ABSTRACT
A study was carried out on the effect of varied levels of dietary aflatoxin on acetylcholinesterase, glucose and total protein concentration in the brain regions of West African Dwarf goats. 20 West African Dwarf goats of about 5-6 months old were used for the trial and they were randomly allotted to four dietary treatments containing 0 (control), 50, 100 and 150 µg aflatoxin/kg diet. The animals were housed individually for the feeding trial in a completely randomised designed and the experiment lasted 12 weeks. At the end of the feeding trial, the animals were sacrificed and brain dissected into different regions. The different regions of the brain studied were medulla oblongata, amygdala, hippocampus, cerebral cortex, mid-brain, cerebellum, pons varoli and hypothalamus. Samples were collected from these regions and homogenised to determine acetylcholinesterase, glucose and total protein concentrations. Result showed that the acetylcholinesterase activity in the brain regions was not significantly influenced by the dietary aflatoxin among the treatments. Glucose concentration was significantly (p<0.05) higher in the hypothalamus of animals fed 50 µg aflatoxin/kg and control diet than those fed 100 and 150 µg aflatoxin/kg. Total protein concentration in the medullar oblongata and the hypothalamic regions of the brain in animals fed 150 ppb was significantly (p<0.05) higher than those on the control diet. The pH of the medulla oblongata, amygdala, hippocampus, cerebral cortex were significantly (p<0.05) higher in goats fed 150 mg/kg than those fed the control diet. However, pH was not significant in the mid brain, cerebellum, pons varoli and hypothalamus among the treatments. This study suggests that dietary aflatoxin up to 100 ppb reduced glucose concentration in the hypothalamus and total protein in the medulla oblongata region of the brain with tendency to impair brain function.
Key words: Aflatoxin, WAD goat brain, acetylcholinesterase, total protein.
Aflatoxins are an important group of mycotoxin produced by the fungi Aspergillus flavus, A. parasiticus and A. nomius (Diaz et al., 2008). Other species of Aspergillus such as A. bombycis, A. ochraceoroseus and A. pseudotamari also produce aflatoxins (Bennett and Klich, 2003). On world-wide scale, the aflatoxins are found in stored food commodities and oil seeds such as corn, peanuts, cottonseed, rice, wheat, oats, barley, sorghum, millet, sweet potatoes, potatoes, sesame, cacao beans, almonds, etc., which on consumption pose health hazards to animals, including aquaculture species of fish, and humans (Abdel-Wahab et al., 2008). The toxin is highly oxygenated, heterocyclic, difuranocoumarin compounds that could be present in human foods and animal feedstuffs. Health effects occur in fish, companion animals, livestock, poultry and humans because aflatoxins are potent hepatotoxins, immunosuppressant, mutagens, carcinogens and teratogens.
In the tropical regions, where the climatic conditions favour luxurious growth of Aspergillus spp, people rely on commodities such as cereals, oilseeds, spices, tree nuts, milk, meat and dried fruits that are potentially contaminated by aflatoxins (Strosnider et al., 2006). Animals are predominantly affected by the Aspergillus spp, metabolite through ingestion of contaminated diet. Aflatoxin is metabolized by cytochrome p450 group of enzymes in the liver, where it is converted to many metabolic products like aflatoxin 8, 9 epoxide, aflatoxicol, aflatoxin Q1, aflatoxin P1, and aflatoxin M1, depending on the genetic predisposition of the species. The amount of aflatoxin 8, 9 epoxide metabolite decides the species susceptibility as this can induce mutations by intercalating into DNA, by forming adduct with guanine moiety in the DNA (Smela et al., 2001).
There is currently lack of information on the aflatoxin effect on the brain of West African Dwarf (WAD) goats. The brain also operates as central control of movement, balance, memory, thought and emotion in the body of an animal (Taylor, 1998). Knowledge of the biochemical composition of the brain is therefore important. Some reports on the biochemical characteristics of the brains of farm animals have been documented, most especially for boars (Egbunike, 1981), pigs (Adejumo and Egbunike, 2001a) and rabbit (Bitto, 2008) but not in relation to aflatoxin effect.
