ABSTRACT
Microtrichia perotitii DC. belongs to the family Asteraceae (Compositeae) and it is a herb found in the West African countries. The aim of this research was to establish pharmacognostic standards for M. perotitii through macroscopic, microscopic, chemo-microscopic and physico-chemical investigations. The macroscopic studies revealed the shape of the leaf as small with acute apex, asymmetric base, pubescent surface with long petiole and a serrated margin. Organoleptically, the leaf is green, slightly bitter and pepperish with an unpleasant odour. The microscopic studies of the leaf showed epidermal cells with irregularly thickened walls, numerous anomocytic stomata, and multicellular covering trichomes on both upper and lower epidermis. The transverse section of the leaf showed that it was dorsiventral with separated elements of vascular bundle. The powdered leaf revealed calcium oxalate crystals (prism and rosette), starch (oval) and xylem (spiral). Quantitative-leaf microscopy revealed the leaf constants as palisade ratio (3.2-3.4); stomatal number (258-285); stomatal index (19.5-24.7); vein-islet number (6.0-8.0) and vein-let termination number (8.0-11.0). The physico-chemical constants of the leaf showed moisture content (12.67 %), total ash (20.33 %), acid –insoluble ash (27.3 %), water- soluble ash (81.18 %), water extractive values (40.9 %) and alcohol extractive values (25.45 %). This is the first time the pharmacognostic parameters of the leaf of M. perotitii were studied and it will be quite useful for its identification, standardization and inclusion in various pharmacopoeias.
Key words: Microtrichia perotitii, macroscopical, microscopical, physico-chemical, pharmacognostic.
Standardization of herbal medicines (drug) is a process of establishing or prescribing a set of peculiar identities or specific characteristics which are generally unique and of unshared qualities. However, amongst various techniques used in identification of plant drugs, pharmacognostic study is always the most reliable. Basic component are standardization and authentication of natural drugs (Chandaz, 2014). Plant used in traditional medicines are authenticated through morphological, physico-chemical and phytochemical analyses (Chandaz, 2014). One of the major challenges of identification of plant is adulteration which simply means replacing the original plant with another one with the intention of increasing either the weight or potency of the product (Rokad et al., 2018). The therapeutic efficacy of any medicinal plant depends generally on the quality and quantity of the chemical constituents it contains (Majumder, 2011; Kumar and Rathinam, 2013). Information about the efficacy of medicinal plants that were passed from generations to generations after sometime could be incorrect, distorted, or even lost and under such circumstances proper scientific evidence must be established through pharmacognostic evaluation (Sumitra, 2014; Evans, 2009). Medicinal plants are the greatest asset to human health and a treasure for discovering new potential compounds with various therapeutic effects. The use of plant drugs locally is becoming increasingly popular especially in modern society because of incidences of adverse effects of synthetic drugs after they have been used. This is a common practice amongst developing countries most especially in Africa where it is their greatest hope (Gireesh et al., 2015; Elujoba et al., 2005). M. perotitii DC. belongs to the family Asteraceae and is widely distributed in West African countries of Nigeria, Senegal, Mali, Port of Guinea, Sierra Leone, Ivory Coast, Ghana and Dahomey (Hutchinson and Dalziel, 1963). In Nigeria it is found in northern part of the country where it is known as Maijankai or Sawun keke in Hausa, Osete in Igbira and Shaware pepe in Yoruba. The herb is an annual plant and is a diffused much branched pubescent varying from 1ft in height. The leaves are obovate, obtuse, cuneately narrowed into petiole, coarsely toothed above ½ - 1½ inch long, â…“ – 1 inch broad. The petiole of lower leaves is 1inch or more at the upper. Other features are in accordance to some members of the family (Daniel, 1877; Andrews, 1954; Watson and Dallwitz, 1992). Traditionally, M. perotitii is used for treating pain related diseases such as toothaches, cuts and burns and rashes in children. Others include, skin disease, rheumatism, diarrhea and jaundice. Earlier phytochemical studies of the leaves revealed the presence of tannins, flavonoids, alkaloids, carbohydrates, cardiac glycosides, saponins, phenolics and terpenoids (Abdullahi et al., 2011). In spite of these medicinal uses of this herb, there are no pharmacognostic reports on the leaves’ constants and other parameters as such this study is aimed at providing pharmacognostic standards of the leaves for the first time.
