ABSTRACT
This is the first to report the antimicrobial effects of extracts of seven endemic medicinal plants. Flower extracts of Onopordum jordanicolum exhibited antibacterial activity against Staphylococcus aureus, MRSA, Klebsiella pneumonia, and Proteus mirabillis. Ethanol extract displayed significant antibacterial activity against S. aureus with the best MIC and MMC values. Onopordum blancheanum flower extracts produced antibacterial activity against S. aureus, Escherichia coli, K. pneumonia, and P. mirabillis. Ethanol extract of Aethionema carneum leaves exhibited antibacterial activity against all test bacteria except MRSA and produced significant antibacterial activity against E. coli with the best MIC and MMC values. Methanol and acetone extracts of Delphinium ithaburense leaves showed significant antibacterial activity against K. pneumonia. Aqueous extract of Lathyrus hirticarpus leaves revealed a broad spectrum of antibacterial activity. Aqueous and acetone extracts of Orchis sancta flowers showed significant antibacterial activity against MRSA and Pseudomonas aeruginosa, respectively. Aqueous and methanol extracts from Papaver umbonatum flowers exhibited significant antibacterial activity against Streptococcus pyogenes with the best MIC and MMC values. For antifungal activity, it was found that Aspergillus brasiliensis and Candida albicans were inhibited by aqueous extracts of A. carneum and P. umbonatum, acetone extract of D. ithaburense, ethanol extract of L. hirticarpus, methanol extracts of O. blancheanum and O. sancta. Interestingly, acetone extract of O. jordanicolum displayed significant antifungal activities against A. brasiliensis and C. albicans with the best MIC and MMC values. Phytochemical screening of promising extracts revealed the presence of alkaloids, flavonoids, saponins, and/or tannins which might be responsible for their antimicrobial activity.
Key words: Antimicrobial, phytochemical, carneum, ithaburense, hirticarpus, Onopordum, sancta, umbonatum.
Traditional medicines that are based on medicinal plants still play an essential role in health care. Medicinal plants are an important source of drugs and a huge number of bioactive medicines against different diseases are developed from plants origin (Sahoo et al., 2010). Secondary metabolites derived from medicinal plants hold great promise in the treatment and prevention of different types of diseases caused by several pathogenic microorganisms. Recently, more than one hundred secondary plant metabolites derived from plants are used worldwide as drugs to treat different diseases (Jagatheeswari et al., 2013). Increasing antibiotic resistance by pathogenic bacteria is developing at an alarming rate, as the rate of discovery for new antibiotics has been on the decline (Hopwood, 2007). Therefore, medicinal plants shift the attention of scientists and researchers to herbal medicine to find effective antimicrobial agents for treatment of diseases caused by multidrug resistant pathogens like bacteria and fungi (Parungao et al., 2007). Although natural products derived from medicinal plants are used for thousands of years to treat different diseases, most medicinal plants around the world are not yet explored for their medicinal activities (Hassan, 2012). Therefore, seven endemic medicinal plants in Jordan (Aethionema carneum, Delphinium ithaburense, Lathyrus hirticarpus, Onopordum blancheanum, Onopordum jordanicolum, Orchis sancta, and Papaver umbonatum) were selected in the present study and evaluated for their antimicrobial activities against human pathogenic bacteria and fungi (Table 1).
Few phytochemical investigations about A. carneum have been described in the literature. This annual plant has a unique character, which produce glucosinolate (mustard oil) compounds (Adigizel, 2000).Nevertheless, the significant antimicrobial activity of this plant is not known. The plant D. ithaburense has been utilized for the treatment of various problems (Table 1). Many species of Delphinium have numerous toxic alkaloids (Gardner and Pfister, 2007). However, few phytochemical investigations about D. ithaburense have been described in the literatures and the antimicrobial effects were not identified. The medicinal plant L. hirticarpus is grown in the northern part of Jordan. This plant is commonly called Indian vetch. Seeds of members of the genus Lathyrus are toxic if ingested in high quantity (Grela et al., 2000). Until today, there is no antimicrobial study related to this plant. Two species of Onopordum from Asteraceae family were investigated in this study, O. blancheanum and O. jordanicolum. The O. blancheanum distributed throughout the Mediterranean areas (Ronel et al., 2009) and it is commonly known as cotton thistle. The O. jordanicolum is a Jordanian local plant and it is commonly called camel thistle. This plant species is found in Jordan Eastern desert. There are no previous studies that assessed the antimicrobial activity of O. blancheanum and O. jordanicolum. The orchid O. sancta belong to the family Orchidaceae. This family is the largest family of the plant kingdom, comprising more than 30,000 species (Jin-Ming et al., 2003).
In Jordan, there is a unique species of orchids called O. sancta commonly known as holy orchid and considered as an elegant perennial plant. The existence of alkaloids in orchid and considered as an elegant perennial plant. The existence of alkaloids in orchid constituents suggests that orchids possess some biological activity against disease and cancer (Bulpitt et al., 2007). Even so, the antimicrobial activity of O. sancta was not evaluated. The native plant to Jordan, P. umbonatum, is grown wildly in many parts of the world. Flowers of P. umbonatum are used for treating various mild pains (Table 1). Recently, it was revealed that active phytochemicals found in P. umbonatum are antioxidants and contain various phenolic compounds (Bernáth, 2006). Nonetheless, no previous antimicrobial investigations of P. umbonatum have been described in the literature. Therefore, no previous studies were performed concerning the use of those medicinal plants selected in this study as sources of antibacterial and antifungal agents. Thus, this study is established to determine the antibacterial and the antifungal activities of those selected medicinal plants.
