ABSTRACT
Breast cancer is a leading cause of deaths among women suffering from cancer in Kenya. The current study was done to determine anticancer activities of medicinal plant extracts against breast cancer cell lines (HCC 1395 and 4T1). Vero cells were used for evaluation of safety of extracts. Thiazoly blue tetrazolium bromide (MTT) assay was used in this study. Reference drugs were 5 fluorouracil and cyclophosphamide. Extract concentrations that inhibited growth of cell growth by half (IC50) were estimated using GraphPad prism version 7 and 90 % of extracts showed anticancer activities. Methanol extracts of Uvariodendron anisatum, Fagaropsis angolensis, Combretum tanaense, Hydnora abyssinica and water extract of F. angolensis exhibited remarkable anticancer activities (IC50Ë‚ 30 µg/ml). Methanol extracts of F. angolensis and H. abyssinica demonstrated high selectivity index (SI ≥3). Evaluation for safety, indicated that about 64% of the extracts under this study were non-toxic (CC50 ˃100 µg/ml). Findings from plants in this study support folklore claims. Phytochemical analysis, bioassay guided fractionation and toxicity studies are underway on extracts of C. tanaense, F. angolensis, H. abyssinica and U. anisatum.
Key words: 4TI, ethnomedicine, HCC 1395, IC50 values, medicinal plants, MTT assay, selectivity index, vero E6
Breast cancer is the most frequently diagnosed and leading cause of cancer deaths among women. It caused about 522,000 deaths in 2012 worldwide and these estimations are expected to double by the year 2030(WHO, 2014). Of all reported cases of cancer in Kenya, breast cancer has a prevalence of 23.3% and is the most common among women (Ministry of Public Health and Sanitation and Ministry of Medical Services, 2011-2016). The reduced access to comprehensive cancer care services, high cost of health services and inadequate cancer specialized health personnel further aggravate the cancer burden. Increasing burden of breast cancer cases in Kenya calls for a number of interventions (Policy Brief, 2011). Furthermore, as a commitment towards realization of vision 2030 in Kenya, policies that address prevention and management of cancers are being implemented. Kenya is also making every effort to reduce pre-mature mortality from non-communicable diseases (NCDs) by one-third as a commitment towards the third goal of sustainable development (ICSU, ISSC, 2015, United Nations (UN), 2015).
It is estimated that 80% of world population use traditional medicine (WHO, 2005; Malki, 2013). In connection with traditional medical systems, about 91% of cancer patients seek complementary alternative medicine (CAM) services worldwide and up to 98% of these patients are those suffering from breast cancer (Leung and Fong, 2007; Mazzio and Soliman, 2009). In other regions of the world, CAM has been useful in the discovery and development of drugs and drug derivatives which are useful clinically in the management of breast cancer. Two of these plants used for more than a century now are Catharanthus roseus (first identified in1950s) as a source of vincristine and vinblastine and Taxux brevifolia is a known source of taxol since 1962 (Evans, 2009). On the other hand, elliptinium isolated from Bleekeria vitensis has also been prescribed for breast cancer treatment for more than ten years now (Shoeb, 2006). Continued search for plant compounds or products is necessary, for discovery of lead compounds with antitumor activities for breast cancer.
Moreover, traditional medicine practices employ plants in treatment, prevention or management of breast cancer (King Saud University, 2016; Prakash et al., 2013). In Kenya, up to 70% of the over 43 million people use traditional medicine in primary health care. Cancer patients use plant based medicines to complement or as alternatives for conventional medicines (Njoroge and Kibunga, 2007). A number of plants are used ethnomedically in the management of breast cancer and some have been documented (Kareru et al., 2007; Ochwangi et al 2014). The plant extracts under this study: Combretum tanaense (Combretaceae), Fagaropsis angolensis (Rutaceae), Hydnora abyssinica (Hydnoraceae), Launaea cornuta (Asteraceae), Prunus and Uvariodendron anisatum (Annoanceae) have been africana (Rosaceae), Spermacoce princeae (Rubiaceae) reported in the literature to manage cancer (Kareru et al., 2007; Kokwaro, 2009; Jeruto, et al., 2008, 2011; Kigen et al., 2013; Ndwigah et al., 2014). The objective of the current study was to establish anticancer activities of selected plant extracts using HCC1395 and 4T1 breast cancer cell lines.
