ABSTRACT
The effect of the time of collection on the quality and quantity of the oil obtained from Severinia buxifolia leaves as well as its antimicrobial activity was studied. The chemical composition of the hydro-distilled oils of the leaves of S. buxifolia (Poir.)Tenore, collected at the four seasons was determined by GC/MS analysis. Moreover, antimicrobial activity was studied, for the oil sample regarding the yield and quality, against selected bacteria and yeast. The highest oil yield was obtained from the leaves collected during winter (0.5%) followed by autumn (0.308%), however, those collected in spring and summer scored almost the same yield; (0.26%) and (0.283%) respectively. Limonene was the most abundant hydrocarbon in winter (35.5%), and amounted to 29.3% in summer, whereas, spring and autumn samples constituted 21.15% and 19.17% of limonene respectively. α-Santalene, accounted to 20.87% in autumn sample followed by the winter sample (18.93%), then 13.56% in the spring sample and recorded its lowest concentration in the summer sample (8.1%). Furthermore, γ-elemene was detected in a lesser extent amounting to 7.75% in the spring sample, 7.33% in autumn sample, 6.28% in the winter sample and 5.54% in the summer sample. Based on the above results, as regards to limonene content, S. buxifolia leaf oil collected in winter was chosen for further antimicrobial study. The agar disc diffusion method was adopted for screening the antibacterial activity of the selected oil sample. Results show moderate effect against Escherichia coli, and Listeria monocytogenes. Nevertheless, it showed weak activity against Staphylococcus aureus, Pseudomonas aeruginosa, Bacillus subtilus, MRSA, and Candida albicans. The MIC of the volatile oil against L. monocytogenes was 4 and 1 µl/ ml against S. aureus, P. aeruginosa, B. subtilus, MRSA and C. albicans.
Key words: Severinia buxifolia (Poir.) Tenore, Rutaceae, GC/MS analysis, limonene, in vitro antimicrobial activity.
Rutaceae is best known for the exotic genus citrus, because of its commercially consumed fruits. Other groups of the same subfamily of Citrus are commonly cultivated as ornamentals in America, including species of
Atalantia,
Clausena,
Murraya, and
Swinglea. Genus
Atalantia includes approximately 20 species (Roskov et al., 2017). Amongst
Atalantia species,
Atalantia buxifolia or
Severinia buxifolia (Poir.) Tenore is the plant of our interest.
S. buxifolia (
Atalantia buxifolia) is commonly known as the thorny, evergreen shrub; it is also known as Chinese box-orange or Box-leaved
Atalantia. The Flora of China has moved this species to
A. buxifolia (Poir.) Oliv. (Wu et al., 2008). In Hainan province of China, the roots of
A. buxifolia are used in folk medicine for the treatment of influenza, cough, malaria, and stomachache by the people of Li nationality (Yang, 2012). Essential oils are complex mixtures including important compounds where each contributes to the beneficial or adverse effects of these oils. Essential oils have massive consumptions as raw materials in several areas, including perfumes, cosmetics, aromatherapy, phototherapy, spices and nutrition Buchbauer, 2000). Accordingly, it was of deep interest to have strong background regarding the essential oil composition since this permits for a better and specially directed application.
Infectious diseases are major causes of death worldwide. Infections with bacteria are associated with high morbidity and mortality especially with immuno-compromised patients (Driscoll et al., 2007; Del Toro et al., 2006).