Plant materials collection
Plant materials and specimens were collected in March, 2014 from different locations of Jordan. The collected plant specimens were identified by a taxonomist specialist, Mr. Refad Khawaldeh, at Jordan Royal Botanical Garden where the voucher specimens are deposited and conserved for future reference; A. carneum (Voucher specimen no. Azzam 079/2014) was collected from Dana Preservation in Tafila, D. ithaburense (Voucher specimen no. Obeidat 082/2014) was collected from Princess Tasneem bint Ghazi Technological Research Station in Al-Salt, L. hirticarpus (Voucher specimen no. Azzam 087/2014) and O. blancheanum (Voucher specimen no. Azzam 093/2014) were collected from Sharhabil Bin Hassneh EcoPark in Irbid, O. jordanicolum (Voucher specimen no. Obeidat 081/2014) was collected from Jordan East desert, and O. sancta (Voucher specimen no. Obeidat 085/2014) and P. umbonatum (Voucher specimen no. Obeidat 090/2014) were collected from the Royal Botanical Garden in Jarash. Collected plant materials (roots, leaves, fruits, and flowers) from each plant species were washed and dried in the shade in an aerated place at room temperature until complete drying. The dried plant material was ground into a fine powder for initiating the extraction process.
Preparation of plant extracts
Each powdered plant material was soaked in each of acetone, Ethanol, methanol, and hot water solvents (plant material to solvent ratio was 1:10, w/v) and extracted for two weeks at room temperature with shaking at 150 rpm. All extracts were filtered through White canvas and filter paper. The collected filtrates of the extracts were dried by evaporation until dryness. The dried extracts were weighed and dissolved in 0.05% dimethyl sulphoxide (DMSO) to a final stock concentration of 200 mg/ml. All crude extracts were purified by filtration through 0.22 mm filter units and kept at -20°C until use.
Test microorganisms
A total of 10 antibiotic-resistant test microorganisms including three Gram positive bacteria (Streptococcus pyogenes ATCC 8668, Staphylococcus aureus ATCC 25923, and Methicillin resistant Staphylococcus aureus ATCC 95047 (MRSA)), five Gram negative bacteria (Salmonella typhimurium ATCC 14028, Escherichia coli ATCC 8739, Pseudomonas aeruginosa ATCC 27253, Klebsiella pneumonia ATCC 7700, and Proteus mirabillis ATCC 12453) and two fungi (Aspergillus brasiliensis ATCC 16404 and Candida albicans ATCC 10231) were used in this study. The antibiotic-resistance patterns for each test microorganism were determined previously by Obeidat (2017).
Preparation of inoculums
Test bacteria and test fungi were grown in nutrient broth (NB) at 37°C for 24 h and in sabouraund dextrose broth (SDB) at 28°C for 48 h, respectively. Bacterial and fungal cultures were serially diluted and adjusted to achieve 2×106 colony forming units (CFU/ml) for bacteria and 2×105 spore/ml for fungi (Ceylan et al., 2008).
Antimicrobial activity
The antibacterial activity of plant extracts was screened in triplicates against test bacteria by using the agar well diffusion method (Perez et al., 1990). An inoculum of 100 μl bacterial suspension was swapped uniformly to solidified nutrient agar (NA) plates and the plates were allowed to dry for 5 min. Holes of 5 millimeters (mm) in diameter were made in the seeded agar using sterile cork borer. Aliquot of 50 μl from each plant extract was added into each hole on the seeded medium and allowed to stand for one hour for proper diffusion and incubated at 37°C for 24 h. The antibacterial activity was evaluated by measuring the inhibition zone diameter in mm around the wells. The antifungal activity of plant extracts was performed in triplicates against test fungi in the same manner as described for bacteria but by using sabouraund dextrose agar (SDA) plates and incubation at 28°C for 48 h.
Determination of the minimum inhibitory concentration and minimum microbial concentration
The minimum inhibitory concentration (MIC) and the minimum microbial concentration (MMC) were determined for plant extracts that showed the most significant antimicrobial activity according to the modified methods previously described by Obeidat (2011). All samples were examined in triplicates.
Phytochemical screening
The phytochemical components of promising extracts (extracts that exhibited broad spectrum antibacterial activity and extracts that showed the most significant antifungal activity) from the selected medicinal plants in this study were performed according to the standard protocols; Mayer’s test for alkaloids (Siddiqui and Ali, 1997), sodium hydroxide test for flavonoids (Roopashree et al., 2008), foam test for saponins (Roopashree et al., 2008), and ferric chloride test for tannins (Iyengar, 1995).
Statistical analysis
All measured inhibition zones were expressed as the mean ± standard error (SE). For statistical evaluation of data for generated inhibition zones, one-way ANOVA (Tukey’s studentized range) was applied using the program IBM SPSS statistics 19.0 for Windows. Significant differences were considered significant at P < 0.05.