Five of the plants were obtained from Embu County (roots of U. anisatum from Kiangombe forest, rhizomes of H. abyssinica from Ishiara Karuri village, barks of F. angolensis, P. africana and aerial parts of L. cornuta from Irangi forest in Embu). The roots of C. tanaense were collected from Mount Kenya University botanical garden in Thika County, while the aerial parts of S. princeae were collected from Mabariri village, Bomwagamo location in Nyamira County. The collected specimens were identified and authenticated with the aid of a taxonomist at the National Museums of Kenya (East Africa Herbarium) where the voucher specimens were prepared and deposited. The plant voucher specimens were as provided in the parentheses, U. anisatum (JMO-1-2015), Hydnora abyssinica (JMO-2-2014), F. angolensis (JMO-3-2015), P. africana (JMO-3-2014), L. cornuta (JMO-1-2014), C. tanaense (JMO-2-2015) and S. princeae (JMO-4-2015).
Extraction and preparation of test extracts
The collected plant samples were air-dried under shade and thereafter they were ground using an electric mill. Methanol extracts were prepared by cold maceration for 48 h, and 250 g of the powders were soaked in 2.5 conical flasks using methanol (1 L). The methanol extracts were filtered and concentrated in vacuo at 50°C and finally dried in an oven at 35°C. Water extracts were obtained by boiling 50 g of the powdered drug in distilled water (0.5 L) for 5 min, the water extracts was then allowed to cool, filtered and then freeze dried. The dry extracts were weighed and stored in a freezer at -20°C. Stock solutions (10 mg/ml) of all extracts were made for anticancer assay, 10 mg of the dry extracts were dissolved in 100 μl of dimethylsulfoxide (DMSO) and then added up to 1000 μl with phosphate buffer solution (PBS). The stock solutions for each extract were then serially diluted by using PBS to obtain working concentrations ranging from 1000 to 0 μg/ml. The preparations were done under sterile conditions and the solutions of extracts were stored at 4°C until use.
Cancer cell lines and cell culture preparations
Human breast cancer cell line (HCC 1395), mouse breast cancer cell line (4TI) and normal kidney epithelial cells from African green monkey (Vero E6) cell line were obtained from the American Type Culture Collection (ATCC) (Rockville, USA). HCC 1395 (ATCC® CRL-2324™) cells were cultured and maintained using Roswell Park Memorial Institute (RPMI-1640). Normal kidney epithelial cells from African green monkey (Vero E6) and 4T1 cells were cultured and maintained using Eagle’s Minimum Essential Medium (EMEM). All cell cultures were supplemented with 100 units/ml penicillin/streptomycin and 10% fetal bovine serum (FBS) and they were maintained at 37°C in a humidified atmosphere of 5% CO2.
Anticancer activities assay of crude extracts
Standard 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was used to evaluate cell viability in the presence and absence of extract(s). The cells were plated in 96- well plates at a density of approximately 2 x104 cells per well and suspended in 100 µl of media. The plates were then incubated for 24 h at 37°C, 5% CO2 and relative humidity of 95% to attach. Thereafter, the extracts and standard drugs were added to the wells at concentrations ranging from 1000 to 0 µg/ml. The experiment was designed in such a manner that experimental blanks (wells containing media and test drug) and negative control (wells containing media and cells) were run simultaneously in triplicates. The plates were once again incubated for 48 h at conditions described above. MTT reagent (10 μl) was added to each well and the plates were further incubated for 4 h after which the supernatant was aspirated. Pure DMSO (100 μl) was added to each well to solubilize MTT crystals. The plates were then read for colour absorbance on an ELISA scanning multiwell spectrophotometer (Multiskan Ex labsystems) at 562 nm. The standard drugs, 5-fluorouracil and cyclophosphamide were used as positive controls. Percentage cell cytotoxicity was calculated using the formula:
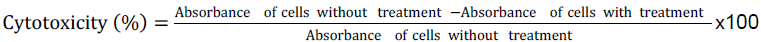
Selectivity index (SI) which indicates the ability of the drug to discriminate against cancerous cell and in favour of normal cells was calculated using the following formula:
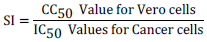
Where, CC50 is the concentration of the extracts that exerted cytotoxic effects on half of the population of normal cells and IC50 is the concentration of the extracts that inhibited growth/proliferation of half of the population of cancerous cells. All the cytotoxicity procedures were performed at Kenya Medical Research Institute (KEMRI) after the approval of the institutional Scientific and Ethics Review Unit.