Experimental location and materials
This study was approved by our institutional committee on the care and use of animals for experiment. The experiment was carried out at the small ruminant unit of the Teaching and Research Farm, University of Ibadan, Ibadan, Nigeria. It is located at latitude 70° 20’ N and longitude 40° 50’ E. It is 200 m above sea level with average day time temperature of 23-27°C and relative humidity of 80-85%.
Clean maize grains were purchased from local market in Ibadan metropolis and sorted. Damaged, coloured and bad kernels were removed and disposed. Other ingredients used for the feeds were purchased from Kesmac Feed and Agric Consult feed mill, opposite University of Ibadan second gate, Ibadan.
Aflatoxin contaminated maize grains
Maize grain served as the aflatoxin carrier in the diets used for this study. The maize grains used for the experiment was inoculated with toxigenic strain Aspergillus flavus predominant in Nigeria. This culturing and inoculation was done at the Plant Pathology Unit, International Institute of Tropical Agriculture, Ibadan, Nigeria. The concentration in the maize grains and the diet was determined as described by Suhagia et al. (2006).
Experimental animals
Total of 20 WAD bucks of 5 months old were purchased for the experiment. The weights of the animals ranged from 7-9 kg. The animals were acclimatized for 28 days at the experimental site for physiological adjustment to feed and environment. Concentrate diet and Gliricidia leaves were fed, and fresh water were provided for the animals throughout the experiment. The animals were vaccinated against peste des pesti ruminants and treated with ivomectin injection against endo and ecto-parasites. Other routine management practices were carried out during the experimental duration.
Experimental diets
Four diets were formulated to meet the nutrient requirement of the animals. Diet 1 which is the control in the experiment and contained cleaned maize without aflatoxin contamination. The contaminated maize grains were used in substitution for the uninfected maize grains to vary the concentration of aflatoxin among diets 2, 3 and 4. All diets were iso-nitrogenous (12.9% CP) and isocaloric (3.57kcal/g DE).
Treatment Layout
Diet 1: Control diet without aflatoxin
Diet 2: Diet containing 50 ppb aflatoxin
Diet 3: Diet containing 100 ppb aflatoxin
Diet 4: Diet containing 150 ppb aflatoxin
Feeding trial
At the end of the acclimatisation period, 20 bucks were weighed and allotted randomly into four treatments such that each treatment has 5 animals housed individually in a completely randomised design. Dietary treatments 1, 2, 3 and 4 were represented with T1, T2, T3 and T4, respectively.
Dietary treatments were offered to the respective animals twice daily at 8.00 a.m. and 12.30 p.m. with their respective diets ad libitum. Feed supply was adequate and responsively supply as the bucks weight changes, since feed consumption would be expected to change with body weight. The experiment lasted for 12 wk, during which animals were fed concentrate as supplement to gliricidia sepium in ratio 2:3 of their body weight.
Brain regions assessment
At the end of the feeding trials the animals were sacrificed, head dissected to harvest the brain and the brain was separated into regions samples were taken from the following regions: amygdala, cerebellum, hypothalamus, hippocampus, pons varoli, mid-brain and medulla oblongata.
Total proteins concentration
The total protein concentrations in the regions were evaluated using the Biuret method as earlier reported by (Adejumo and Egbunike, 2001b). An automatic dispenser was used to measure 5 mL of Biuret reagent into a test tube and 10 mL of the homogenate will be added. The mixture was incubated at room temperature of about 25ºC for 30 min. After incubation, the incubated mixture was poured into a clean cuvette. The side of the cuvette was thoroughly wiped with tissue paper before it was placed inside spectrophotometer at wavelength of 540 nm to determine the protein concentration. The blank was used to standardize the spectrophotometer. The standard was prepared using 0.1 mL of total protein standard and 5 mL of Biuret reagent.
