Hemizygia bracteosa (Benth.) Briq (Lamiaceae) is used in the treatment of mental disease, memory loss, HIV, malaria and had antimicrobial and hypoglycemic properties. To the authors’ knowledge, no scientific study has been done on these properties except antimicrobial and hypoglycemic properties. The phytochemical investigation focused on the quantification of phenolic compounds, flavonoids, tannins, anthocyanins and reducing sugars. Gas chromatography-mass spectrometry (GC-MS) analysis was performed on derivatized and non-derivatized extracts for the determination of chemical composition. Phytochemical composition evaluation shows phenolics content between 9.3 and 24.8 mg eq GA/g, a flavonoids content of 9 to 10.5 mg eq Q/g, an anthocyanins content of 0.0005 to 0.0025 mg eq C3G/g, a reducing sugar content of 126.1 to 123.7 mg eq G/kg, and undetected tannin content. GC-MS results revealed the presence of sugars, alcohols, acids, sesquiterpenes, amino acids, ester, lactone, phenolic. It also revealed that acetylcholinesterase inhibition was between 16.9 and 36.5%; xanthine oxidase inhibition between 3.8 and 60.2% ; ï¡-amylase inhibition between 6.1 and 8.7%, anti-inflammatory activity between 4.7 and 8.1%, anti-cancer activity between 24 and 96.4% for MCF7 ; 33.1 and 96.8% for HCT116 ; antioxidant activity between 2.1 and 13.2%. The results of acetylcholinesterase inhibition of ethyl acetate and methanolic extracts may justify the use of the plant in the treatment of mental illnesses and memory loss. But H. bracteosa should be used with caution because of its very high anti-cancer activity that could induce cytotoxicity.
Hemizygia bracteosa (Orthosyphon bracteatus) (Benth. Baker] (Lamiaceae) is a herbaceous plant native to tropical region and is abundant in West Africa. This plant is widely used in folklore to treat mental illness, memory loss, to prevent and treat HIV infection, AIDS symptoms and as a stimulant for dance (Mujovo, 2009; Stafford,2009). H. bracteosa is also used as an anti-mosquito (anti-Plasmodium falciparum) to treat malaria (Burkill, 1995). A previous work has also shown its antimicrobial and hypoglycemic properties (Konfo et al., 2014). The above various medicinal uses of H. bracteosa suggest that this plant could possess some biological activities including antioxidant, anti-cancer, anti-inflammatory, anti-diabetic, acetylcholinesterase inhibitory and xanthine oxidase properties. However, these biological activities of H. bracteosa have not been explored as suggested by the literature review. Therefore, this study aimed to study the chemical composition of H. bracteosa and to screen its biological activities including antioxidant, anti-cancer, anti-inflammatory, anti-diabetic, acetylcholinesterase inhibitory and xanthine oxidase properties.
Chemicals and plant material
Fresh leaves of H. bracteosa were harvested in July 2014, in Savalou located in the center part of Benin. The plant was botanically identified by the National Herbarium of the University of Abomey Calavi (no. AA 6543/HNB) where voucher specimens are deposited. All chemicals and reagents used in this study were of analytical grade and obtained from Sigma-Aldrich-Fulka chemical company (Saint Quentin, France).
Preparation of crude extract
Sufficient plant material was harvested, rinsed with water and spread in the laboratory's air-conditioned drying room at 16°C until the plant material was dry. The dried plant was ground in a blender to obtain powder. The powder was extracted successively with solvents of increasing polarity including cyclohexane, dichloromethane, ethyl acetate and methanol. 100 g of the powder obtained are mixed with 1 L of cyclohexane. The mixture was left in continuous maceration with stirring. The extract was then filtered. The organic portion was evaporated to dryness with a rotary evaporator at 25°C. The residue was extracted again with 1 L of cyclohexane and the extraction procedure repeated until exhaustion grounds. The residue was successively extracted using 1 L of other solvents of increasing polarity namely dichloromethane, ethyl acetate and methanol. The extracts obtained were stored at -20°C for analysis.
Phytochemical studies
Quantification of phenolic content
Total phenolics content was determined by Folin-Ciocalteu method with modifications (Bekir et al., 2013). 20 μL of extract solution Total phenolics content was determined by Folin-Ciocalteu method with modifications (Bekir et al., 2013). 20 μL of extract solution (50 mg/L DMSO) were added to 100 µL of Folin-Ciocalteu reagent (0.2 N). After 5 min of incubation at ambient air away from the light, 80 µL of sodium carbonate solution (Na2CO3; 75 g/L) was added and incubated for 15 min at room temperature away from light. The absorbance was measured at 765 nm against a blank where Folin-Ciocalteu reagent was replaced by distilled water. Gallic acid (0 to 11.5 mg/L) was used as standard reference for calibration curve. All tests were carried out in triplicate. The concentration was obtained from a calibration curve and expressed in mg equivalent of gallic acid per g of dry mass.