Data analysis
Raw data for MTT assay was transfered for processing using Microsoft Excel 2013 to compute optical densities and IC50. Statistical analysis was performed with GraphPad Prism Version 7 to express the IC50 of the three independent experiments as mean ± standard error of the mean (SEM). Anticancer activities of the crude extracts was analyzed with respect to the American National Cancer Institute criteria where crude extracts that had IC50< 50 µg/ml were considered active and those with IC50< 30 µg/ml as remarkably active (Boik, 2001; Wratchanee et al., 2010; Siti et al., 2011). The safety levels of the extracts were estimated by determination of CC50 values; extracts that had CC50˃100 µg/ml were considered safe. The SI values were calculated and selectivity was denoted as high, moderate or non-selective where the values were ≥3, between 1 and 3, and Ë‚1, respectively.
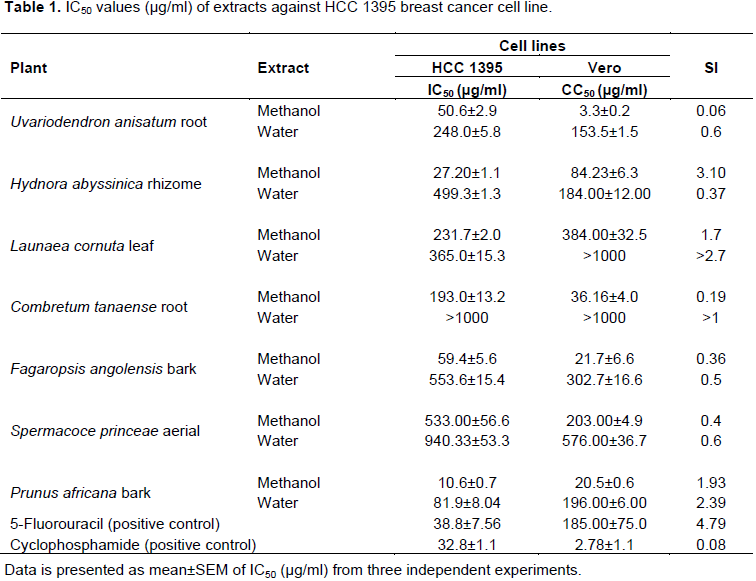
Anticancer activities of extracts against HCC 1395 breast cancer cell line
Investigations of anticancer activities of plant extract against human breast cancer cell line (HCC 1395) gave results as indicated in Table 1. Methanol extracts of the H. abyssinica rhizome and P. africana bark were the most promising with estimates of IC50 values being 27.20±1.1 and 10.6±0.7 µg/ml, respectively. Other extracts that had high activity were U. anisatum root methanol, F. angolensis bark methanol and P. africana bark water (IC50= 50.6±2.9, 59.4±5.6 and 81.9±8.04 µg/ml, respectively). Nine extracts exhibited low anticancer activities against HCC 1395 cell line (100 ≤IC50≤1000 µg/ml) as shown in Table 1 and C. tanaense water extract was considered inactive (IC50>1000 µg/ml).The results reveal that two methanolic plant extracts were more potent against HCC 1395 breast cancer cell line as compared to the reference drugs (cyclophosphamide and 5 fluorouracil). H. abyssinica rhizome (IC50=27.20±1.1 µg/ml) and P. africana bark (IC50=0.6±0.7) were more active against HCC1395 human breast cancer cell line. The IC50 for 5-fluorouracil was 32.8±1.1 and that of cyclophosphamide was 38.8±7.56 µg/ml against the same breast cancer cell line.
