not shown) in the GTE treated group indicating that, through protection of dyslipidemia, GTE seems to avoid central obesity and abnormal accumulation of lipids in the adipose tissue.
The authors have not declared any conflict of interests.
The authors thank Addis Ababa University for thesis research support offered to Tesaka Wendimnew. They also thank the College of Health Sciences for allowing them to use their laboratory facilities.
REFERENCES
|
Abebe M, Kinde S, Belay G, Gebreegziabhier A, Challa F, Gebeyehu T, Nigussie P, Tegbaru B (2014). Antiretroviral treatment associated hyperglycemia and dyslipidemia among HIV infected patients at Burayu Health Center, Addis Ababa, Ethiopia: a crosssectional comparative study. BMC Res. Notes 7:380-387.
Crossref
|
|
|
|
Andrea M, Sabrina C, Barbara R, Daniela F, Giuseppe P, Eleonora D, Franco B, Stefano F (2010). The Bile Acid Sensor FXR Protects against Dyslipidemia and Aortic Plaques Development Induced by the HIV Protease Inhibitor Ritonavir in Mice. PLoS ONE 5:e13238
Crossref
|
|
|
|
Arshad HR, Fahad MA, Khaled SA, Saleh SA, Masood AK (2015). Implications of green tea and its constituents in the prevention of cancer via the modulation of cell signalling pathway. BioMed. Res. Int. pp. 1-12.
|
|
|
|
Balentine DA, Wiseman SA, Bouwens LC (1997). The chemistry of tea flavonoids. Crit. Rev. Food Sci. Nutr. 37:693-704.
Crossref
|
|
|
|
Bhaskaran K, Hamouda O, Sannes M, Boufassa F, Johnson AM, Lambert PC, Porter K CASCADE Collaboration (2008). Changes in the risk of death after HIV seroconversion compared with mortality in the general population. JAMA 300:5-59.
Crossref
|
|
|
|
Bursill CA, Abbey M, Roach PD (2007). A green tea extract lowers plasma cholesterol by inhibiting cholesterol synthesis and upregulating the LDL receptor in the cholesterol-fed rabbit. Atherosclerosis 193:86-93.
Crossref
|
|
|
|
Chacko SM, Thambi PT, Kuttan R, Nishigaki I (2010). Beneficial effects of green tea: a literature review. Chin. Med. 5:13-19.
Crossref
|
|
|
|
Chantry CJ, Hughes MD, Alvero C, Cervia JS, Meyer WA (2008). Lipid and glucose alterations in HIV-infected children beginning antiretroviral therapy. Pediatrics 122:129-38.
Crossref
|
|
|
|
Chen Qi (2006). Chinese traditional medicine pharmacological research methodology. Guangzhou, Peoples's Medical Publishing Company.
|
|
|
|
Ebrahimzadeh MA, Nabavi SM, Nabavi SF, Eslami B (2009). Free radical scavenging ability of methanolic extract of Hyoscyamus squarrosus leaves. Pharmacologyonline 2:796-802.
|
|
|
|
Feeney ER, Mallon PW (2011). HIV and HAART associated dyslipidemia. Open Cardiovasc. Med. J. 5:49-63.
Crossref
|
|
|
|
Fisher SD, Miller TL, Lipshultz SE (2006). Impact of HIV and highly active antiretroviral therapy on leukocyte adhesion molecules, arterial inflammation, dyslipidemia and atherosclerosis. Atherosclerosis 185:1-11.
Crossref
|
|
|
|
Flint OP, Lubinski LJ, Parker RA, Noor M (2005). Comparative effects of atazanavir versus other HIV protease inhibitors on glucose and amino acid stimulated insulin secretion. Antivir. Ther. 10:32.
|
|
|
|
Govarthanan M, Rajinikanth R, Kamala-Kannan S, Selvankumar T (2015). A comparative study on bioactive constituents between wild and in vitro propagated Centella asiatica. Genet Eng. Biotechnol. J. 13(1):25-29.
Crossref
|
|
|
|
Gsponer T, Petersen M, Egger M, Phiri S, Maathuis MH, Boulle A (2012). The causal effect of switching to secondline ART in programmes without access to routine viral load monitoring. AIDS 26:57-65.
Crossref
|
|
|
|
Gyamfi MA, Yonamine M, Aniya Y (1999). Free radical scavenging action of medicinal herbs from Ghana Thonningia sanguine on experimentally induced liver injuries. Gen. Pharmacol. 32:661-67.
Crossref
|
|
|
|
Koo SI, Noh SK (2007). Green tea as inhibitor of the intestinal absorption of lipids: potential mechanism for its lipid-lowering effect. J. Nutr. Biochem. 18:179-183.
Crossref
|
|
|
|
Lee GA, Rao MN, Grunfeld C (2005). The effects of HIV protease inhibitors on carbohydrate and lipid metabolism. Curr. HIV/AIDS Rep. 2:39-50.