Quantification of total flavonoids
Quantification of the flavonoids was carried out according to trichloride aluminum method (Bekir et al., 2013). 100 μL of extract solution (50 mg/L DMSO) was added to 100 μL of methanolic solution of aluminuim trichloride (2%) and incubated for 15 min. The absorbance was read at 415 nm against a blank. All measurements are performed in triplicate. Quercetin is the reference used. Flavonoid content was calculated using the standard calibration curve and expressed in milligrams equivalent of quercetin per gram of dry mass (mg EQ/g).
Quantification of tanins
Tanins content was determined according to the vanillin method (Naczk et al., 2000). 100 μL of sulfuric vanillin solution (1%, 7M H2SO4) was mixed with 50 μL of the extract solution (50 mg/L of DMSO). The mixture was incubated for 15 min at 25°C in the absence of light. The absorbance was read at 500 nm against its blank. The reference used was catechine. All measurements were performed in triplicate. Tanin content was calculated using standard (catechin) calibration curve and expressed as milligrams equivalent of catechin per gram of dry mass (mg ECat/g).
Quantification of anthocyanins
The assay was carried out according to the differential pH method described by Bekir et al. (2013). Two reaction mixtures, one consisting of 100 μL of extract solution (50 mg/L DMSO) and 100 μL of buffer solution [pH = 1 (0.2 M KCl - HCl 0.2 M)] and the other consisting of 100 μL of extract solution (50 mg/L DMSO) and 100 μL of another buffer solution [pH = 4.5 (0.2 M acetic acid - 0.2 M sodium acetate)] were incubated for 15 min at ambient temperature and protected from light. Then, the absorbance of each mixture was measured at nm and 700 nm. All measurements were performed in triplicate. The anthocyanin content was calculated according to the equation:
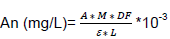
A = sample absorbance = [(abs510-abs700)pH=1 – (abs510-abs700)pH=4.5; M = molar mass of cyanidin 3-glucoside = 449.2 g/mol; DF = sample dilution factor = 10 to 50; e = molar absorption coefficient of the majority anthocyanin = 26900 L.mol-1cm-1; L = optical path = 0.52 cm; 10-3 = conversion factor from g to mg
Quantification of reducing sugars
100 μL of extract solution (50 mg/L DMSO) was mixed with 150 μL of 3,5-dinitrosalycilic acid (0.05 M), homogenized and then incubated in bain-marie at 100°C for 5 min. An addition of 750 μL of distilled water was followed by stirring. Finally, the absorbance was read at 540 nm against a blank and a negative control. Glucose was the reference used. All experiments were performed in triplicate. Reducing sugar content was calculated according to glucose calibration curve and expressed as milligram equivalent of glucose per gram of extract (mg eq G/g)
Preparation of extracts for GC-MS analysis
All extracts were analysed before and after derivatization. The derivatisation was carried out according to the method adopted by Tzing and Ding (2010) with some modifications. 5 mg of each extract were solubilized in 1 mL of a tetrahydrofural solution. Then, 150 μL of N,O-bis(trimethylsilyl) trifluoroacetamide (BSTFA), 1.5 μL of chlorotrimethylsilane were added to the mixture. The mixture was stirred, bubbled with nitrogen for 20 s and then incubated at 40°C for 15 min. Finally, 20 μL of the reaction mixture are taken for analysis by GC-MS.
GC-MS analysis
The analysis by GC-MS was carried out using a chromatograph Mark Varian 3400 Cx (Ullis, France) equipped with a fused silica capillary column DB-5MS (5% phenylmethylpolysyloxane, size 30 m x 0.25 mm, 0.25 mm film thickness) and a Varian Saturn GC-MS 4D mass spectrometer. The chromatographic analysis conditions were: raising the temperature from 60 to 260°C with a first gradient of 5°C/min, 15 min to reach 260°C; a second gradient of 40°C/min to reach 360°C; the duration of the analysis was 30 min; the samples were solubilized in petroleum ether, the carrier gas is helium (99.999% purity) at a rate of 1 ml/min and the injector at 200°C. The conditions of analysis by mass spectrometry were: the emission current of the mass spectrometer was 10 μA, the voltage of the electron multiplier varies between 1400 and 1500V; the temperature of the trap was 150°C and that of the transfer line was 170°C, the mass digitization was 40-650 atomic mass unit.