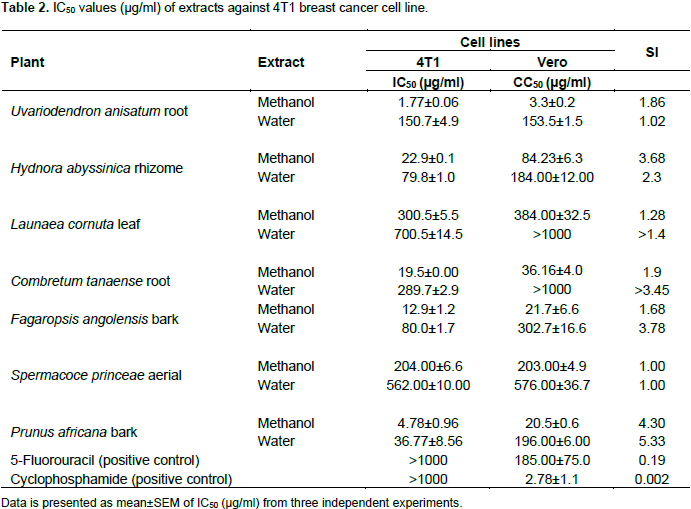
Anticancer activities of extracts against 4T1 breast cancer cell line
Five extracts demonstrated remarkable activities against mouse breast cancer cell line (4T1); they had IC50 estimates at concentrations below 30 µg/ml and these extracts were all methanolic extracts of U. anisatum root, H. abyssinica rhizome, C. tanaense root, P. africana bark and F. angolensis bark. The water extracts obtained from H. abyssinica rhizome, P. africana bark and F. angolensis bark had activities at concentrations 30≤IC50≤100 µg/ml and was regarded to have high activity. All the other remaining six extracts under this study demonstrated low activity as indicated in Table 2. The IC50 values for the active extracts against 4T1 cell lines, U. anisatum root methanolic extract (IC50=1.77±0.06 µg/ml), P. africana bark methanolic extract (IC50=4.78±0.96 µg/ml), F. angolensis bark methanolic extract (IC50=12.9±1.2 µg/ml), C. tanaense (IC50=19.5±0.0 µg/ml) and H. abyssinica rhizome methanolic extract (IC50=22.9±0.1 µg/ml) are shown. Interestingly, it was observed that the standard reference drugs (5-fluorouracil and cyclophosphamide) were inactive against 4T1 breast cancer cell line.
Safety of extracts against vero cell line
Nine out of the 14 extracts exhibited CC50 values which were greater than 100 µg/ml; these included, methanoland water extracts obtained from L. cornuta leaves and S. princeae aerial part; water extracts of U. anisatum root, H. abyssinica rhizome, F. angolensis bark, C. tanaense root and P. africana bark. Most of the methanol extracts of plants whose water extracts have been depicted as safe were found to be toxic against vero cells; they demonstrated CC50 values ranging from 3.3±0.2 to 84.23 ±6.3 µg/ml as indicated in Table 2. U. anisatum root methanol extract was found to be highly toxic; it demonstrated the lowest CC50 values against vero cell line (CC50=3.3±0.2 µg/ml). Methanol extracts of P. africana bark (CC50=20.5±0.6 µg/ml), F. angolensis bark (CC50=21.7 ±6.6 µg/ml) C. tanaense root (CC50=36.16±4.0 µg/ml) and H. abyssinica rhizome (CC50=84.23±6.3 µg/ml) were all considered toxic since they had CC50 values at concentrations that were less than 100 µg/ml. The standard reference drugs, 5-fluorouracil and cyclophosphamide had varied toxicity levels against the vero cell line; 5-fluorouracil (CC50>100 µg/ml) was considered nontoxic, while cyclophosphamide was found to be toxic with CC50 values estimated at 2.78±1.1 µg/ml (Table 1).
Selectivity index (SI)
The calculation of selectivity index
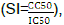
, was used to establish the ability of the extracts to discriminate their effect against normal and cancer cell lines. The calculated SI values are indicated in Tables 1 and 2 for HCC 1395 and 4T1 breast cancer cell lines, respectively. Two methanol extracts,
H. abyssinica rhizome (SI=3.10) and
P. africana bark (SI=1.93) demonstrated moderate selectivity index following their effect on HCC 1395 breast cancer cell line. All other extracts under this study were non selective in their activity in relation to HCC 1395 breast cancer cell line.