Crossref
|
|
|
|
Loest HB, Noh SK, Koo SI (2002). Green tea extract lowers the lymphatic absorption of cholesterol and a-tocopherol in ovariectomized rats. J. Nutr. 132:1282-1288.
Crossref
|
|
|
|
Muramatso K, Fukuyo M, Hara Y (1986). Effect of green tea catechins on plasma cholesterol level in cholesterol-fed rats. J. Nutr. Sci. Vitaminol. 32:613-622.
Crossref
|
|
|
|
Parker RA, Flint OP, Mulvey R, Elosua C, Wang F (2005). Endoplasmic reticulum stress links dyslipidemia to inhibition of proteasome activity and glucose transport by HIV protease inhibitors. Mol. Pharmacol. 67:1909-1919.
Crossref
|
|
|
|
Purnell JQ, Zambon A, Knopp RH, Pizzuti DJ, Achari R, Leonard JM, Locke C, Brunzell JD (2000). Effect of ritonavir on lipids and post-heparin lipase activities in normal subjects. AIDS 14:51-57.
Crossref
|
|
|
|
Rabia SA, Masood SB, M Tauseef Sultan, Zarina M, Shakeel A, Saikat D, Vincenzo De Feo, Muhammad ZUH (2015). Preventive role of green tea catechins from obesity and related disorders especially hypercholesterolemia and hyperglycemia. J. Transl. Med. 13:79.
Crossref
|
|
|
|
Rani R, Nagpal D, Gullaiya S, Madan S, Agrawal SS (2014). Phytochemical, Pharmacological and Beneficial Effects of Green Tea. IJPPR 6:420-426.
|
|
|
|
Riddle TM, Kuhel DG, Woollett LA, Fichtenbaum CJ, Hui DY (2001). HIV protease inhibitor induces fatty acid and sterol biosynthesis in liver and adipose tissues due to the accumulation of activated sterol regulatory element-binding proteins in the nucleus. J. Biol. Chem. 276(40):37514-37519.
Crossref
|
|
|
|
Rouleau D, Fortin C, Trotier B, Lalonde R, Lapointe N, Cote P (2011). Antiretroviral therapy for adults infected with HIV. Guidelines for health care profesionals from Quebec HIV care committee. Can. J. Infect Dis. Med. Microbiol. 22:52-60.
Crossref
|
|
|
|
Shafran SD, Mashinter LD, Roberts SE (2005). The effect of low-dose ritonavir monotherapy on fasting serum lipid concentrations. HIV Medicine 6:421-425.
Crossref
|
|
|
|
Shrestha S, Ehlers SJ, Lee JY, Fernandez ML, Koo SI (2009). Dietary green tea extract lowers plasma and hepatic triglycerides and decreases the expression of sterol regulatory element-binding protein-1c mRNA and its responsive genes in fructose-fed, ovariectomized rats. J. Nutr. 139(4):640-645.
Crossref
|
|
|
|
Solerâ€Rivas C, Espín JC, Wichers HJ (2000). An easy and fast test to compare total free radical scavenger capacity of foodstuffs. Phytochem. Anal. 11(5):330-338.
Crossref
|
|
|
|
Sprinz E, Lazzaretti RK, Kuhmmer R, Ribeiro JP (2010). Dyslipidemia in HIV-infected individuals. Br. J. Infect Dis. 14:575-588.
Crossref
|
|
|
|
Tassiopoulos K, Williams P, George R, Marilyn C, James O, John F (2008). Association of Hypercholesterolemia Incidence with Antiretroviral Treatment, Including Protease Inhibitors, Among Perinatally HIV-Infected Children. J. AIDS. 47:607-614.
Crossref
|
|
|
|
Tran H, Robinson S, Mikhailenko I, Strickland DK (2003). Modulation of the LDL receptor and LRP levels by HIV protease inhibitors. J. Lipid Res. 44(10):1859-1869.
Crossref
|
|
|
|
Wang S, Noh SK, Koo SI (2006a). Epigallocatechin gallate and caffeine differentially inhibit the intestinal absorption of cholesterol and fat in ovariectomized rats. J. Nutr. 136:2791-2796.
Crossref
|
|
|
|
Wang S, Noh SK, Koo SI (2006b). Green tea catechins inhibit pancreatic phospholipase A2 and intestinal absorption of lipids in ovariectomized rats. J. Nutr. Biochem. 17:492-498.
Crossref
|
|
|
|
Xu JZ, Yeung SYV, Chang Q, Huang Y, Chen ZY (2004). Compar-ison of antioxidant activity and bioavailability of tea epicatechins with their epimers. Br. J. Nutr. 91:873-881.
Crossref
|
|
|
|
Zaveri NT (2006). Green tea and its polyphenolic catechins: Medicinal uses in cancer and non-cancer applications. Life Sci. 78:2073-2080.
Crossref
|