Collection and preparation of the herb
The herb M. perotitii was collected from Rigasa village, Kaduna State, Nigeria in the month of April 2016. The fresh herb was compared with specimen authenticated by Mal. Musa M. of the herbarium unit, Department of Biological sciences, Ahmadu Bello University, Zaria and was given a voucher number 998 for future references. The fresh herb was allowed to dry under the shade for three weeks until all the leaves have fallen-off the stalk. They were reduced to coarse powder with traditional pestle and mortar. Both fresh and the coarse powder were used for the studies.
Macroscopic study
The fresh leaves of M. perotitii were subjected to morphological studies in order to evaluate appearance such as surface, dimensions, point of attachment, lamina including composition, inclusions, shape, base, venation, margin and apex as well sensory profile that is colour, odour, texture and taste. The methods described by Brain and turner (1975), WHO (2007 and Evans (2009) were adopted.
Microscopic study
Both fresh and powdered leaves were studied.
Qualitative examination
For this study, the surface of the leaf of M. perotitii was prepared by peeling –up the upper and lower epidermis with sterilized forceps while the transverse section was prepared with a sharp razor blade. Each of the preparations was cleared of opaque materials by boiling them in 70% chloral hydrate solution. Small quantity of the powdered leaves was also prepared by clearing them in 70% chloral hydrate solution after being boiled in a test-tube on a Bunsen flame. Epidermal and other anatomical features were observed from the surfaces and transverse section of the leaf. The standards methods of Brain and Turner (1975), WHO (1984, 2007) and Evans (2009) were adopted.
Chemo-microscopic examination
For this study, the powdered leaves of M. perotitii were first cleared of obscured materials by boiling them in 70% chloral hydrate solution. Fragments of the leaves were transferred onto clean microscopic slide with dilute glycerol to prevent dehydration or hardening. Detecting reagents for metabolites such as starch, lignins, cellulose, calcium oxalate crystals, fixed oils and fat, calcium carbonates, mucilages and tannins were applied for their presence in cells inclusions or cell wall. These examinations were carried out according to the methods described by Brain and Turner (1975), WHO (2007) and Evans (2009).
Quantitative leaf examination
For this study, fresh leaf of M. perotitii was prepared by peeling–off both the upper and lower epidermis and clearing them by in boiling 70% chloral hydrate solution. The cleared leaf was mounted on clean microscopic slide and covered with cover slip. A set-up camera Lucida at x10 objective (1 small stage micrometer division = 10 µm; calibration factor = 2.7) was set for the determination of stomatal number, stomatal index, vein-islet number, veinlet termination number and palisade ratio. The length and width of stomata and trichomes were measured by calibrating the eyepiece micrometer using stage micrometer as described by Evans (2009).
Physical constant examination
For this study, various physico-chemical parameters were determined for the powdered leaves of M. perotitii and these included; moisture content, total ash, acid-insoluble ash, water soluble ash, water soluble extractive values and alcohol extractive values. The evaluation was carried out according to the methods described (WHO, 1998; Evans, 2009; Pratima and Mathad, 2011).
Statistical analysis
The data obtained were expressed as mean ± standard error of mean (SEM), and n represents the number of replicates in a particular experiment.
Macroscopic examination
Macroscopically, the leaf of M. perotitii was observed to be small with pubescent surface and alternately arranged along the smooth and soft stalk. Its apex is cute, the base is unequal and the margin is serrated while the venation is pinnate. The dimension of the leaf was 3.6 to 3.7 mm in length and 1.6 to 2.1 in breadth. The leaf when it is fresh is always greenish with unpleasant odour while its taste is slightly bitter and pepperish (stringent) (Figure 1). The summary of the macroscopic and organoleptic observations are given in Table 1.