L. cornuta leaf water extract was the only water extract that was observed to have high selectivity index (SI>2.7). However, it had low activity against HCC 1305 cell line. The standard reference drugs demonstrated varying SI values with 5-fluorouracil having high selectivity (SI=4.79), whereas cyclophosphamide was non selective; it had SI values of 0.08 in relation to HCC 1395 breast cancer cell line as shown in Table 1.
Higher selectivity indices were recorded on 4T1 breast cancer cell line, water extracts of F. angolensis bark (SI=3.78) and P. angolensis bark (SI=5.33). Methanol extracts also had high activity on this same cell line, H. abyssinica rhizome (SI=3.68) and P. africana bark (S=4.3) as shown in Table 2. All the other extracts exhibited moderate selectivity indices with values ranging from 1.00 to 2.3, it is also noted that SI values for water extract of C. tanaense root were high (SI>3.45) on 4T1 breast cancer cell line. The SI values for the standard reference drugs as indicated in Table 2 shows that both 5-fluorouracil and cyclophosphamide were non-selective in the case of 4T1 breast cancer cell line.
The strength of anticancer activities of the extracts in this study varied in relation to extracts and breast cancer cell line; extracts of H. abyssinica rhizome demonstrated anticancer activities against HCC 1395 and 4T1 breast cancer cell line. A study conducted by Yagi et al. (2012) on cytotoxicity of water and 70% ethanol extracts of Hydnora johannis root, showed that the extracts had anticancer activity against human mouth epidermoid carcinoma (KB) cell line. Low toxicity of H. abyssinica rhizome extracts was reported against vero cell line; this is in agreement with the study done by Koko et al. (2009) and Yagi et al. (2012) which establish the safety of 80% ethanol extract of H. abyssinica against 3T3 mouse fibroblast cell line; 70% ethanol extract of H. johannis root against MRC5 (derived from non-cancer human fetal lung), respectively. Other toxicity studies that were done by Osman (2010) using rats, showed that water extract of H. abyssinica was non-toxic at dose of 1600 mg/kg and the safety studies are consistent with the current studies. P. africana bark extracts were found to be active against HCC 1395 and 4T1 breast cancer cell line; anticancer activity against HCC 1395 breast cancer cell line is reported for the first time in the current study.
Studies done by Nabende et al. (2015) using 4T1 breast cancer cell line showed that methanol extract of P. africana bark was active while the water extract was inactive. Contrastingly, in the current study, water extract was found to be active against 4T1 breast cancer cell line. In other anticancer studies, ethanolic extract of P. africana bark was found to have significant anticancer activities against PC3 and LNCaP human prostate cancer cell lines with IC50 values estimated at about 2.5 µl/ml (Shenouda et al., 2007). The water extract of P. africana bark is reported to be safe in this study; this finding is consistent with that of Karani et al. (2013), where estimated CC50 values of P. africana bark were 104.08 µg/ml. It is known that extracts with CC50 of more than 100 µg/ml are considered to be safe in cytotoxic studies. The anticancer activities of the remaining five plants in the current study: F. angolensis, C. tanaense, U. anisatum, L. cornuta and S. princeae has not been previously reported. Methanol extracts of two plants: F. angolensis bark and U. anisatum root exhibited considerable anticancer activities on breast cancer cell lines (4T1 and HCC 1395).
C. tanaense root methanol extracts were active against 4T1 and inactive against HCC 1395 breast cancer cell lines. Except for water extracts of F. angolensis which had high activity, all the other water extracts demonstrated low activities against the anticancer cell lines. The activities of methanol extracts of L. cornuta and S. princeae were also reported to be low. The variations in anticancer activities that was observed in different extracts, indicate that methanol was a better solvent for extracting compounds with anticancer activities. Most of the extracts in this study were non-toxic except for methanol extracts of U. anisatum root which was rated highly toxic, followed by F. angolensis bark, H. abyssinica rhizome and lastly C. tanaense root. It was established that the extracts that had high activities against cancer cell lines were toxic to the normal cell lines. The active extracts, H. abyssinica rhizome, U. anisatum root, P. Africana bark, F. angolensis bark and C. tanaense root exhibit differential selectivity and therefore demonstrated ability to distinguish between cancer and normal cell lines in this study. Previous studies done by Nabende et al. (2015), established that methanol extract of P. africana bark distinguished normal vero cell line from 4T1 breast cancer and CT26 human colon cancer cell lines by SI values of 7.26 and 1.11, respectively, this means that the methanol extract of P. africana bark had high cytotoxic effect against cancer cell line, on the other hand, it has low toxicity against normal cell line.