The proposed strategies to avoid and govern infectious diseases include public health improvements in sanitation and hygiene, as well as the use of antimicrobial agents (WHO, 2001). The resistance of microorganisms to antibiotic has become an important alarm to the patients as well as a scientist (Westh et al., 2004) in addition to the side effects of these antibiotics. This directed researchers to explore new chemotherapeutic agents to combat the infections caused by drug-resistant microbes and to reduce the harm caused by antibiotics (Bocanegra-Garcı´a et al., 2009; Giamarellou, 2006) . Volatile oils obtained from plants have been recognized for many years as antimicrobial agents. Direct addition of essential oil from aromatic plants to food products evidenced antimicrobial effect (Costa et al., 2015). Nowadays, due to consumer complaint from artificial preservatives, attention to volatile oils and their application in food preservation has been considered. Many reports dealt with the wide range of application for essential oils as antiseptic, antibacterial, antiviral, antioxidant, anti-parasitic, antifungal, and insecticidal agents (Burt, 2004; Dorman and Deans, 2008). Hence, essential oils can serve as a powerful device to minimize the bacterial resistance (Stefanakis et al., 2013). This work was carried out to evaluate the oil produced from the plant and the effect of seasonal fluctuation on the yield and composition of the essential oil of the leaves, furthermore comparing the identified components and the major constituents detected in the studied samples, with the aim to rationalize the effect of climate conditions on the yield and quantity. The antimicrobial activity of the oil sample giving the highest yield and quality was studied.
Plant material
Microbial strains
The tested bacteria and fungi were supplied by the Antimicrobial Unit, National Research Center, Egypt. Bacterial strains were
Bacillus subtilus (ATCC6051)
, Staphylococcus aureus (ATCC 6538)
, Methicillin-resistant Staphylococcus aureus (MRSA) (laboratory collection)
, Listeria monocytogenes (ATCC2180-1A) representing pathogenic gram positive bacteria
, and
Escherichia coli (ATCC 8739) and
Pseudomonas aeruginosa(ATCC 9027) representing pathogenic gram negative bacteria and
Candida albicans (RCMB 05035) representing fungi.
Ciprofloxacin (Pharco Pharm. Cairo Egypt, and Amphotericin B obtained from Sigma-Aldrich (Merk) was used as reference drugs.
Preparation of the volatile oil
For preparation of essential oil, fresh leaf samples were collected at three months intervals along
the four seasons (winter, spring, summer and autumn) between May 2013 to June 2014 and separately subjected to hydro distillation in a Clevenger’s apparatus for 3 h, according to the procedure described in the Egyptian Pharmacopeia
. The obtained oils were separately dried over anhydrous sodium sulphate and carefully stored in a refrigerator for further chemical and biological studies. The percentage yields were calculated on a dry weight basis, and the oils were kept in a refrigerator for further analysis. The specific gravities and refractive indices were determined according to the Egyptian Pharmacopoeia (CAPA, 2005) procedures. All stated values were the average of three determinations.
Determination of percentage yield of the volatile oil
The yield of the volatile oils was calculated as weight/weight (g/kg), on fresh weight basis.
GC/MS analysis of the volatile oil content
Volatile oil prepared from S. buxifolia leaves were subjected to GC/MS analysis. Volatile oil prepared was subjected to GC/MS analysis. The injection volume was 1 µl/ml and the instrument was controlled by the Shimadzu Class-5000 Version 2.2 software containing a NIST62 (National Institute of Standards and Technology) MS library. Volatiles were separated on a DB5-MS column (30 m length, 0.25 mm inner diameter, and 0.25 ml film (J&W Scientific, Santa Clara, California). Injections were made in the split mode for 30 s, and the gas chromatograph was operated under the following conditions: injector 220°C and column oven 40°C for 3 min, then programmed at a rate of 12°C/min to 180°C, kept at 180°C for 5 min, and finally ramped at a rate of 40°C/min to 220°C and kept for 2 min; He carrier gas was at 1 mL/min. The transfer line and ion–source temperatures were adjusted at 230 and 180°C, respectively. The HP quadrupole mass spectrometer was operated in the electron ionization mode at 70 eV. The scan range was set at 40 to 500 m/z. The percentages of different components in each oil sample were determined by computerized peak area measurements relative to each other. Volatile components were identified using the procedure described (Farag and Wessjohann, 2012). The peaks were first deconvoluted using AMDIS software (www.amdis.net) and identified by its retention indices (RI) relative to n-alkanes (C6–C20), mass spectrum matching to NIST, WILEY library database.