Glucose concentration
The glucose concentrations in the regions of the brain were evaluated using the method as earlier reported by (Bitto et al.,
2009). 10 mL of the homogenate was introduced into a test tube and l mL of the glucose reagent was added. The mixture was incubated at 37°C for 10 min. Some of the incubated mixture was poured in a clean cuvette and read at wavelength of 500 nm.
Acetylcholinesterase concentration
The regions were homogenized in 0.1 mL of phosphate buffer (pH 7.4) using Elvenjem glass homogeniser and latter assay for acetylcholinesterase (AChE) concentration was determined according to the calorimetric method as reported by Egbunike (1981) which measure the rate of hydrolysis and acetyl thiocholine iodine substrate to thiocholine and acetate using 5:5 dithiobis-2-nirobenzoate (DTNB) as the colour reagent. 2.6 mL of the buffer was pipette inside the cuvette. 100 mL of DTBN and 0.4 mL of the homogenate were added and the mixture was placed inside the spectrophotometer and this was standardized to zero after which 20 mL of substrate was added. The initial absorbance was read and after 4 mins the final reading was taken at a wavelength of 405 nm.
Data analysis
Data obtained were subjected to analysis of variance at p = 0.05 and means were separated using Duncan’s multiple range tests of SAS (1999). SAS/STAT® (version 8.0) (SAS Institute, Cary, North Carolina, United States).
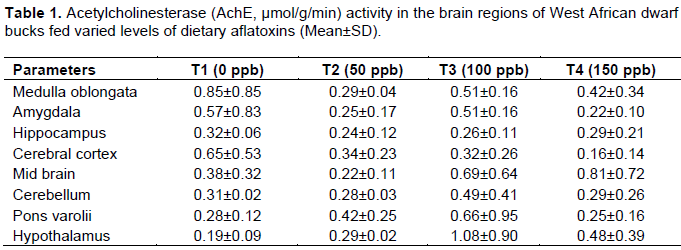
Acetylcholinesterase activity in brain regions of WAD goats
The effect of aflatoxin on the AChE activity in the brain regions of WAD bucks is as shown in Table 1. The result obtained showed that there was no significant difference the AChE concentration among the dietary treatments. The AChE in medulla oblongata, amygdala, hippocampus, cerebral cortex, mid-brain, cerebellum, pons varolis and hypothalamus was not significantly influenced by dietary aflatoxin among the treatments. The AChE observed in treated animals was not significantly different from the control.
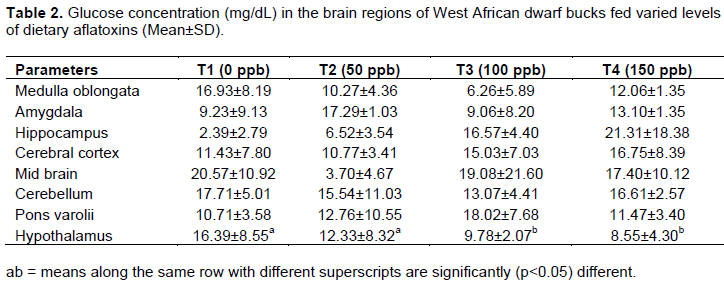
Glucose concentration in the brain regions of WAD goats
The glucose concentration in the brain regions of WAD bucks fed varied levels of dietary aflatoxin is as shown in Table 2. The result obtained showed no significant difference in all the brain regions examined except hypothalamus among the treatments. It was observed that the glucose concentration in the hypothalamus of goats fed treatment 2 was not significantly different from those fed control diet. However, the value obtained for animals on T2 and T1 were significantly (p<0.05) higher than those fed treatments 3 and 4. The glucose concentration in hypothalamus of goats fed treatment 3 was not significantly different from those fed treatment 4.