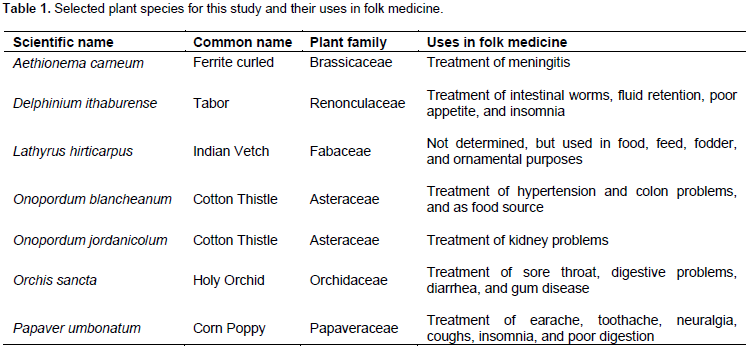
Antimicrobial activities were estimated by measuring the average diameters of the formed inhibition zones around wells. The inhibitory effects of plant extracts prepared by different solvent (water, Ethanol, methanol, and acetone) from A. carneum, D. ithaburense, L. hirticarpus, O. blancheanum, O. jordanicolum, O. sancta, and P. umbonatum were investigated against 10 antibiotic-resistant test microorganisms including eight bacteria and two fungi. In general, most plant extracts of selected plants in this study showed a broad spectrum of antimicrobial activity. Although all plant parts of selected plants were screened for the potential antimicrobial activity, it was found that antimicrobial activity was produced from leaves of A. carneum and D. ithaburense from flowers of L. hirticarpus, O. blancheanum, O. jordanicolum, O. sancta, and P. umbonatum (Table 2). It was found that flower extracts of O. jordanicolum were the best source for antimicrobial agents. All test microorganisms were affected by at least one extract. It was found that all flower extracts of O. jordanicolum exhibited antibacterial activity against Gram-positive bacteria except acetone extract which did not show antibacterial activity against S. pyogenes (Table 2). Methanol and water extracts were significantly the most active extracts against S. aureus and MRSA, respectively (Table 2).
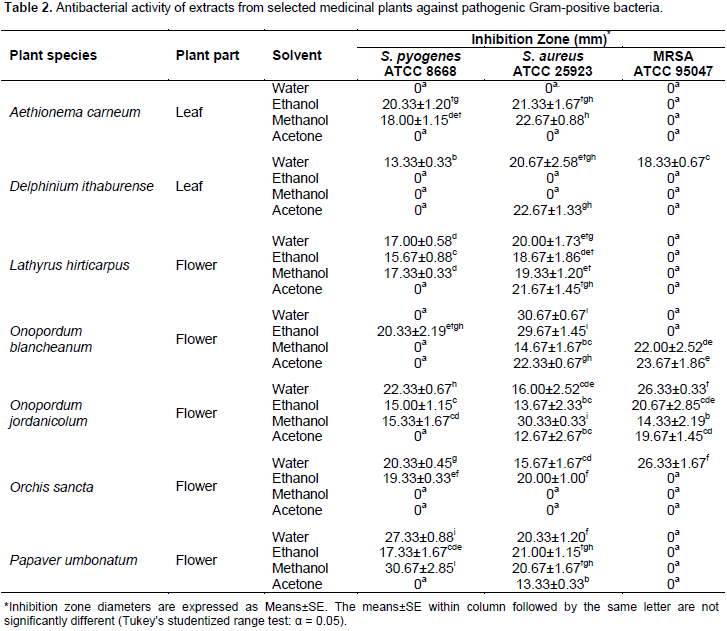
Ethanol extract of O. jordanicolum exhibited antibacterial activity against Gram-negative bacteria (Table 3). On the other hand, water and ethanol extract of O. blancheanum, which belongs to the same plant genus, showed a broad spectrum of antimicrobial activity with significant antibacterial activity against S. aureus. As shown in Table 2, the aqueous and methanol extracts of P. umbonatum flowers exhibited significant antibacterial activity against S. pyogenes. Remarkably, it was found that leaf extracts of D. ithaburense (methanol and acetone extracts) and A. carneum (water and ethanol extract) produced significant antibacterial activity against K. pneumonia (Table 3). It was observed that S. typhimurium (Gram-negative bacterium) followed by MRSA (Gram-positive bacterium) and P. aeruginosa (Gram-negative bacterium) are the least sensitive among all test microorganisms used in this study (Table 2 and 3). The bacterium S. typhimurium was affected by only three extracts (water and ethanol extract of A. carneum and ethanol extract of O. jordanicolum); ethanol extract of A. carneum gave the most significant antibacterial activity (Table 3), whereas eight extracts inhibited MRSA growth (Table 2).