Evaluation of the antimicrobial activity
In-vitro qualitative screening susceptibility test
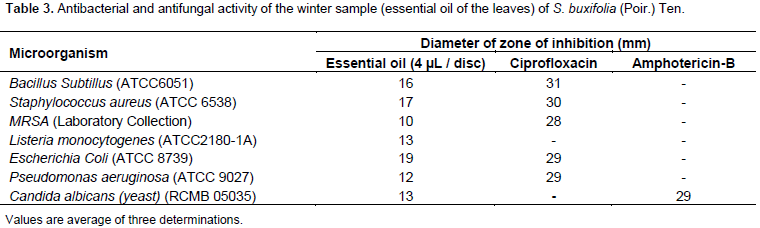
The selected volatile oil sample of the leaves of S. buxifolia under investigation was screened for their antimicrobial activity against representatives of G+ bacteria: B. subtillus, S. aureus, Methicillin-resistant S. aureus (MRSA), L. monocytogenes, and G-E. coli, P. aeruginosa, and yeast (C. albicans) applying the agar disc diffusion according to CLSI guidelines (2009). The selected volatile oil was tested by impregnating sterile discs of Whatmann filter paper 1 (5 mm diameter) in 20 µl of the oils. 20 µl of dimethyl sulfoxide was used as a negative control. The reference standards ciprofloxacin and amphotericin-B were dissolved separately in dimethyl sulfoxide at a concentration of 20 µg/µl. The discs were then placed onto the surface of the plates containing the solid bacterial medium (Mueller–Hinton agar) or the fungal medium (Dox’s medium) which has been heavily seeded with the spore suspension of the tested microorganisms. The plates were incubated at 37°C for 25 h in case of bacteria and at 25°C for 48 h in case of fungi. After incubation, the inhibition zones were measured in mm.
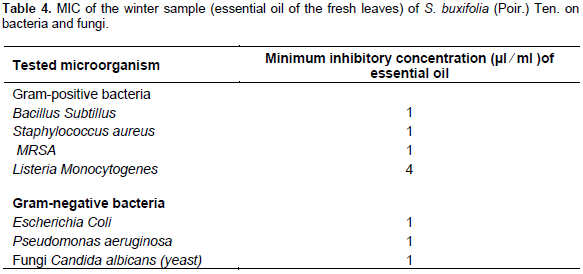
Determination of the minimum inhibitory concentration (MIC)
Minimum inhibitory concentration (quantitative assay) was evaluated for the volatile oil of S. buxifolia, based on the results obtained for the antimicrobial screening. Accordingly, B. subtilus, S. aureus, MRSA, L. monocytogenes, E. coli, P. aeruginosa and yeast (C. albicans) for S. buxifolia leaf oil. In brief, stationary phase cultures of bacterial strains were prepared at 37°C and used to inoculate fresh 5.0 ml culture to an OD600 of 0.05. The 5.0 ml culture was then incubated at 37°C until an OD600 was achieved from which standardized bacterial suspensions were prepared to a final cell density of 6 105 CFU/ml. Serial dilutions from the volatile oils were prepared and mixed with 5.0 ml of the standardized bacterial suspension then added to the plates and incubated for 24 h at 37°C. The colony forming units (CFU) were counted for each dilution (NCCLS, 2000).
Agar dilution method
The tested samples were serially diluted in molten medium equilibrated at 50°C with 2% glucose. One ml was added to each well in a 24-well plate with a flat bottom and allowed to solidify. The centre of each well was inoculated with 10 ml of the bacterial suspension. Drug free growth control was included. MIC was determined after 48 h at 35°C. MICs were defined as the lowest concentration that had granular appearing micro-colonies of growth instead of filamentous radiating colonies on solid agar.
This is the first report dealing with the effect of seasonal variation of the essential oils obtained from the fresh leaves of S. buxifolia (Poir.) Tenore. Investigation of the oil obtained from the leaves of S. buxifolia at different seasons; winter, spring, summer and autumn revealed qualitative and quantitative differences. The highest oil yield was obtained from the leaves collected during winter (0.5%) followed by autumn (0.308%), while, those collected in spring and summer counted almost the same yield; 0.26 and 0.283% respectively (Table 1). GC/MS analysis of the essential oil obtained from S. buxifolia leaves at four different seasons (Table 2) indicated that the identified constituents varied as regards to their abundance and concentration. The total identified compounds were 95.93, (89.82, 90.16 and 88.44% in autumn, spring, winter and summer respectively. The total number of constituents identified under the adopted conditions was 59 among which 15 were detected in the four oil samples under investigation. The rest of constituents appeared, however, unevenly distributed in the analyzed oils.