Phenolics constituents were identified by comparing their retention times obtained on the apolar column DB-5MS with those of the C5-C24 alkanes of the literature, mass spectra obtained with those of the NIST 08 library (National Institute of Standards and Technology) and reported in published articles. The percentage composition of the extract was carried out according to the method of Normalization of GC peak areas, assuming that the mass response factors are the same for all compounds. The results are mean values of ​​two extract injections, without taking into account correction factor. Only volatile compounds were identified.
Biological screening
All extracts were tested at a concentration of 50 mg/L of DMSO 5% in the wells.
Antioxidant activity assay
The antioxidant scavenging activity of extracts was studied using 1,1-diphenyl-2-picrylhydrazyl free radical (DPPH) as described by Bekir et al. (2013). 20 µL of extract solution were mixed with 180 µL DPPH methanolic solution (0.2 mM). The resulting mixture was incubated at 25°C for 25 min in the dark. Upon incubation, the absorbance of the mixture was measured at 524 nm against a blank. Negative control where the extract solution was replaced by solubilization solvent and its blank were also tested. Ascorbic acid was used as positive control. All tests were carried out in triplicate. The free radical-scavenging activity of each extract solution was expressed as percentage of inhibition and was calculated using the formula:
Inhibition (%) = 100 – (A(Blank) – A(sample))/A(Blank)
Acetylcholinesterase inhibition assay
The evaluation of acetylcholinesterase inhibition of extracts was carried out according to the Ellman’s colorimetric method as previously reported by Bekir et al. (2013) with modifications. 50 µL of phosphate buffer A [(Na2HPO4, 12H2O) 0.1M pH = 8], 25 μL of extract solution (50 mg/L and 5% DMSO in the well), 125 µL solution of dithiobisnitrobenzoate (3 mM) dissolved in buffer C [(Na2HPO4, 12H2O) 0.1 M pH = 7], and 25 μL of the acetylcholinesterase (2.8 U/mL) dissolved in buffer B [(Na2HPO4, 12H2O), (0.02M; pH = 7)] were added to the 96-well microplate. The mixture was incubated for 15 min at 25°C followed by addition of 25 µL of acetylthiocholine iodide. The mixture was again incubated for 10 min. Then, the absorbance was read at 412 nm against a blank. The same procedure was done with a negative control (0% acetylcholinesterase inhibition activity) where the sample was replaced by solubilization solvent and a blank, as well as the positive control which is galanthamine. The experiments were done in triplicate. Anticholinesterase inhibitory activity was expressed as percent of inhibition calculated as:
Inhibition (%) = [(A(control) – A(sample))/A(control)] x 100
Xanthine oxidase inhibition assay
The anti-xanthine oxidase inhibition activity was realized according to method described by Lin et al. (2000) with many modifications The mixture consisted of 60 µL of phosphate buffer [(Na2HPO4) (70 mM, pH=7.5)], 50 µL of extract solution (50 mg/L and 5% DMSO in the well) and 30 μL of xanthine oxidase (0.1 U/mL) was performed in wells of a 96-well and incubated at 25°C in a spectrophotometer for 15 min. This is followed by addition of 60 μL of xanthine (150 µM) and 5 min of incubation in the spectrophotometer, after which the absorbance was read at 295 nm against a blank. A negative control where extract solution is replaced by solubilization solvent and its blank were also tested (0% xanthineoxidase inhibition activity). Allopurinol was used as positive control. The assay was done in triplicate. The percent of xanthine oxidase inhibition was calculated using the following equation:
Inhibition (%) = [(A(control) – A(sample))/A(control)] x 100
a-Amylase inhibition assay
The assay was carried out following Jyothi et al. (2011) method with some modifications. 50 µL of extract solution (50 mg/L and 5% DMSO in the well) and 50 µL of a-amylase (0.02 U/mL) was incubated at 25°C for 15 min. 100 µL of starch (1%) was added to the mixture followed by a further incubation for 3 min at 25°C. Addition of 100 µL of DNS (3,5-dinitrosalycilic acid) reagent (12 mL of distilled water, 12 g of sodium potassium tartrate dissolved in 8 mL of sodium hydroxide (2 M); 0.4379 g of 3,5-dinitrosalycilic acid dissolved in 20 mL of distilled water) was done to stop the reaction. This mixture was again incubated for 10 min at 100°C. Finally, the reaction mixture was diluted by adding 1 mL of phosphate buffer [NaH2PO4: 2H2O (pH = 6.9)] containing sodium chloride 6.7 mM) and the absorbance was read at 540 nm against a blank. Blank was performed by replacing enzyme by buffer. A negative control sample was performed in the same manner by replacing the sample by solvent (0% a-amylase inhibition activity). Acarbose was used as positive control. a-Amylase inhibition activity was expressed as percentage inhibition and calculated using the formula:
Inhibition (%) = [(A(control) – A(sample))/A(control)] x 100
5-Lipo-oxygenase inhibition assay
Evaluation of the inhibition activity of 5-lipoxygenase is carried out according to the method described by Bekir et al. (2013). A reaction mixture composed of 150 μL of sodium phosphate buffer [(Na2HPO4, 2H2O), (KH2PO4), (NaCl), (pH = 7.4)], 20 μL of extract solution (50 mg/L and 5% DMSO in the well), 60 µL of linoleic acid (3.5 mM), 20 μL of enzyme, was incubated at 25°C for 10 min against a blank. The absorbance was measured at 234 nm. A negative control where the extract solution is replaced by the solvent solubilizing the extract, and a blank was also tested (0% 5-lipo-oxygenase inhibition activity). Nordihydroguaiaretic acid was used as positive control. The tests are carried out in triplicate. The inhibition activity of 5-lipoxygenase expressed as percentage inhibition is calculated according to the formula:
Inhibition (%) = [(A(control) – A(sample))/A(control)] x 100
Cytotoxicity evaluation
Anti-cancer activity of the extracts was evaluated on human breast cancer cells (MCF-7) and human colon cancer cells (HCT116) according to the method described by Natarajan et al. (2011) with modifications. 100 µL of cells suspension was deposited in the wells of the 96-well plate at a rate of 3.104 cell/well. 100 µL of culture medium was added to have a sufficient volume for growth. The plate was incubated at 37°C for 24 h. The culture medium was gently removed taking care not to detach the adherent cells. 100 µL of culture medium containing the extract were added to 100 µL of culture medium in order to have 50 mg/L for concentration of extract in the wells. The contact with the cells was carried out at 37°C for 48 h. After removal of culture medium and PBS washing, 50 µL of MTT solution (1 mg/mL in PSB) were added to each well and then the plate was incubated for 40 min at 37°C. The MTT was gently removed and then 50 µL of DMSO were added to each well for solubilization of the formazan crystals formed. Homogenization within the spectrophotometer and reading of the optical density at 605 nm completed the test. The experiments were repeated three times and the results were compared with cells grown only in the presence of culture medium. Controls were carried out in parallel with anti-cancer drug, Tamoxifen.
Statistical analysis
All data were expressed as means ± standard deviations of triplicate measurements. The confidence limits were set at P<0.05. Correlations were carried out using the correlation and regression in the EXCEL program.
Extraction yield
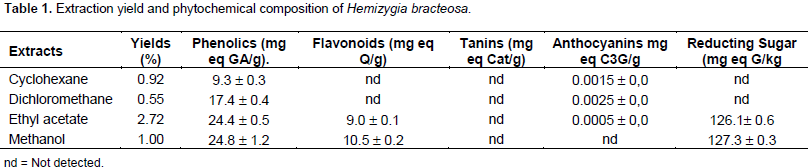
The yield of each extract is shown in Table 1. Ethyl acetate extract had the highest yield which was 2.72% followed by methanol (1.00%) and cyclohexane extracts (0.92%). The lowest yield was obtained with dichloromethane extract (0.55%). To the best of the authors knowledge, there is no data on the yields of the various extracts.
Chemical composition
Phenolic compounds content
Table 1 shows phenolic compounds contents of H. bracteosa extracts. The lowest content, 9.3 ± 0.3 mg equivalent gallic acid/g, was obtained with cyclohexane extract. The highest content, 24.8 ± 1.2 mg equivalent gallic acid/g, was obtained with methanol extract. These results showed that ethyl acetate and methanol extracts have more phenolic content than non polar extract, suggesting that the compounds are polar. This low content could influence the biological activities of the plant because polyphenols are revealed to be responsible for several activities. There is lack of information on phenolics content of genus, Hemizygia. However, a comparison with some Lamiaceae (Khomdram and Singh, 2011) showed that, H. bracteosa contains fewer phenolics compounds than Elsholzia blanda (46.28 ± 0.543), Elsholzia stachyodes (32.07 ± 0.566), Hyptis suaveolens (32.07 ± 0.363), Ocimum americanum (26.15 ± 0.808) and Ocimum basilicum (39.31 ± 0.439). This difference could be explain by the difference between the organs, extraction technique, extraction solvents and nature of the plants.