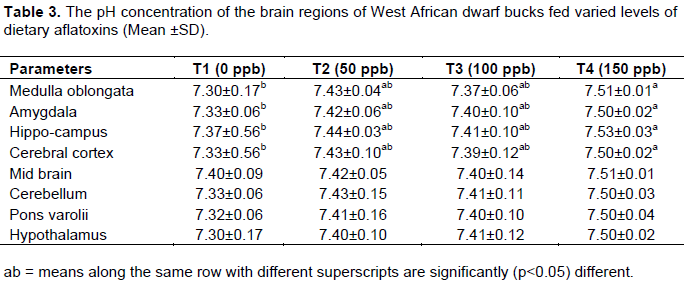
The pH and total protein concentration in brain region of WAD goats
The pH of the brain regions of WAD bucks fed dietary aflatoxin is as shown in Table 3. No significant difference was observed in the pH of mid-brain, cerebellum, pons varoli, hypothalamus of the animals among the treatments. However, there was significant (p<0.05) difference in the pH value of medulla oblongata, amygdala, hippocampus and cerebra among the treatments. The pH in these brain regions followed the same trend, and that of animal fed Treatment 4 were not significantly different from those fed treatments 2 and 3 but were significantly (p<0.05) higher than those bucks on the control diet.
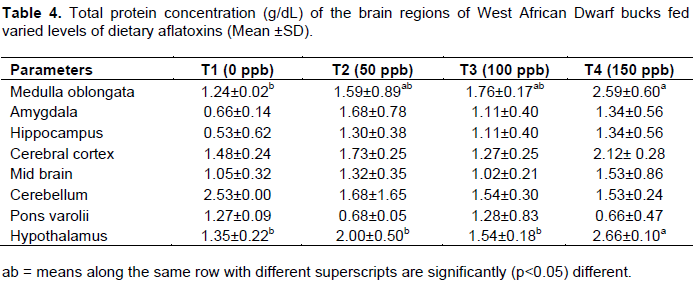
The total protein concentration within the brain regions of the bucks fed varied level of dietary aflatoxin is as shown in Table 4. The amygdala, hippocampus, cerebral cortex, cerebellum and pons varoli of the animal fed dietary aflatoxin were not significantly different among the treatments.
However, total protein concentration in medullar oblongata of the animal on treatment 1 was significantly (p<0.05) lower than those on treatment 4 while those on treatments 2 and 3 were not significantly different from each other. Also, the hypothalamus of the animal fed treatment 4 which has the highest dose of aflatoxin recorded total protein concentration which was significantly (p<0.05) higher than goats fed treatments 1, 2 and 3. However, the total protein in hypothalamus of goats fed treatments 2 and 3 was not significantly different from the control.
The ability of aflatoxin producing fungi (Aspergillus flavus) to grow on wide range of food and feed stuffs under certain condition constitutes a threat to both animals and human (Sayed and Abeer, 2013). In developing countries where food availability often times are considered before food safety, there is a lack of legislation on acceptable limits for aflatoxin and population are undoubtedly exposed to high amount of aflatoxin (Williams et al., 2004). The AChE activity that was not significantly different among the brain regions for all the treatments indicated that the inclusion of aflatoxin level up to 150 ppb does not affect normal synthesis and catabolism of neurotransmitters (AChE) which could presumably be tolerated by the animal since it does not affect any brain region biochemicals. This could probably be that goat has higher resistance to aflatoxicosis than sheep and cattle. Adejumo et al. (2005) reported from the study on sex differences in acetylcholinesterase activity in Red sokoto bucks and does that the AChE maintains its characteristically low concentration in Red sokoto bucks brain. However, AChE concentration was highest in the mid brain, medulla oblongata, hippocampus of pig brain (Adejumo and Egbunike, 2002).