The findings of this study provide a scientific justification for the traditional use of these plants. The anticancer activity of 90% of these plants against 4T1 and HCC 1395 breast cancer cell lines is being reported for the first time in the current study. Methanolic extracts of C. tanaense (root), P. africana (bark), H. abyssinica (rhizome), F. angolensis (bark) and U. anisatum (root) possess high anticancer activities. Out of the five extracts with high potency, the methanol extract of H. abyssinica rhizome was considered the safest followed by methanol extracts of C. tanaense root, F. angolensis bark, P. africana bark and U. anisatum root in decreasing order of safety. Presently, these extracts are being investigated by bioassay guided fraction to establish the compounds that are responsible for activity against breast cancer cell lines.
The authors have not declared any conflict of interests.
The authors thank the Center for Viral Research (CVR), Kenya Medical Research Institute for provision of the cell lines. They also extend their gratitude to Mr. Muchiri of the Department of Tissue culture at VCR, KEMRI for assisting in the storage and maintenance of the cell lines. Zablon Malago and John Nzivo of Pharmaceutical Chemistry Laboratory, Mount Kenya University, Gervason Moriasi of Medical Biochemistry Laboratory, Mount Kenya University and Gilbert Mukindia of Kenyatta University Laboratory are also appreciated for their technical assistance.
REFERENCES
|
Boik J (2001). Natural Compounds in Cancer Therapy. Oregon Medical Press, Minnesota, USA.
|
|
|
|
Evans WC (2009). Trease and Evans' Pharmacognosy. (15th Edition), London: WB Saunders Company Ltd.
|
|
|
|
|
Jasneef A, Swatantra BS, Shivshanker P (2013). Phytochemical screening and physicochemical parametersof crude drugs: A brief review. Int. J. Pharm. Rev. 2(12):53-60.
|
|
|
|
|
Jeruto P, Mutai C, Catherine L, Ouma G (2011). Phytochemical constituents of some medicinal plants used by the Nandis of South Nandi district. J. Anim. Plant Sci. 9(3):1201-1210.
|
|
|
|
|
Jeruto P, Mutai C, Catherine L, Ouma G, Otieno D (2008). An ethnobotanical study of medicinal plants used by the Nandi people in Kenya. J. Ethno. Pharmacol. 116(1):370-376.
Crossref
|
|
|
|
|
Karani LW, Tolo FM, Karanja SM, Khayeka- Wandabwa C (1023). Safety of Prunus africana and Warbugia ugandensis in asthma treatment. South African Journal of Botany 88:183-190
Crossref
|
|
|
|
|
Kareru PG, Kenji GM, Gachanja AN, Keriko JM, Mungai G (2007). Traditional medicines among the Embu and Mbeere people of Kenya. Afr. J. Tradit. Complement Altern. Med. 4(1):75-86.
Crossref
|
|
|
|
|
Kigen KG, Ronoh KH, Kipkore WK, Rotich KJ (2013). Current trends of traditional herbal medicine practice in Kenya: A Rev. 2(1):32-37.
|
|
|
|
|
Kokwaro JO (2009). Medicinal plants of East Africa (Third edition) Kenya: East Africa Literature Bureau.
|
|
|
|
|
King Saud University (2016). Natural cures for breast cancer treatment. Saudi Pharmaceutical Journal 24:233-240
Crossref
|
|
|
|
|
Leung PC, Fong H (2007). Alternative Medicine for Cancer. Ann. Tradit. China Med. Vol. 3. World Scientific Publishing Co. Pte. Ltd.