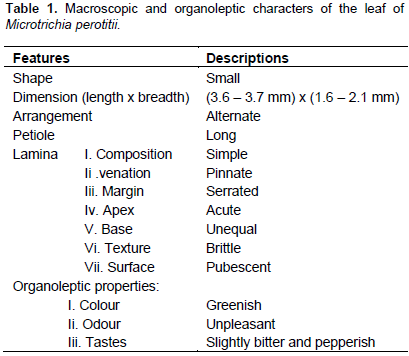

Microscopic examination
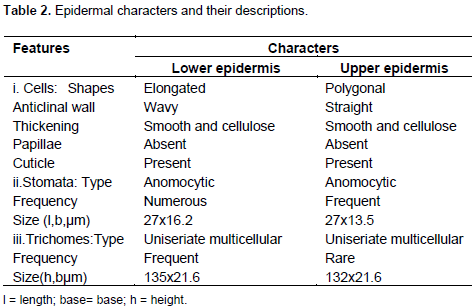
The result of microscopic evaluation of the leaf of M. perotitii showed typical characteristics of leaves in terms of taxonomic importance that is the presence of lower and upper epidermis, xylem, phloem, vascular bundles, mesophyll, collenchyma and trichomes. The observed shapes of the cells showed that they appeared elongated at the lower epidermis while they are diagonal at the upper epidermis. The anticlinal walls are wavy at the lower epidermis while they are straight at upper epidermis. The stomata were anomocytic type and were more frequent at the lower epidermis. Uniseriate multicellular trichomes were identified at both the upper and lower surfaces but were rare at the upper surface (Figure 2 to 6). A summary of the features are summarized in Table 2.
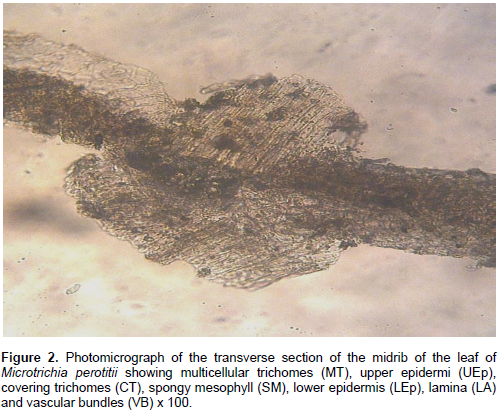
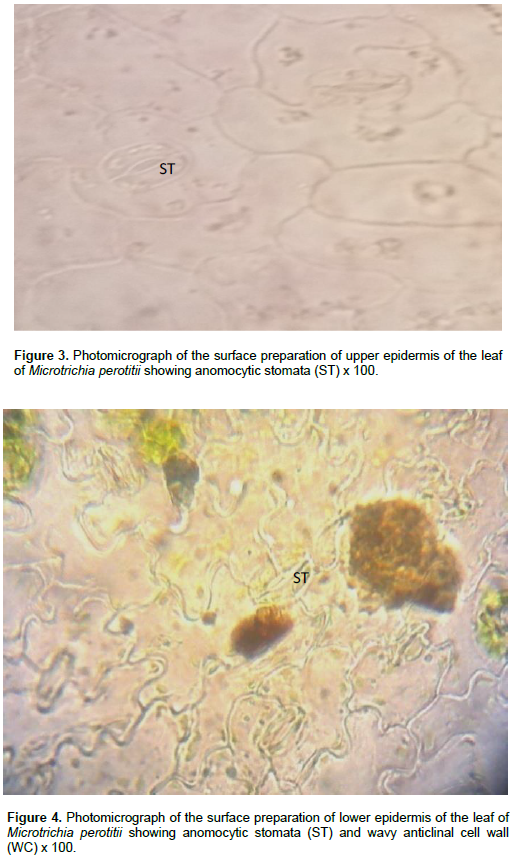
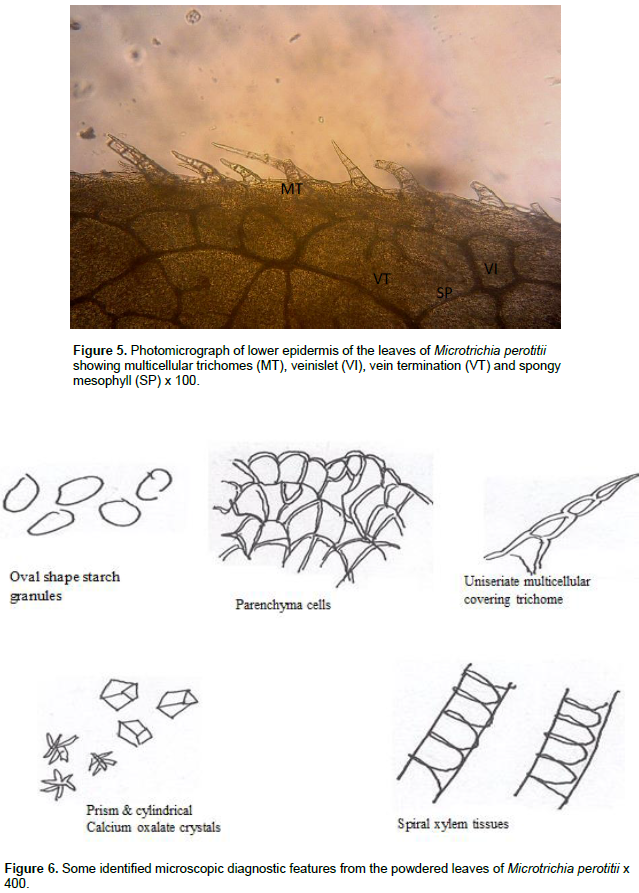
Chemo-microscopic evaluation
The chemo-microscopic evaluation of the powdered leaf of M. perotitii revealed the presence of cellulose cell wall, lignified cell wall, calcium oxalate crystals, tannins and fat and oils (Table 3).
Quantitative evaluation
The result of quantitative evaluation of the leaf of M. perotitii is presented in Table 4.
Physico-chemical examination
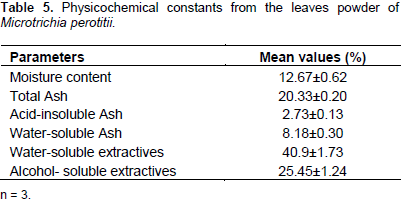
The result of the physical constant evaluation is presented in Table 5. The value of moisture content that is loss on drying has indicated the drug cannot easily be deteriorated by fungal or microbial attacks.