The lack of micro doses of dietary aflatoxin effect on the AChE activity in the cerebellum may be due to its involvement in locomotion and muscular activities which are maintained in the animal up till old age (Adejumo and Egbunike, 2004). In addition, the cerebellum itself is characterised by typically low AChE activities (Adejumo and Egbunike, 2002). The normal level of AChE reported for Red Sokoto goats was 4.32±0.36 µmol/g/min (Adejumo and Egbunike, 2002).
The significant difference that was observed in the protein concentration in medulla oblongata and hypothalamus could be due to change in protein synthesis or metabolism and could be attributed to under nutrition or poor nutrient utilization as reported in these same animals (Ewuola et al., 2013). Aflatoxin has been reported to binds and interferes with enzymes and substrates that are needed in the initiation, transcription and translation process involved in protein synthesis. This may also be indicative of aflatoxin effect on the brain development as protein in the brain is important for it functions such as repair of worn-out tissues for growth, muscles development and it also binds to some minerals to ensure bioavailability of minerals for proper utilisation (Adejumo et al., 2005).
The total protein levels in the brain regions in the study were generally higher than the values reported for the brain regions of male porcine by Adejumo and Egbunike (2001, 2001a) and Bitto (2008) for rabbit bucks. This disparity may be due to species differences in biochemical characteristics of brain regions. Also, total proteins in the brain undergo major changes during development (Tucek et al., 1990) and such changes have been found to be unaffected by genetic or species effects amongst some ruminant (Adejumo et al., 2005).
There were no significant differences in glucose concentration of the different brain regions, except hypothalamus. The significant difference in the hypothalamus may be due to its involvement in glucose transport, energy production and glycogenesis which was also observed by Sayed and Abeer (2013) male Sprague rat fed aflatoxin. It could also indicate that WAD bucks may have increased activities in their brain with respect to hormone secretion (Taylor et al., 1998) since hypothalamus is involved in the release of gonadotropin releasing hormones in the brain.
The glucose concentration in the hypothalamus region of animal fed 100 and 150 µg/kg aflatoxin significantly lower than those fed 50 µg/kg aflatoxin and the control diet could be an indication of hypothalamic hypoglycaemia probably induced by the toxin. This implies that glucose is low, psychological process requiring mental effort may be impaired. The result corroborates the finding of Ikegwuonu (1983) who reported that nerves tissue requirement for glucose molecules were reduced during aflatoxicosis. Glucose has earlier been reported to be the obligatory energy substrate for fuelling brain and it is entirely oxidised to carbon iv oxide and water for optimal use (Magistretti et al., 1999; Kong et al., 2002).
The alteration in the pH of medulla oblongata, amygdala, hippocampus and cerebral cortex among the treatments could be attributed to treatment effect induced by the toxin. However the observe values across the treatments were within the physiological range of 7.2-7.5 reported by Bermeryer (1974).
Aflatoxin does not have any significant effect on the brain pH, values obtained ranges from 7.3-7.5 in all the dietary treatments. The AChE is reported to be optimally active within a pH range of 7.2-8.5 (Bergmeryer, 1974). At a level beyond this range, AChE is inactivated (Bergmeryer, 1974), although WAD goats have been adjudged to be tolerable to aflatoxin.
Based on the results of this study, the acetylcholinesterase activity and total protein concentration for the treatments were not influence across the brain regions by the micro doses of aflatoxin up to 150 µg/kg. However, the animals on 100 and 150 µg/kg suffer hypothalamic hypoglycaemic condition in the brain which may impair psychological process affect mental effort like coordination. This study suggests that dietary aflatoxin above 50 ppb reduced glucose concentration in the hypothalamus and total protein in the medulla oblongata region of the brain.
The authors have not declared any conflict of interests.