Flavonoids content
Flavonoids were quantified only in ethyl acetate extract and in methanol extract with respective values ​​of 9 ± 0.1 mg eq Q/g and 10.50 ± 0.2 mg eq Q/g dry mass (Table 1). Information on flavonoids content of H. bracteosa plant or on species of the genus Hemizygia is scare. Nevertheless these results can be compared with those of other species in the family. Thus, the value of the methanol extract is lower than those obtained with ethanolic extracts (80%) of the aerial parts of the eight Lamiaceae species studied by Khomdram and Singh (2011). This difference could be explain by the difference of extraction method, plant organ, edaphic, climatic and environmental conditions.
Tanin content
Tanin content was not detected. However, comparison with eight species of Lamiaceae showed that they contain them (Khomdram and Singh 2011). This difference can be explained by difference of extraction technic, plant organs, and climatic and environmental conditions.
Anthocyans content
Only dichloromethane extract contains a tiny amount of 0.0023 ± 0 mg eq cyanidine-3-glucoside/g of dry mass (Table 1). No data is available on the anthocyanin content of H. bracteosa in the literature or on the genus Hemizygia.
Reducing sugars content
The determination of reducting sugar content showed that only the ethyl acetate and methanol extracts contained respectively 126.1 ± 0.6 and 127.3 ± 0.3 mg eq glucose/kg (Table 1). Reducing sugars have not been quantified in the less polar extracts. This determination of the sugar content of H. bracteosa would be the first in the genus Hemizygia.
Compounds identified before and after derivatisation
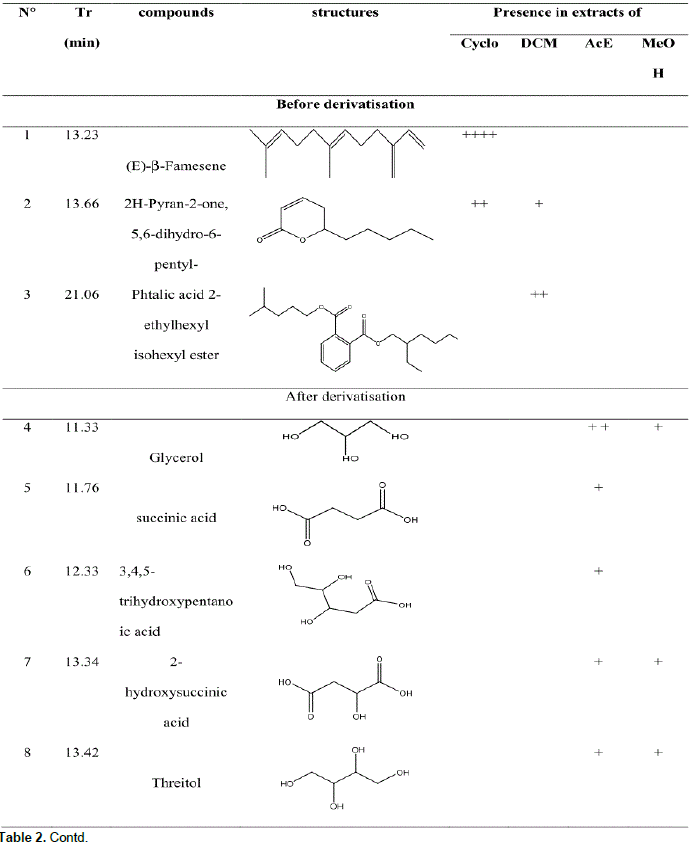
GC-MS analysis before and after derivatization of H. bracteosa extracts identified sugars, alcohols, acids, sesquiterpene, amino acid, ester, lactone and a single phenolic compound called catechollactate (Table 2). Sesquiterpene (E) -b-famesene was very abundant in the cyclohexane extract. It was the major compound of the essential oil of the aerial part of the plant (Kpoviessi et al., 2016). The only phenolic compound identified and its low abundance could account for the low phenolic content.