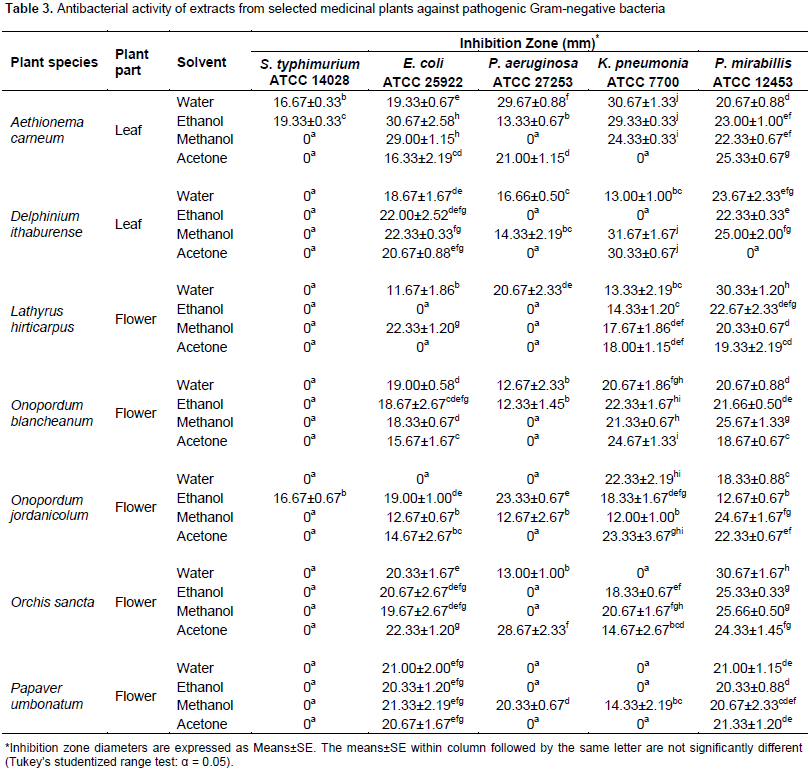
Interestingly, all extracts of O. jordanicolum exhibited anti-MRSA activity. The highest significant anti-MRSA activity was produced from aqueous extracts of O. jordanicolum and O. sancta flowers. It was found that only 13 extracts (out of 32) exhibited antibacterial activity against P. aeruginosa (Table 3). The most significant antibacterial activity against P. aeruginosa was obtained from aqueous extract of A. carneum and acetone extract of O. sancta. However, the most sensitive bacteria were P. mirabillis and E. coli, which were affected by most plant extracts (Table 3). The growth of P. mirabillis was inhibited by all plant extracts except acetone extract of D. ithaburense. The bacterium E. coli was insensitive to only three plant extracts; ethanol and acetone extracts of L. hirticarpus and aqueous extract of O. jordanicolum. For antifungal activity, it was observed that the test fungi was inhibited by few plant extracts (Table 4). For instance, it was observed that only water extract of A. carneum leaves, ethanol extract of L. hirticarpus flowers, and methanol extract of O. sancta flowers exhibited antifungal activity against A. brasiliensis and C. albicans (Table 4). However, it was found that all flower extracts of O. jordanicolum exhibited antifungal activity.
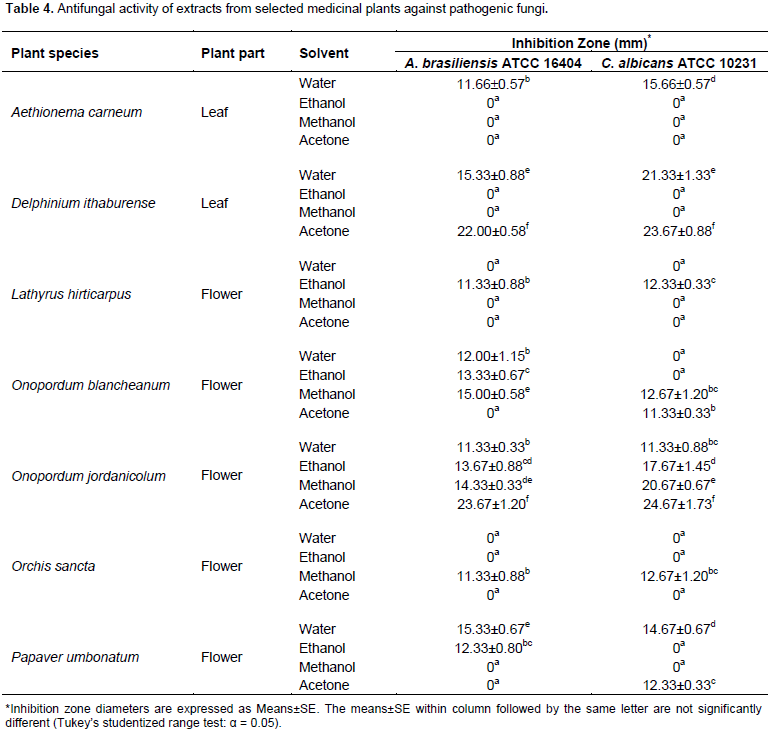
Furthermore, acetone extract of O. jordanicolum flowers produced the highest significant antifungal activity against A. brasiliensis and C. albicans. It was interesting to notice that acetone extract of D. ithaburense leaves also exhibited significant antifungal activity against both test fungi (Table 4). It was found that methanol extract of O. blancheanum and aqueous extract of P. umbonatum exhibited high antifungal activities. Significant antibacterial activities, expressed as MIC and MMC, of promising plant extracts against test bacteria are shown in Table 5. Extracts of selected plants were among the most active with MIC values ranging from 4 to 32 mg/ml and MMC values ranging from 8 to 64 mg/ml. The antibacterial activities with the best MIC and MMC values were produced significantly by ethanolic extracts of A. carneum against E. coli and O. blancheanum against S. aureus and by methanolic extracts of O. jordanicolum against S. aureus and P. umbonatum against S. pyogenes (Table 5). Whereas, MIC and MMC values of the most active antifungal plant extracts ranged from 32 to 64 mg/ml and from 64 to 128 mg/ml, respectively. The antifungal activities with the best MIC and MMC values were produced significantly by acetone extracts of O. jordanicolum followed by D. ithaburense against A. brasiliensis and C. albicans.