The pharmacognostic analysis of M. perotitii carried-out will help in establishing its botanical identity. The standardization of the herbal medicines is necessary to assure the quality of the drug and will also help in checking and preventing substitution and adulteration of foreign material, that is by mixing or substituting the original drug material with other spurious, substandard, defective, spoiled, useless other parts of the same or different plants (Rokad et al., 2018). It is a common knowledge that plant drugs are used locally for treatment of various disease conditions without recourse to standardization in other to establish the correct identity of the drug (Periyanayagam and Karthikeyi, 2013). The macroscopic evaluation is a technique of qualitative evaluation based on the study of morphological and sensory profiles of drugs. Therefore, the macroscopic characters of M. perotitii studied can serve as diagnostic parameters especially its organoleptic characteristics (Singh et al., 2010; Sathis et al., 2011). The organoleptic characteristics of the leaf were unique being bitter and pepperish with unpleasant odour. Microscopic evaluation is one of the simplest and cheapest methods to establish the correct and accurate identity for a plant drug (Patel and Zaveri, 2011). The microscopic evaluation of the leaf of M. perotitii showed that the leaf has anomocytic stomata occurring at both adaxial and abaxial epidermis thus indicating efficient gaseous exchange for photosynthesis and loss of water (Shaukat et al., 2010). Multicellular trichomes also appeared on both surfaces of the leaves although not so frequent at the upper surface. The presence and types of trichomes are useful diagnostic features and they contribute to plant resistance against herbivory and reduction of the rate of transpiration in the plants (Ahlam and Bouran, 2011; Priyanka et al., 2011). The transverse section of the leaf indicated that it is dorsiventral and showed the appearance of a typical leaf structure. Calcium oxalate crystals were observed and they may be involved in the dispersing of light to the chloroplasts in the photosynthetic parenchyma cells of the leaves.