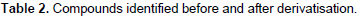
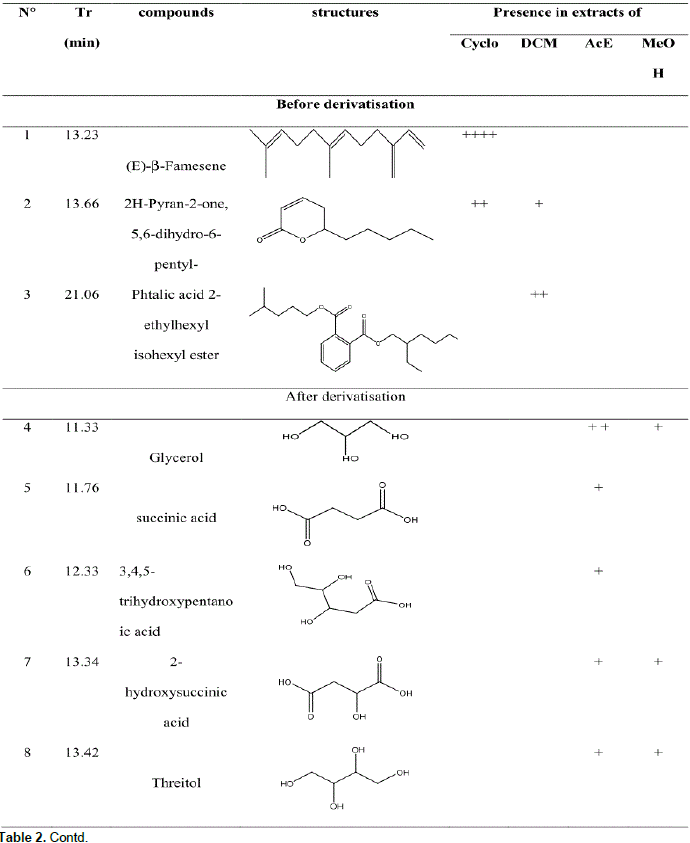
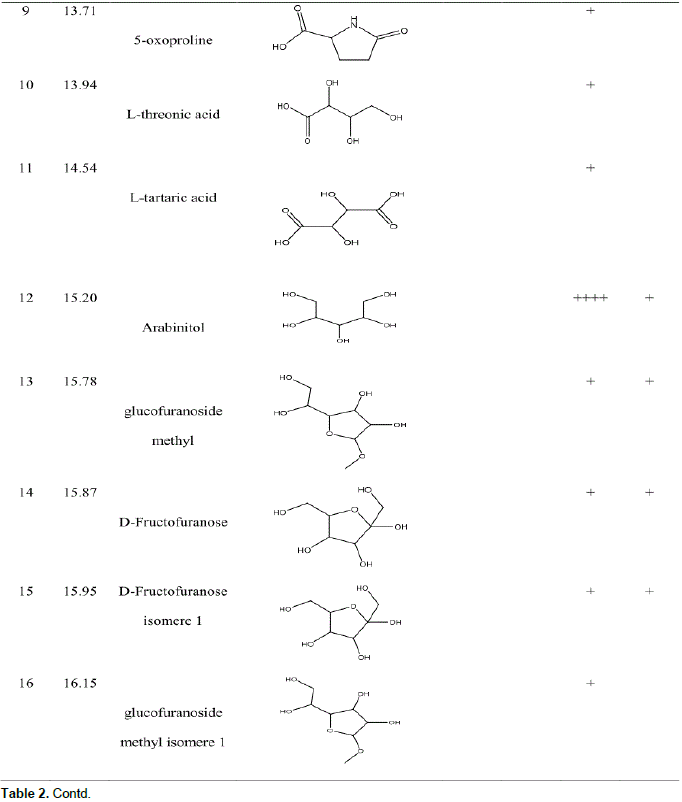
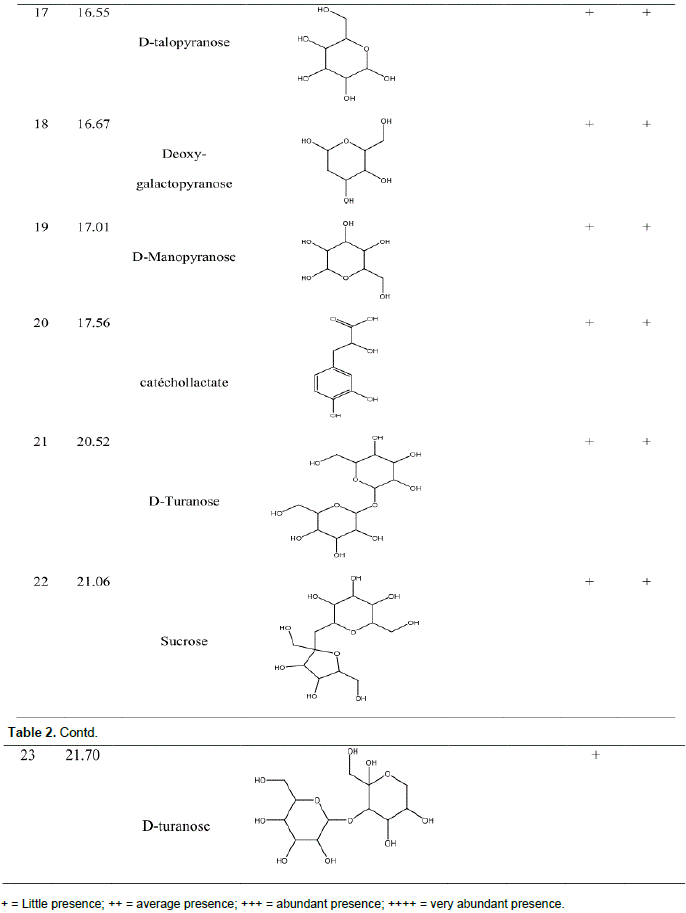
Biological activities
The antioxidant activity
The results of DPPH essay (Table 3) showed that H. bracteosa extracts were poor in antioxidant. The antioxidant activity of extracts was low. The best activity was obtained with methanol extract which presented 13.2 ± 0.6% of inhibition. Cyclohexane extract is the lowest in antioxidant (2.1 ± 0.2% of inhibition). Methanol is the best antioxidant extraction solvent. Ascorbic acid (5.3 mg/L) was used as the positive control. It presented 53.01 ± 0.4% of inhibition. This low activity could be explained by the poverty of phenolic compounds. Phenolic compounds could be responsible for this activity. Antioxidant activity of H. bracteosa leaves methanolic extract was different from of ethanolic extract of Coriandrum sativum plant which gave 0.21 ± 0.1 % at 0.1 mg/ml (100 mg/L) (Maya and Sarada, 2014). H. bracteosa at 50 mg/L was rich in antioxidant with 13.2 ± 0.6 % than C. sativum.
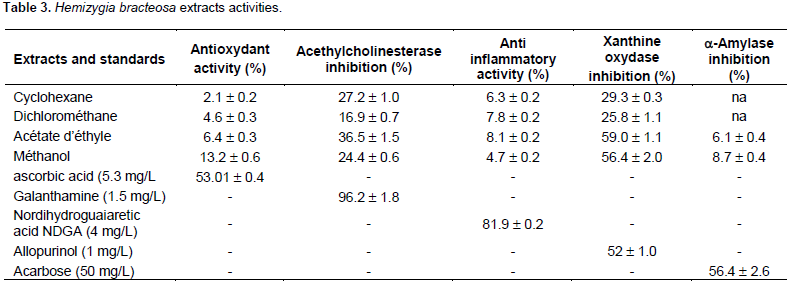
The acetylcholinesterase inhibition
These results (Table 3) show that the H. bracteosa extracts have moderate activity ranging from 16.9 ± 0.7% (dichloromethane extract) to 36.5 ± 1.5% (Ethyl acetate extract). The results could justify its use in the treatment of memory loss in pupils and the elderly. Percent of