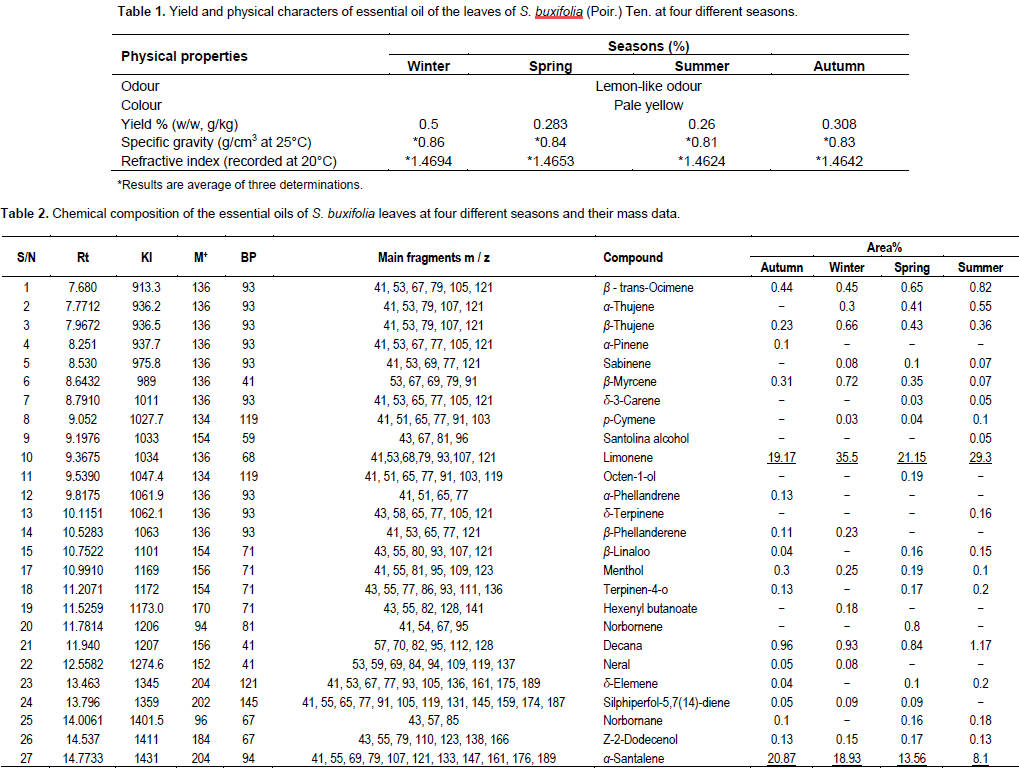
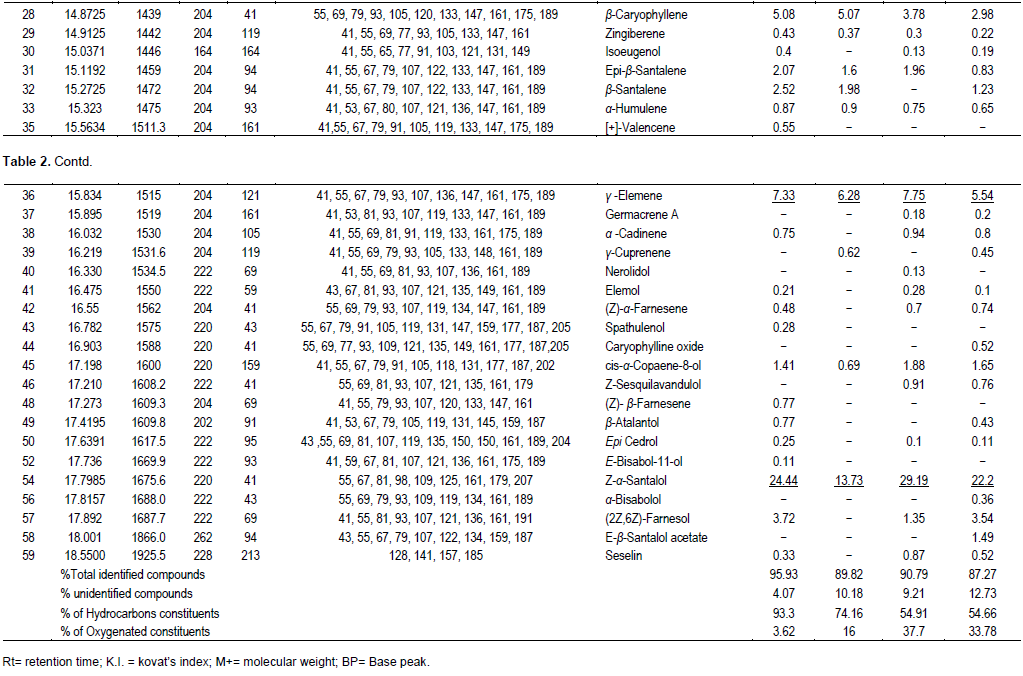
Hydrocarbons were the most abundant compounds detected, amounting the highest concentration in autumn (93.3%), followed by those collected in winter (74.16%). Nevertheless, spring and summer samples constituted the same amount of hydrocarbons; 54.91 and 54.66%, respectively. Monoterpene hydrocarbons constituted the most dominant chemical group among the four studied seasons; limonene was the most abundant in winter (35.5%), and amounted to 29.3% in summer, whereas, spring and autumn samples constituted 21.15 and 19.17% of limonene respectively. Next to monoterpenoids, sesquiterpenoids were the most detected compounds in the volatiles studied at different seasons from the leaves of S. buxifolia, with α-santalene accounting up to 20.87% in autumn sample followed by the winter sample (18.93%), then reaching 13.56% in the spring sample and finally, recorded the lowest concentration in the summer sample(8.1%) relative to the other samples. Furthermore, γ- elemene was found but in a lesser extent than α-santalene amounting to (7.75%) in the oil collected in spring, (7.33%) in the autumn sample, (6.28%) in the winter sample and (5.54%) in the summer sample. Throughout our study in the four seasons; the number of oxygenated compounds ranged from 16 to 37.7%; spring sample recorded the highest content of oxygenated compounds (37.7%) with Z-α-santalol constituting the major sesquiterpene alcohol (29.19%), the oxygenated compounds amounted up to 3.62 % in autumn, where Z-α-santalol was the major compound (24.44%), followed by summer (33.78%) with same major sesquiterpene (22.2%), however winter showed the lowest oxygenated compounds recorded in all studied oil samples (16%), Z-α-santalol amounting to 13.37%.