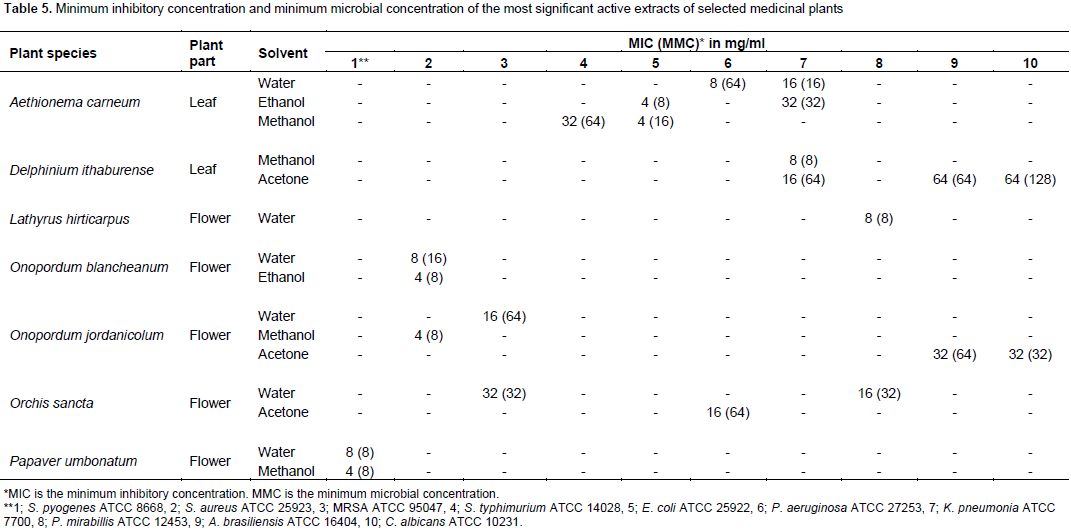
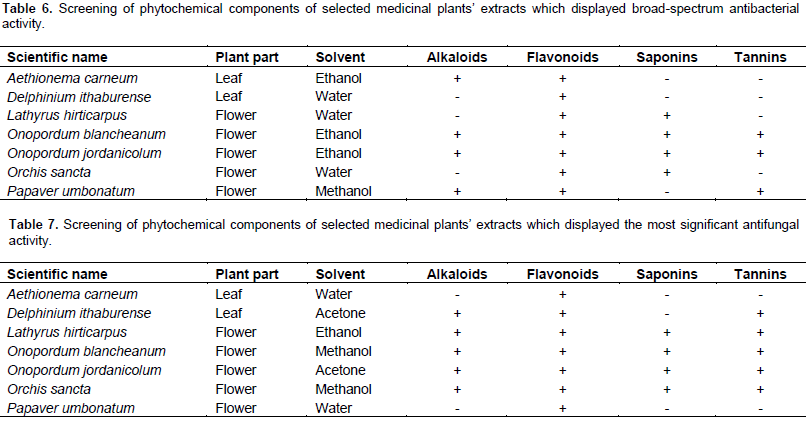
The preliminary phytochemical screening of extracts with broad-spectrum antibacterial activity revealed the presence of alkaloids in ethanol extract of A. carneum leaves, O. blancheanum and O. jordanicolum flowers as well as in methanol extract of P. umbonatum flowers (Table 6), flavonoids in all selected medicinal plant extracts, saponins in aqueous extracts of L. hirticarpus and O. sancta flowers in addition to ethanol extract of O. blancheanum and O. jordanicolum flowers, tannins in ethanol extract of O. blancheanum and O. jordanicolum flowers and in methanol extract of P. umbonatum flowers (Table 6). The screening of phytochemical constituents of extracts with the most significant antifungal activity is illustrated in Table 7. The presence of alkaloids and tannins was detected in all extracts except aqueous extracts of A. carneum leaves and P. umbonatum flowers. Flavonoids were detected in all promising plant extracts. Saponins were found in methanol extracts of O. blancheanum and O. sancta, acetone extract of O. jordanicolum, and ethanol extract of L. hirticarpus flowers.
Antibiotic resistance of many human pathogens including bacteria and fungi has become a major clinical and public health problem. Therefore, the antimicrobial activity of seven endemic medicinal plants that have not been evaluated previously was investigated and determined in the current study. Four solvents were used in the extraction of bioactive compounds from different plant parts. Generally, it was revealed that most prepared extracts from leaves of A. carneum and D. ithaburense and from flowers of L. hirticarpus, O. blancheanum, O. jordanicolum, O. sancta, and P. umbonatum exhibited promising antibacterial and antifungal activities. Thus, this study provides valuable information on the abilities of the extracts of selected medicinal plants to yield bioactive compounds that could be potentially used to treat diseases caused by multidrug-resistant bacteria and fungi. The efficacy of plant extracts evaluated as antimicrobial agents was dependent on the solvent of extraction. In general, ethanol was the best extracting solvent from A. carneum leaves and from flowers of O. blancheanum and O. jordanicolum, produced broad-spectrum and/or significant antibacterial activity (Tables 2 and 3). Whereas, extraction of D. ithaburense leaves, L. hirticarpus flowers, and O. sancta flowers by hot water was found to be the best method for extraction of antibacterial active compounds while methanol appeared to be the appropriate solvent for extraction of antibacterial agents from P. umbonatum flowers.
For production of broad spectrum antifungal activity, it was observed that acetone is the best extracting solvent from most selected medicinal plants. Obeidat et al. (2012) reported that ethanol is the best extracting solvent for plant leaves. Alzoreky and Nakahara (2003) found that both methanol and acetone proved to be suitable solvents for extraction of bioactive inhibitory effects from medicinal plants. Eloff (1998) and Cowan (1999) revealed that methanol was more efficient than acetone in extracting phytochemicals from plant materials. In accordance with those contradictory findings and in agreement with Obeidat et al. (2012), the results of this study indicate that the antimicrobial efficacy depends on selected plant species, plant part, solvent type, test microorganism, and phytochemical components. The obtained results show that most plant extracts displayed antibacterial activity against all test bacteria except S. typhimurium which was affected only by three extracts. In contrast to previous findings (Ripa et al., 2009; Obeidat et al., 2012), this study showed in general no significant differences in susceptibility of Gram-positives and Gram-negative bacteria to plant extracts but unfortunately most extracts produced no or even limited antibacterial activity against S. typhimurium.