galanthamine (1.5 mg/L) inhibition which was used as positive control was 96.2 ± 1.8 mg/L. No data on acetylcholinesterase inhibition are available in the literature. Methanolic extract of H. bracteosa leaves would be more active than Lavandula augustifolia ethanolic extract which is a Lamiaceae, (Ferreira et al., 2006) because 0.5 mg/ml (500 mg/L) of L. augustifolia, is 10 times the concentration of H. bracteosa which gave 26.6 ± 9.5% inhibition. H. bracteosa at 50 mg/L was inhibited at 24.4 ± 0.6%.
5-lipoxygenase inhibition
The evaluation of 5-lipoxygenase inhibition H. bracteosa extracts anti-inflammatory activity gave Results (Table 3) which show a very low activity. The lowest value, 4.7 ± 0.2%, was obtained with methanolic extract. The highest value was 8.1 ± 0.2% inhibitions, it was obtained with ethyl acetate extract. The positive control used was nordihydroguaiaretic acid (NDGA) (4 mg/L) presenting percent of inhibition of 81.9 ± 0.2% of inhibition. The results of this study were the first in the literature. No comparison can be made with genus Hemizygia. As compared to Canthium parviflorium studied by Sirigiri et al. (2014), H. bracteosa was less active. While H. bracteosa at 50 mg/L showed 4.7 ± 0.2% inhibition, C. parviflorium another Lamiaceae showed 59% inhibitionat of 25 μg/mL (25 mg/L). H. bracteosa extract was more concentrated but had a lower percentage and C. parviflorium is less concentrated but with a higher percentage.