The crystals were thought to have a physiological role sequestering excess calcium within plant cells (Popescu et al., 2010). Starch granules were observed and appeared in oval shape. Although the starch is the main form in which plants store carbon, they are sometimes converted into sugar by amyloplats when the plant needs energy (Alison, 2010). Chemo-microscopic evaluation of the leaf showed proteins, starch, cellulose and tannins including calcium oxalate crystals and lignins which are indications of the presence of alkaloids, flavonoids and glycosides in the leaves (Prabhu et al., 2009). Quantitative microscopic evaluation of the leaf of M. perotitii has provided values for palisade ratio, stomatal number, epidermal layer, stomatal indices, vein islet number and veinlet termination number. These information set genuity and standard for the herb as well as distinguishing it from co-generic species that may be closely related and cannot easily be characterized by general microscopy (Veeranjaneyulu and Rama, 1984). The physico-chemical evaluation of the leaves of M. perotitii could serve as a significant role in standardization and quality control by means of purity, stability and phytochemical composition of the herb (Bharat and Parabia, 2010). The moisture content for M. perotitii calculated by loss was 12.67% which is less than 14% standard requirement for crude drugs. This is an indication that the powder of this herb can be stored for a longer period of time without spoilage (Ahmad et al., 2012; Kadam et al., 2012; Mubo et al., 2014). The total ash value for M. perotitii lies within fair limits and thus signified its quality and purity and gives idea about the total inorganic content in it (Ugur and Selima, 2011; Woratouch et al., 2011).
The acid- insoluble ash value of 2.73±0.13% obtained for the leaves is an indication that the herb was in good physiological condition thus contained less extraneous matter in the form of contamination with silicious materials (earth and sand) and provides information about non-physiological ash produced due to adherence of inorganic dirt and dust to the crude drug. The water-soluble ash was 8.18±0.30% and this parameter is used to detect the presence of materials exhausted by water (Schoffstall, 2000; Singh and Sharma, 2010; Adedapo et al., 2011; Pratima and Pratima, 2011; Ahamad et al., 2012; Kamalakannan et al., 2012 ; Kunle et al., 2012; Veena and Pracheta, 2013; Sangram et al., 2015). Extractive values of plants give useful estimation of chemical constituents in the drug as well as a measure of the stability of phytocompounds in the plant drug in a given solvent. The value for the water soluble extractive for the leaves of M. perotitii were much higher than that of the alcohol thus indicating higher polarity and more penetration of the water molecules into the cellular membrane in order to extract all inter-cellular components of the plant material. The leaves therefore, exhibited more amount of water soluble components compared to alcohol extract and may provide estimation of specific constituents soluble in a particular solvent (Periyanayagam et al., 2013; Vipin et al., 2015). All the results obtained for the pharmacognostic evaluation of the leaves of M. perotitii were compared to those of some members of Asteraceae in order to maintain a standard since this is the first report about the leaves in the literature.
The pharmacognostic study on the leaves of M. perotitii is being reported for the first time in literature. The findings will be useful in the identification of the herb, detection of adulterants and its monograph. It will also provide avenue for further studies on microscopic evaluation of the leaves.
The authors declare that they have no conflict of interest.
The authors wish to acknowledge the contributions and support of our various institutions for providing the enabling environment to carry out this research. Need to be mentioned are all the technical staff of our research laboratories.
REFERENCES
|
Abdullahi M N, Ibrahim H, Ilyas N (2013). Evaluation of Phytochemical Screening and Analgesic Activity of Aqueous Extract of The Leaves of Microtrichia perotitii DC (Asteraceae) in Mice using Hotplate Method. Med. Plant Res. 3:537-543.
Crossref
|
|
|
|
Abdullahi MN, Ibrahim H, Ilyas N (2011). Phytochemical Screening and Biological Studies of the Leaves of Microtrichia perotitii DC (Asteraceae). Br .J. Med. Plants 1(3):88-97.
Crossref
|
|
|
|
Adedapo A, Jimoh F, Afolayan A (2011). Companson of the nutritive value and Biological activities of the Acetone, Methanol and water extracts of the Leaves of Bidens pilosa and chenopodium album. Acta polon Pharmaceu. Drug Res. 68(1):83-92.
|
|
|
|
Ahlam SE, Bouran IA (2011). Microscopical studies on the Leaf and Petiole of Vernonia amygadlina Del. Adv. Aplied. Sci. Res. 2(2):398-406.
|
|
|
|
Ahmad J, Showkat M, Naquri KJ (2012). Preliminary Pharmacognostical Standardisation of Aerial parts of Artemisia absinthium L. Int. Res. J. Pharm. 3(1):217-220.
|
|
|
|
Alison MS (2010). Starch and Starch granules. John Innes Centre, Norwich, UK. eLS. Wiley online Library. John Wiley and Sons.