The antimicrobial activity of A. carneum is evaluated for the first time in this study. It was found that ethanol and methanol extracts of A. carneum leaves exhibited antibacterial activity against Gram-positive bacteria S. pyogenes and S. aureus. However, none of the extracts displayed anti-MRSA effects. Aqueous and ethanol extract were found to produce antibacterial activity against all test Gram-negative bacteria. Significant antibacterial activity was given from water and ethanol extract against K. pneumonia. The best significant antibacterial activity against P. aeruginosa was obtained from water extract of A. carneum. Interestingly, the least sensitive bacterium S. typhimurium was inhibited by water and ethanol extract of A. carneum. All A. carneum extracts exhibited antibacterial activity against P. mirabillis and E. coli. In addition, ethanolic extract of A. carneum showed significant antibacterial activity against E. coli with the best MIC and MMC values. For antifungal activity, it was found that A. brasiliensis and C. albicans were inhibited by water extract of A. carneum. Preliminary phytochemical screening of active extracts obtained from A. carneum leaves demonstrated the presence of alkaloids and flavonoids in ethanolic extract that exhibited a broad spectrum antibacterial activity and flavonoids in aqueous extract that exhibited the most significant antifungal activity. Therefore, the antimicrobial activity of A. carneum could be attributed to the presence of flavonoids and alkaloids in leaf extracts.
Although no antimicrobial investigations about
D.
ithaburense have been described in the literatures, its antibacterial and antifungal activities are identified in the current study. Water extract of
D.
ithaburense leaves, which was found to contain flavonoids, produced a broad spectrum antibacterial activity against all test bacteria except
S.
typhimurium. Methanol and acetone extracts of
D.
ithaburense leaves showed the highest significant antibacterial activity against
K.
pneumonia. In some related species, it was revealed that green synthesis of silver nanoparticles using
D.
denudatum root extract exhibits antibacterial activities (
Suresh et al., 2014). The essential oil from the flowers of
D.
formosum showed moderate antibacterial activity against
E. faecalis and
S.
aureus, no antibacterial activity against
E.
coli, and no anticandidal activity (Güleç et al., 2007). However in the current study, leaf extracts of
D.
ithaburense exhibited both antibacterial activity against
E.
coli and anticandidal activity against
C.
albicans. The bacterium
E.
coli was found to be sensitive to all
D.
ithaburense extracts, while acetone extract of
D.
ithaburense leaves that contains different phytochemicals including alkaloids, flavonoids, and tannins exhibited the most significant antifungal activity against
A.
brasiliensis and
C.
albicans.
The medicinal plant L. hirticarpus was not previously screened for its potential as a source of antimicrobial agents. It was found that water extract prepared from L. hirticarpus flowers exhibited antibacterial activity against all test bacteria except MRSA and S. typhimurium. This broad spectrum antibacterial activity might be due to detected flavonoids and saponins in L. hirticarpus flowers. Moreover, it was found that only MRSA and S. typhimurium were insensitive to all extracts of L. hirticarpus flowers. Even though MRSA was resistant to L. hirticarpus extracts, S. aureus was sensitive to all plant extracts. Likewise, K. pneumonia and P. mirabillis were inhibited by all plant extracts. It was observed that ethanol extract of L. hirticarpus flowers exhibited antifungal activity against A. brasiliensis and C. albicans. Interestingly, alkaloids, flavonoids, saponins, and tannins were detected in ethanol extract. Thus, the significant antifungal activity of ethanol extract of L. hirticarpus flowers could be due to the presence of these phytochemical compounds. Two Onopordum species were investigated in this study, O. blancheanum and O. jordanicolum. Those Onopordum species were not examined previously for their effectiveness as antimicrobial agents. It was revealed that flower extracts of O. jordanicolum produced broad spectrum antimicrobial activities and appeared to be the best source for antimicrobial agents among tested plant species in this study.
Ethanol extract of O. jordanicolum flowers showed antibacterial activity against all test bacteria with the best MIC and MMC values produced by methanol extract for S. aureus. Furthermore, all O. jordanicolum extracts produced antibacterial activity against S. aureus, MRSA, K. pneumonia, and P. mirabillis. Both test bacteria S. aureus and MRSA, which belongs to the same species were inhibited significantly by methanol and aqueous extracts of O. jordanicolum flowers, respectively. Interestingly, it was detected that all flower extracts of O. jordanicolum displayed antifungal activity against A. brasiliensis and C. albicans with the best MIC and MMC values for acetone extract. For O. blancheanum, aqueous and ethanol extract of O. blancheanum flowers displayed significant antibacterial activity against S. aureus with the best MIC and MMC values for ethanol extract. All O. blancheanum extracts produced antibacterial activity against S. aureus, E. coli, K. pneumonia, and P. mirabillis. It was noticed that methanol extract exhibited antifungal activity against A. brasiliensis and C. albicans. Zare et al. (2014) illustrated that methanol extract of the related species O. acanthium showed significant antibacterial activity against S. epidermidis and M. luteus, while n-hexane extract demonstrated negligible to no inhibitory activity against Gram-negative and Gram-positive bacteria. Ugur et al. (2011) reported that chloroform and ethanol extract of O. caricum has a potential to inhibit the growth of S. maltophilia and S. aureus.