These findings support the idea that the seasonal variation can influence the quantifier of oxygenated compounds present in the oil. Comparing the results to that reported (Scora and Ahmed, 1994), there is no great difference between the number of compounds identified; 59 components were also identified, however, the difference was clear in the detected compounds. The major reported components were α -santalene (24%), trans β -santalol (21%), germacrene-B (10%), and β- caryophyllene (7%) and the monoterpene limonene (19%) in one tested sample. On the other hand, another report showed variation (Dongxu et al., 2011) from our study as major identified components were isocyclocitral (41.598 %), guaiacol (15.234%), β-eudesmol (10.790%), thujopsene (2.581%), 1,7,7-trimethyl-bicyclohept-2-one (5.841%), santalol (3.702%), ferruginol (2.657%) at one sample. Herein, we have evidenced variation in the tested samples as regards to yield and components which were affected by the seasonal effect. Furthermore, there was an obvious impact on the climax of each season on the volatile constituents of each cultivated plant. Previous reports evidenced that limonene possesses antifungal, bacteriostatic and bactericidal activities (Dorman and Deans, 2000; Dambolena et al., 2008; Jaroenkit et al., 2011; Chee et al., 2009). Moreover, it was also suggested to be used as a food preservative (Vuuren and Viljoen, 2007).