The xanthine oxidase inhibition
Analysis of xanthine oxidase inhibition results (Table 3), showed an activity between 3.8 ± 0.1% (dichloromethane extract) and 60.2 ± 0.9% inhibition (methanolic extract). The xanthine oxidase inhibition of H. bracteosa is summarized in Table 3. The most polar extracts showed the best inhibitions. Allopurinol which was the positive control used, presented 1 mg/L inhibition of 52 ± 1.0%. There is no data on this activity in the literature. A comparison with Shivraj and Se (2014) work showed that leaves methanolic extract of H. bracteosa was more active than root methanolic extract of Rapidx negundo. Indeed, two extracts were tested at the same concentration of 50 mg/L but the percentage inhibition of H. bracteosa which is 60.2 ± 0.9 was greater than that of Vitex negundo which was 45.8 ± 0.2%. This difference could be explained by the difference of organs, the technic, edaphic conditions, climatic conditions and geographical area. H. bracteosa may be indicated for the treatment of gout disease.
The a-amylase inhibition
The evaluation of anti a-amylase activity of H. bracteosa extracts gave results shown in Table 3. The results show that all extracts exhibited a-amylase inhibition. Methanol extract showed the highest inhibition with 8.7 ± 0.4%. The lower inhibition, 6.1 ± 0.4% was obtained with ethyl acetate extract. Cyclohexane and dichloromethane extracts showed no activity. This shows that the bioactive compounds responsible for this activity are present only in the polar extracts. Polar solvents are the best solvents extracting these bioactive compounds.
Acarbose (50 mg/L) is the positive control used which presented 56.4 ± 2.6% inhibition. The leaves exhibited low activity, while the whole plant was hypoglycemic in Wistar rats (Chabi et al., 2015). Although, origin of the two plants is the same city, the difference in activity can be explained mainly by the difference in the organs used (leaves in the current study and whole plant in the study carried out by Chabi et al. (2015), and also by the type of extract (aqueous extract in the study of Chabi et al. (2015) and extract with cyclohexane, dichloromethane, ethyl acetate and methanol in the current investigation) and the mode of evaluation of the activity (in vivo in Chabi et al. (2015) and in vitro in the current study).
H. bracteosa was less active than the methanolic extracts of Phlomis bruguieri, Phlomis rigida, Stachys byzantina and Scutellaria toumefortii which presented at 15 mg/mL (1500 mg/mL), an inhibition percentage respectively, of 49.7 ± 5.7; 94.8 ± 3.3; 86.1 ± 2.8 and 52.1 ± 0.1 (Hedieh et al., 2014). It takes 30 times the concentration of H. bracteosa to have these high percentages. On the other hand, it would be less active than other Lamiaceae whose methanolic extracts showed 60.24 ±0.69% for Mangifera indica, 47.30 ± 1.27% for Azadirachta indica, 47.12 ± 0.40% for Phyllanthus amarus (Dineshkumar et al., 2010). These differences can be explained by concentration, geographical location, edaphic conditions, climatic conditions and extraction technique.
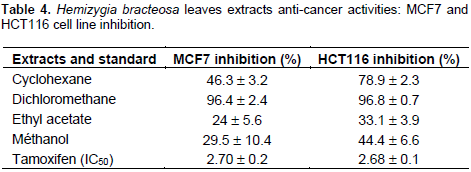
The anti-cancer activity
Table 4 shows the results of the anti-cancer activity. The table reveals that all extracts had an activity on cancer cells. Ethyl acetate extract was the least active on MCF7 (24 ± 5.6%) and HCT116 (33.1 ± 3.9%) cells. Dichloromethane extract was most active on both MCF7 (96.4 ± 2.4%) and HCT116 (96.8 ± 0.7%) lines. No data on anti-cancer on MCF7 and HCT116 lines exist in the literature. The apolar extracts were more active than polar extracts. This can be explained by the affinity of the compounds responsible of this activity with apolar solvents. As the extracts strongly inhibit the growth of cancer cells, they will therefore be toxic at this concentration for normal cells. This cytotoxicity is corroborated by the study carried out by Chabi (2015) which highlights the toxicity of the plant at certain doses on normal cells of liver and kidneys of Wistar rats. H. bracteosa should therefore be taken with caution. Further study is needed to identify and isolate the compounds responsible for the manufacture of medicines. What was involved in the search for anti-cancer drugs was weaking havoc in the populations today.
This study showed that H. bracteosa leaves have low concentration of phenolic compounds, contain bio-active substances which are sugars, alcohols, acids, phenolic compounds, sesquiterpenes, amino acid, ester and lactone. The leaves have low antioxidant activity. It also showed a moderate xanthine oxidase inhibition, acetylcholinesterase inhibition and a strong anti-cancer activity. It is cytotoxic, therefore, its use must be with caution. This work is a preliminary assessment study that deserves further investigations to identify the bioactive substances through several fractionation of extracts.
The authors have not declared any conflict of interests.
The authors thank the government of Benin for providing scholarship to support this study. They also thank Professor Jalloul Bouajila for his contribution.