Thus, the antimicrobial results in this study are competitive and more promising than that reported previously for other Onopordum species. It was clearly observed that extracts of both Onopordum species investigated in this study contain various phytochemical types including alkaloids, flavonoids, saponins, and tannins that are known to exhibit anti- bacterial and antifungal activities. The orchid O. sancta is a unique species in Jordan. However, its antimicrobial activity was not studied before this study. The four extracts of O. sancta flowers exhibited antibacterial activity against E. coli and P. mirabillis. Significant anti-MRSA activity was produced from aqueous extract of O. sancta flowers. Acetone extract of O. sancta gave the highest significant antibacterial activity against P. aeruginosa. It was found that only methanol extract of O. sancta flowers showed antifungal activity against A. brasiliensis and C. albicans. On the other hand, it was reported that methanolic extract of the related species O. latifolia showed antifungal activity against C. albicans and antibacterial activity against multidrug-resistant clinical isolates including E. coli, S. aureus, and Enterococcus sp. (Avasthi et al., 2013). Alkaloids, flavonoids, saponins, and tannins were detected in methanol extract and only flavonoids and saponins were detected in water extract of O. sancta. This result is in agreement with Avasthi et al. (2013) who demonstrated that phytochemical analysis of the active fractions obtained from O. latifolia contained flavonoids, steroids, and tannins. Therefore, the antimicrobial activity of O. sancta might be attributed to the presence of these phytochemicals.
The antimicrobial activity of P. umbonatum has not been performed. Therefore, this plant was selected in this study for determination of its antimicrobial activity. Water and methanol extract prepared from P. umbonatum flowers exhibited significant antibacterial activity against S. pyogenes with the best MIC and MMC values. Although there is no antibacterial activity against MRSA, the other related test bacteria S. aureus was found to be sensitive to all flower extracts of P. umbonatum. Similarly, E. coli and P. mirabillis were inhibited by all extracts of P. umbonatum. In addition, methanol extract exhibited antibacterial activity against P. aeruginosa and K. pneumonia. Aqueous extract produced the best antifungal activity against A. brasiliensis and C. albicans. This result is in accordance with Kostic et al. (2010) who indicated that ethanol extract of the related species P. rhoeas exhibited antifungal activity against C. albican and antibacterial activity against E. coli, S. aureus, and P. aeruginosa. Alkaloids, flavonoids, and tannins were detected in methanol extract of P. umbonatum, and only flavonoids were detected in the aqueous extract. This is in agreement with the previous analysis of the phytochemical constituents of P. umbonatum by Bernáth (2006) who revealed the presence of flavonoids and clinically useful alkaloids.
The result of this study shows the existence of different phytochemicals in selected medicinal plants such as alkaloids, flavonoids, saponins, and tannins that are known to exhibit various antimicrobial activities. It was previously reported (Cushnie and Lamb, 2005; Lim et al., 2006; Maatalah et al., 2012) that alkaloids, flavonoids, saponins, and tannins possessing various antimicrobial activities including antibacterial and antifungal activities. Therefore, the antimicrobial activity of promising medicinal plant extracts investigated in this work could be due to the presence of these phytochemicals. In conclusion, the results of this study represent the first report on the antibacterial and antifungal activities of aqueous, ethanol, methanol, and acetone extracts prepared from seven endemic medicinal plants (A. carneum, D. ithaburense, L. hirticarpus, O. blancheanum, O. jordanicolum, O. sancta, and P. umbonatum) against human frequent pathogens. The promising extracts from selected plants in this study may be used in the development of future drugs and in the treatment of infectious diseases caused by human pathogenic bacteria and fungi that exhibited antibiotic resistance. Further phytochemical analysis is required to characterize all bioactive constituents and their amounts in active medicinal plant extracts.
The authors have not declared any conflict of interests.
The author thanks the Deanship of Scientific Research, Al-Balqa Applied University for financing this project and Mrs. Ghadeer Alazzam for technical assistance.
REFERENCES
|
Adigizel N (2000). Aethionema R. Br. In: Flora of Turkey and the east Aegean Islands, Güner A, Özhatay N, Ekim T, Bazer KHC (Eds.), Edinburgh University Press, Edinburgh. pp. 31-34.
|
|
|
|
Alzoreky NS, Nakahara K (2003). Antibacterial activity of extracts from some edible plants commonly consumed in Asia. Int. J. Food Microbiol. 80:223-230.
Crossref
|
|
|
|
Avasthi AS, Ghosal S, Purkayastha S (2013). Study of antimicrobial activity of Orchis Latifolia. Int. J. Pharm. Biol. Sci. 4(4):B638-B646.
|
|
|
|
Bernáth J (2006). Poppy: The genus Papaver. Gordon and Breach Publishing Group.
|
|
|
|
Bulpitt JC, Li Y, Bulpitt PF, Wang J (2007). The use of orchids in Chinese medicine. J. R. Soc. Med. 100(12):558-563.
Crossref
|
|
|
|
Ceylan O, Okmen G, Ugur A (2008). Isolation of soil Streptomyces as source antibiotics active against antibiotic-resistant bacteria. Asian J. Biol. Sci. 2:73-82.
|
|
|
|
Cowan MM (1999). Plant products as antimicrobial agents. Clin. Microbiol. Rev. 12:564-582.
|
|
|
|
Cushnie TPT, Lamb AJ (2005). Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 26:343-356.
Crossref
|
|
|
|
Eloff JN (1998). Which extractant should be used for the screening and isolation of antimicrobial components from plants? J. Ethnopharmacol. 60:1-8.
Crossref
|
|
|
|
Gardner DR, Pfister JA (2007). Toxic alkaloid concentrations in Delphinium nuttallianum, Delphinium andersonii, and Delphinium geyeri in the intermountain region. Rangeland Ecol. Manage. 60:441-446.