|
|
|
|
Andrews FW (1954). The Flowering Plants of the Sudan, Vol.3 Compositae-Graminae. Published for the Sudan Govt. by T. Bande and co Ltd. Arbroath. Scotland. pp. 1-63.
|
|
|
|
Bharat G, Parabia MH (2010). Pharmacognostic evaluation of the bark and seeds of Mimusop selengi L. Int. J. Pharm. Pharmaceut. Sci. 2:110-113.
|
|
|
|
Chandaz S (2014). Importance of pharmacognostic study of medicinal plants. An overview. J. Pharmcog. Phytochem. 2(5):69-73.
|
|
|
|
Daniel O (1877). Flora of Tropical Africa. Vol III Umbelliferae to Ebenaceae. Published under the Authority of the first commissioner of Her Majesty's works. By L. Reeve and Co., 5 Henrietta Street, Covent. Garden London. pp. 302-303.
|
|
|
|
Elujoba AA, Odeleye OM, Ogunyemi CM (2005). Traditional medicinal development for medical and dental healthcare delivery system in Africa. Afr. J. Trad. Med. 2(1):46-61.
|
|
|
|
Evans WC (2009). Trease and Evans. Pharmacognosy. 16th edition. W. B. Saunders. Toronto Harcourt Pub. Ltd
View
|
|
|
|
Gireesh MA, Sandeep RP, Vinayak U, Pramod JH, Harsha VH (2015). Pharmacognostic evaluation of Achyranthes coynei: Leaf. Egyp. J. Basic Apld. Sci. 2:25-31.
|
|
|
|
Hutchinson J, Dalzie JM (1963). Flora of West Tropical Africa, 2nd edn Vol 12. Crown Agent for overseas Government and Administration Millbank, London SW 1:325-397.
|
|
|
|
Kadam PV, Vadav KN, Navasare VS, Bhilwade SK, Patil MJ (2012). Eclipta alba: A Phytopharmacognostic study. Int. J. Pharm. Phytopharmacol. Res. 1(6):350-353.
|
|
|
|
Kamalakannan P, Kavitha R, Elamathi R, Deepa T (2012). Sridhar, S. Study of Phytochemical and Antimicrobial potential of Methanol and Aqueous extracts of Aerial Parts of Elephantopus scaber L. Int. J. Cur. Pharmceut. Res. 4(1):18-21.
|
|
|
|
Kunle OA, Oluyemisi F, Egharevba HO, Ahmadu PO (2012). Standardization of Herbal Medicines: A review. Int. J. Biodiv. Cons. 4(3):101-112.
Crossref
|
|
|
|
Majumder P (2011). Evaluation of pharmacognostic, phytochemical profile along with quantitative physicochemical standards on the root of medicinal herb Peporemia pellucid. (L) HBK. J. Pharmaceut. Biomed. Sci. 10(10):1-3.
|
|
|
|
Mubo AS, Tolulope AO, Mike OS (2014). A Pharmacobotanical Study of two Medicinal species of Fabaceae.Asian Pac. J. Trop. Biomed. 4(2):131-136.
Crossref
|
|
|
|
Patel S, Zaveri M (2011). Pharmacognostic study of the Roots of Justica gendarussa Burm. J. Trad. Med. 6(2):61-72.
|
|
|
|
Periyanayagam K, Gopalakrishnan S, Karthikeyan V (2013). Evaluation of Pharmacognostical and Phytochemical properties of the Leaves of Psidium guajava Linn- Chittidhar variety. Innov. J. Health Sci. 1(3):10-13.
|
|
|
|
Periyanayagam K, Karthikeyan V (2013). Pharmacognostical, SEM and XRF profile of the leaves of Artocarpus heterophyllus L. (Moraceae) - A contribution to combat the NTD. Innov. J. Life Sci. 1(1):23-28.
|
|
|
|
Popescu ML, Mihaela D, Diana DU (2010). Contributions to the Pharmacognostical and Phytobiological study on Taxacum officinale (L) weber. Parmacia. 58(5):646-653.
|
|
|
|
Prabhu K, Karar PK, Ponudural K, Hemalatha S (2009). Pharmacognostic investigation of the leaves and stem of vibrurnum erubescens wall D.C. Trop. J. Pharmaceut. Res. 6:557-566.