Since winter sample recorded the maximum yield and constituted the highest percentage of limonene, therefore, winter sample was selected as a representative to undergo further in the vitro antimicrobial study. There was a correlation between the inhibition zone diameter of the agar diffusion method (qualitative method) (Rashed and Butnariu, 2014a) and MIC (quantitative method) values of broth dilution method (Rashed and Butnariu, 2014b). The selected essential oil showed a maximum zone of inhibition with minimum MIC value among all tested microorganisms. The selected volatile oil sample of S. buxifolia leaves exhibited moderate antibacterial and antifungal activity at the given concentrations when compared to ciprofloxacin and amphotericin B respectively as a standard (Table 3). Moreover, the oil exhibited activity against most of the tested bacteria with a MIC of 1% v/v except for L. monocytogenes. This activity might be attributed to the high concentration of limonene in the sample; the mechanism of action for the antibacterial activity was previously reported; the inactivation of certain strain of E. coli by (+)-limonene, in a medium adjusted at certain pH, the effect of the synergistic lethal effect on combining (+)-limonene with heat and Pulse Electric field (PEF) treatments to inactivate E. coli (Espina et al., 2013). Referring to Table 4, the MIC of the volatile oil of the leaves of S. buxifolia against L. monocytogenes recorded 4 µl/ml, while 1 µl/ml against other bacterial strains. Therefore, this volatile oil could be considered as moderate antibacterial and antifungal agent.
The authors declare that they have no conflict of interest.
REFERENCES
|
Bocanegra–García V, Del Rayo Camacho–Corona M, Ramírez–Cabrera M, Rivera G, Garza–González E (2009). The bioactivity of plant extracts against representative bacterial pathogens of the lower respiratory tract. BMC Res. Notes 2:95.
Crossref
|
|
|
|
Buchbauer G (2000). The detailed analysis of essential oils leads to the understanding of their properties. J. Perfumer Flavorist. 25:64-67.
|
|
|
|
Burt S (2004). Essential oils: Their antibacterial properties and potential applications in foods-A review. Int. J. Food Microbiol. 94:223-2531.
Crossref
|
|
|
|
Chee HY, Kim H, Lee MH (2009). In vitro antifungal activity of limonene against Trichophyton rubrum. Mycobiology 37:243-246.
Crossref
|
|
|
|
Clinical and Laboratory Standards Institute, CLSI (2009). Performance standards for Antimicrobial Susceptibility Testing 27th ed. CLSI supplement M100. Wayne, PA: Clinical and Laboratory Standards Institute; 2017.
View
|
|
|
|
Costa DC, Costa, HS, Albuquerque TG, Ramos F, Castilho MC, Sanches-Silva A (2015).Advances in phenolic compounds analysis of aromatic plants and their potential applications. Trends Food Sci. Technol. 45:336-354.
Crossref
|
|
|
|
Dambolena JS, Lo’pez AG, Ca’nepa MC, Theumer MG, Zygadlo JA (2008). Inhibitory effect of cyclic terpenes (limonene, menthol, menthone and thymol) on Fusarium verticillioides MRC 826 growth and fumonisin B1 biosynthesis Toxicon. 51:37-44.
Crossref
|
|
|
|
Del Toro M, Rodriguez–Bano J, Martinez–Martinez L, Pascual A, Perez–Canoa R, Perea E, Muniain M (2006). Epidemiology, clinical features and prognosis of infections due to Stenotrophomonas maltophilia. Enferm Infect. Microbiol. Clin. 24:4-9.
|
|
|
|
Dongxu J, Yuanbin L, Baiyin H, Changqing L, Youliang X, Xiaoping L (2011). Analysis of the Essential Oil in Atalantia Buxifolia by GC–MS. Tradit. Chin. Drug Res. Clin. Pharmacol. 22(1):R284.1
|
|
|
|
Dorman HJD, Deans SG (2000). Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J. Appl. Microbiol. 88:308-316.