Crossref
|
|
|
|
Grela ER, Studziński T, Winiarska A (2000). Lathyrism in people and animals. Med. Wet. 56(9):558-562.
|
|
|
|
Güleç C, Yayli N, Yesilgil P, Terzioglu S, Yayli N (2007). Chemical composition and antimicrobial activities of the essential oil from the flowers of Delphinium formosum. Asian J. Chem. 19(5):4069-4074.
|
|
|
|
Hassan BAR (2012). Medicinal plants (Importance and uses). Pharmaceut. Anal. Acta 3:e139.
|
|
|
|
Hopwood D (2007). How do antibiotic-producing bacteria ensure their self-resistance before antibiotic biosynthesis incapacitates them? Mol. Microbiol. 63:937-940.
Crossref
|
|
|
|
Iyengar MA (1995). Study of Crude Drugs, 8th Ed., Manipal Power Press, Manipal, India. P2.
|
|
|
|
Jagatheeswari D, Deepa J, Ali HSJ, Ranganathan P (2013). Acalypha indica L- an important medicinal plant: a review of its traditional uses, and pharmacological properties. Int. J. Res. Bot. 3(1):19-22.
|
|
|
|
Jin-Ming K, Nogoh-Khang G, Lian-Sai C, Tet-Fatt C (2003). Recent advances in traditional plant drugs and orchids. Acta Pharm. Sin. 24(1):7-21.
|
|
|
|
Kostic DA, Mitic SS, Mitic MN, Zarubica AR, Velickovic JM, Dordevic AS, Randelovic SS (2010). Phenolic contents, antioxidant and antimicrobial activity of Papaver rhoeas L. extracts from Southeast Serbia. J. Med. Plants Res. 4(17):1727-1732.
|
|
|
|
Lim SH, Darah I, Jain K (2006). Antimicrobial activities of tannins extracted from Rhizophora apiculata barks. J. Trop. For. Sci. 18(1):59-65.
|
|
|
|
Maatalah MB, Bouzidi NK, Bellahouel S, Merah B, Fortas Z, Soulimani R, Saidi S, Derdour A (2012). Antimicrobial activity of the alkaloids and saponin extracts of Anabasis articulate. J. Biotechnol. Pharm. Res. 3(3):54-57.
|
|
|
|
Obeidat M (2017) Isolation and characterization of extremely halotolerant Bacillus species from Dead Sea black mud and determination of their antimicrobial and hydrolytic activities. Afr. J. Microbiol. Res. 11(32):1303-1314.
Crossref
|
|
|
|
Obeidat M (2011). Antimicrobial activity of some medicinal plants against multidrug resistant skin pathogens. J. Med. Plants Res. 5(16):3856-3860.
|
|
|
|
Obeidat M, Shatnawi M, Al-alawi M, Al-Zu`bi E, Al-Dmoor H, Al-Qudah M, El-Qudah J, Otri I (2012). Antimicrobial Activity of Crude Extracts of Some Plant Leaves. Res. J. Microbiol. 7(1):59-67.
Crossref
|
|
|
|
Parungao MM, Maceda EBG, Villano MAF (2007). Screening of antibiotic-producing actinomycetes from marine, brackish and terrestrial sediment of Samal Island, Philippines. J. Res. Sci., Comput. Eng. 4(3):29-38.
|
|
|
|
Perez C, Pauli M, Bazerque P (1990). An antibiotic assay by agar-well diffusion method. Acta Biol. Med. Exp. 15:113-115.
|
|
|
|
Ripa F, Haque M, Nahar L, Islam M (2009). Antibacterial, cytotoxic and antioxidant activity of Passiflora edulis Sims. Eur. J. Sci. Res. 31(4):592-598.
|
|
|
|
Ronel M, Khateeb S, Lev-Yadun S (2009). Protective spiny modules in thistles of the asteraceae in Palestine. J. Torrey Bot. Soc. 136(1):46-56.
Crossref
|
|
|
|
Roopashree TS, Dang R, Rani RHS, Narendra C (2008). Antibacterial activity of antipsoriatic herbs: Cassia tora, Momordica charantia and Calendula officinalis. Int. J. Appl. Res. Nat. Prod. 1(3):20-28.
|
|
|
|
Sahoo N, Manchikanti P, Dey S (2010). Herbal drugs: Standards and regulation. Fitoterapia 81(6):462-471.
Crossref
|
|
|
|
Siddiqui AA, Ali M (1997). Practical Pharmaceutical chemistry, 1st Ed., CBS Publishers and Distributors, New Delhi, India. pp. 126-131.
|
|
|
|
Suresh G, Gunasekar PH, Kokila D, Prabhu D, Dinesh D, Ravichandran N, Ramesh B, Koodalingam A Siva GV (2014). Green synthesis of silver nanoparticles using Delphinium denudatum root extract exhibits antibacterial and mosquito larvicidal activities. Spectrochim. Acta A Mol. Biomol. Spectrosc. 157:61-66.
Crossref
|
|
|
|
Ugur A, Sarac N, Duru ME (2011). Chemical composition and antimicrobial activity of endemic Onopordum caricum. Middle-East J. Sci. Res. 8(3):594-598.
|
|
|
|
Zare K, Nazemyeh H, Lotfipour F, Farabi S, Ghiamirad M, Barzegari A (2014). Antibacterial activity and total phenolic content of the Onopordon acanthium L. seeds. Pharm. Sci. 20(1):6-11.
|