|
|
|
|
Pratima H, Pratima M (2011). Pharmacognostic Evaluation and Phytochemical Analysis of Leaves of Cajanus cajan L. J. Adv. Dev. Res. 2(2): 181-185.
|
|
|
|
Priyanka Y, Deepak S, Nayaka S (2011). Microscopic studies of Tridex procumbens L. Int. J. Biomed. Res. 2:508-517.
|
|
|
|
Kumar AR, Rathinam KS (2013). Pharmacognostical Studies on Artemisia annual. Int. J. Res. Pharm. Biotechnol. 1(1):64-67.
|
|
|
|
Rokad N, Pande J, Chanda S (2018). Pharmacognostic and phytochemical studies of Ipomoea pes-caprae, An halophyte from Gujarat. J. Pharmacog. Phytochem. 7(1):11-18.
|
|
|
|
Sangram KP, Debajyoti D, Chandan D (2015). Phytochemical Screening, Pharmacognostic study and Physicochemical Evaluation of Leaf of Crotalaria pallida Aiton Der Pharmacia Let. 7(10):239-247.
|
|
|
|
Sathis KD, David B, Prashanthi G, Harani A (2011). Pharmacognostical Evaluation Study on Crotalaria jumcea Linn. Amer.-Euras. J. Sci. Res. 6(3):139-145.
|
|
|
|
Schoffstall AM, Gaddis BA, Druelinger ML (2000). Microscale and Miniscale Organic Chemistry Laboratory Experiments. McGraw Hill companies USA pp. 86-103.
|
|
|
|
Shaukat M, Huma S, Manyam A, Shahnaz G, Ghazala HR (2010). Parmacognostic Studies on Fresh Mature Leaves of Holoptetea integritolia (ROXB) Plarich. Pax. J. Bot. 42(6):3705-3708.
|
|
|
|
Singh A, Saharan AV, Kumawat IC, Khatri A, Bhandari A (2014). A Pharmacognostical Study of Vernonia cinerea Less (Asteraceae) and Evaluation of Anti-inflammatory and Antibacterial activities of Stem. Egyp. Pharmceut. J. 13(2):104-112.
Crossref
|
|
|
|
Singh S, Machawal L, Chauhan MG (2010). Pharmacognostic study of male leaves of Trichosanthes dioica.Roxb with special emphasis on microscopic technique. J. Pharmacog. Phytothera. 2(5):71-75.
|
|
|
|
Sumitra C (2014). Importance of pharmacognostic study of medicinal plants: An overview. J. Pharmacog. Phytochem. 2(5):69-73.
|
|
|
|
Ugur C, Selima K (2013). Nitrate, Moisture and Ash Contents of Edible wild plants. J. Cell. Plant Sci. 2011; 2(1):1-5.
|
|
|
|
Veena S, Pracheta S (2013). Microscopic Studies and Preliminary Pharmacognostical Evaluation of Euphorbia neriifolia L. Leaves. Ind. J. Nat. Prod. Res. 4(4):348-357.
|
|
|
|
Vipin CP, Bhuwanendra S, Arshad A (2015). Pharmacognostic Evaluation of Leaves and Stem of Murraya koenigii. UK. J. Pharmaceut. Biosci. 3(1):37-41.
|
|
|
|
Veeranjaneyulu K, Rama VSD (1984). Stomatal frequency and Resistance of some Tropical members of Asteraceae. Proc. Indian Natl. Sci. Acad. 50(3):317-320.
|
|
|
|
Watson L, Dallwitz M (1992). The Families of Flowering Plants: Description, Illustration, Identification and Information 1992; Accessed 18th August, 2008.
|
|
|
|
Woratouch T, Thatree P, Suchada S (2011). Pharmacognostic Evaluation of Lagerstroemia Species leaves. J. Med. Plant Res. 5(8):1330-1337.
|
|
|
|
World Health Organisation (1998). Quality control Methods for Medicinal Plants Materials. England. pp. 10-21, 22-45.
|
|
|
|
World Health Organisation (2007). Guidelines for assessing quality of herbal medicines with reference to contaminants and residues. England. pp. 210-215.
|