Crossref
|
|
|
|
Driscoll JA, Brody SL, Kollef MH (2007). The epidemiology, pathogenesis and treatment of Pseudomonas aeruginosa infections. Drugs 67:351-168.
Crossref
|
|
|
|
Egyptian Pharmacopoeia (2005). 4th edition, Central Administration of Pharmaceutical Affairs (CAPA), Ministry of Health and Population, Cairo, Egypt.
|
|
|
|
Espina L, Gelaw TK, De Lamo–Castellvı´ S, Pagan R, Gonzalo D (2013). Mechanism of Bacterial Inactivation by (+)–Limonene and Its Potential Use in Food Preservation Combined Processes. Plos One. 8(2):e56769
Crossref
|
|
|
|
Farag MA, Wessjohann LA (2012). Volatiles Profiling in Medicinal Licorice Roots Using Steam Distillation and Solid–Phase Microextraction (SPME) Coupled to Chemometrics J. Food Sci. 77:C1179-1184.
Crossref
|
|
|
|
Giamarellou H (2006). Treatment options for multidrug–resistant bacteria. Expert Rev. Anti-Infect. Ther. 4:601-618.
Crossref
|
|
|
|
Jaroenkit P, Matan N, Nisoa M (2011). In vitro and in vivo activity of citronella oil for the control of spoilage bacteria of semi dried round scad (Decapterus maruadsi) Int. J. Med. Aromat. Plants. 1: 234-239
|
|
|
|
National Committee for Clinical Laboratory Standards, NCCLS (2000). and Methods for dilution antimicrobial susceptibility tests of bacteria that grow aerobically Approved Standard. M7–A5. Wayne, PA.
|
|
|
|
Rashed K, Butnariu M (2014a). Antimicrobial and Antioxidant Activities of Bauhinia racemosa Lam. and Chemical Content, Iran. J. Pharm. Res. 13(3):1073-1080.
|
|
|
|
Rashed KN, Butnariu M (2014b). Isolation and antimicrobial and antioxidant evaluation of bio-active compounds from Eriobotrya japonica stems. Adv. Pharm. Bull. 4(1):75-81.
|
|
|
|
Roskov Y, Abucay L, Orrell T, Nicolson D, Kunze D, Flann TC, Bailly N, Kirk P, Bourgoin T, DeWalt RE, Decock W, De Wever A (2016). In Species 2000 & ITIS Catalogue of Life at database World Plants.
|
|
|
|
Scora RW, Ahmed M (1994). The Leaf oils of Severinia buxifolia (Poir.) Tenore. J. Essent. Oil Res. 6(4):363-367.
Crossref
|
|
|
|
Stefanakis MK, Touloupakis E, Anastasopoulos E, Ghanotakis D, Katerinopoulos HE, Makridis P (2013). Antibacterial activity of essential oils from plants of the genus Origanum. Food Control 34:539-546.
Crossref
|
|
|
|
Vuuren SFV, Viljoen AM (2007). Antimicrobial activity of limonene enantiomers and 1,8–cineole alone and in combination. Flavour Fragr. J. 22:540-544.
Crossref
|
|
|
|
Westh H, Zinn CS, Rosdahl VT, Group SS (2004). An international multicenter study of antimicrobial consumption and resistance in Staphylococcus aureus isolates from 15 hospitals in 14 countries. Microb. Drug Resist. 10:169-176.
Crossref
|
|
|
|
World Health Organization (WHO) (2001). WHO Global strategy for containment of antimicrobial resistance. Geneva, Switzerland.
|
|
|
|
Wu ZY, Raven PH, Hong DY (2008). Flora of China. (Oxalidaceae through Aceraceae). Vol.11 Science Press, Beijing, and Missouri Botanical Garden Press, St. Louis.
View
|
|
|
|
Yang T (2012). Study on the Bioactive Components from the Roots of Atalantia buxifolia″, Master's thesis, Hainan University, CLC: R284.
|