Crossref
|
|
|
|
|
Malki AM (2013). Cancer treatment strategies. OMICS Group eBooks.
|
|
|
|
|
Mazzio AE, Soliman KFA (2000). In Vitro screening for tumoricidal properties of International Medicinal Herbs. J. Phytother. Res. 23(3):385-398.
Crossref
|
|
|
|
|
Ministry of Public Health and Sanitation and Ministry of Medical Services (2011-2016). National Cancer Strategy 2011-2016. Republic of Kenya.
|
|
|
|
|
Nabende PN, Karanja SM, Mwatha JK, Wachira SW (2015). Anti-proliferative activity of Prunus africana, Warbugia stuhlmanni and Maytenus senegalensis extracts in breast and colon cancer cell lines. Eur. J. Med. Plant 5(4):366-376.
Crossref
|
|
|
|
|
Ndwigah N, Stanely N, Amugune K, Beatrice K, Thoithi N, Grace N, Mwangi W, Mwangi W, Mugo N, Hannington N, Kibwage O (2014). Antibacterial and antifungal activity of Dombeya torrida (JF Gmel) and Hydnora abyssinica (A. Braun). Afr. J. Pharmcol. Therap. 3(1):14-18.
|
|
|
|
|
Njoroge GN, Kibunga JW (2007). Herbal medicine acceptance, sources and utilization for diarrhoea management in a cosmopolitan urban area (Thika, Kenya). Afr. J. Ecol. 45(1):65-70.
Crossref
|
|
|
|
|
Policy Brief (2011). On the situational analysis of cancer in Kenya (2011). Republic of Kenya: National Assembly.
|
|
|
|
|
Siti SMM, Nurhanan MY, Muhd Haffiz J, Mohd Ilham A, Getha K, Asiah O, Norhayati I, Lili Sahira H, Anee S (2011). Potential anticancer compound from Cebera Odollam. J. Trop. Forest. Sci. 23(1):89-96.
|
|
|
|
|
World Health Organization (WHO) (2014). Global status report on non-communicable diseases. Geneva. ISBN: 9789241564854. pp. 154-181.
|
|
|
|
|
Wratchanee M, Vithoon V, Wanna C, Arunporn I, Kesara NB (2010). Cytotoxic activity of Thai medicinal plants against human cholangiocarcinoma, laryngeal and hepatocarcinoma cells in vitro, BCM Compliment. Altern. Med. 10(55):1-8.
|
|
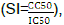 , was used to establish the ability of the extracts to discriminate their effect against normal and cancer cell lines. The calculated SI values are indicated in Tables 1 and 2 for HCC 1395 and 4T1 breast cancer cell lines, respectively. Two methanol extracts, H. abyssinica rhizome (SI=3.10) and P. africana bark (SI=1.93) demonstrated moderate selectivity index following their effect on HCC 1395 breast cancer cell line. All other extracts under this study were non selective in their activity in relation to HCC 1395 breast cancer cell line. L. cornuta leaf water extract was the only water extract that was observed to have high selectivity index (SI>2.7). However, it had low activity against HCC 1305 cell line. The standard reference drugs demonstrated varying SI values with 5-fluorouracil having high selectivity (SI=4.79), whereas cyclophosphamide was non selective; it had SI values of 0.08 in relation to HCC 1395 breast cancer cell line as shown in Table 1.
, was used to establish the ability of the extracts to discriminate their effect against normal and cancer cell lines. The calculated SI values are indicated in Tables 1 and 2 for HCC 1395 and 4T1 breast cancer cell lines, respectively. Two methanol extracts, H. abyssinica rhizome (SI=3.10) and P. africana bark (SI=1.93) demonstrated moderate selectivity index following their effect on HCC 1395 breast cancer cell line. All other extracts under this study were non selective in their activity in relation to HCC 1395 breast cancer cell line. L. cornuta leaf water extract was the only water extract that was observed to have high selectivity index (SI>2.7). However, it had low activity against HCC 1305 cell line. The standard reference drugs demonstrated varying SI values with 5-fluorouracil having high selectivity (SI=4.79), whereas cyclophosphamide was non selective; it had SI values of 0.08 in relation to HCC 1395 breast cancer cell line as shown in Table 1.