REFERENCES
|
Abdel-Wahab M, Mostafa M, Sabry M., El-Farrash M, Yousef T (2008). Aflatoxins a risk factor for hepatocellular carcinoma in Egypt, Mansoura Gastroenterology Center study, (September-October 2008). Hepato-gastroenterology 55(86-87):1754-1759.
|
|
|
|
Adejumo DO, Egbunike GN (2001a). Age dependent change in the total protein concentrations in the brain regions and hypophyses of the pig, Int. J. Agric. Rural Dev. 5:19-26.
|
|
|
|
|
Adejumo DO, Egbunike GN (2001b). Effect of ovariectomy and testosterone and oestrogen administration on acetylcholinesterase activity and total protein in the brain regions and adenohypophyses of large white gilts. Trop. Anim. Prod. Invest. 4:77-81.
|
|
|
|
|
Adejumo DO, Egbunike GN (2002). Regional variation in acetylcholinesterase activity and total protein in the brain and hypophyses of large white boars managed under a hot humid environment. ASSET series A. 2(1):49-53.
|
|
|
|
|
Adejumo DO, Egbunike GN (2004). Changes in acetylcholinesterase activities in the developing and aging pig brain and hypophyses. Int. J. Agric. Rural Dev. 5:46-53.
|
|
|
|
|
Adejumo DO, Ladokun AO, Sokunbi OA (2005). Species differences in acetylcholesterase activity and total protein concentration in the brain and hypophyes of Red sokoto goats and Gudali Cattles, Asset Series A. 5(1):121-127.
|
|
|
|
|
Bennett JW, Klich M (2003). Mycotoxins. Clin. Microbiol. Rev. 16:497-516.
Crossref
|
|
|
|
|
Bergmeryer HV (1974). Method of enzymatic analysis. 2nd Ed. vol. 2:831-853 Academic press inc. New York.
|
|
|
|
|
Bitto II (2008). Total protein and cholesterol concentration in brain regions of rabbits fed pawpaw peel meal. Afr. J. Biomed. Res. 11:73-78
|
|
|
|
|
Bitto II, Arubi JA, Gumel AA (2009). Reproductive Tract Morphometry and Some Haematological Characteristics of Female Rabbits Fed Pawpaw Peel Meal Based Diets. Afr. J. Biomed. Res. 9(3):199-204
Crossref
|
|
|
|
|
Diaz DE Hopkins BA, Leonard LM, Hagler WM Jr, Whitlow LW (2008). Effect of fumonisin on lactating dairy cattle. J. Dairy Sci. 83(abstr.):1171.
|
|
|
|
|
Egbunike GN (1981). Regional distribution of acetylcholinesterase activity in the brain and hypophyses of crossbred European boars reared in the humid tropics. Acta Anat. 110:248-252.
Crossref
|
|
|
|
|
Ewuola EO, Jimoh OA, Bello AD (2013). Growth response and nutrient digestibility of West African Dwarf goats fed micro doses of dietary aflatoxin. Sci. J. Anim. Sci. 2(11):316-322.
|
|
|
|
|
Ikegwuonu FI (1983). The neurotoxicity of aflatoxin B1 in the rat. Toxicology 28:247-257.
Crossref
|
|
|
|
|
Kong J, Shepel PN, Holden CP, Mackiewicz M, Pack AI, Geiger JD (2002). Brain Glycogen Decreases with Increased Periods of Wakefulness: Implications for Homeostatic Drive to Sleep. J. Neurosci. 22(13):5581-5587.
|
|
|
|
|
Magistretti PJ, Pellerin L, Rothman DL, Shulman RG (1999). Energy on demand. Science 283:496-497
Crossref
|
|
|
|
|
SAS (1999) Statistical Analysis Software. SAS/STAT user's guide. IAS Inst Inc. Cary, New York.
|
|
|
|
|
Sayed MR, Abeer MW (2013). Impact of 90 days oral dosing naturally occurring aflatoxin mixture on Sprague Rat neurochemistry and behavioural pattern middle east. J. Sci. Res. 14(2):228-232.
|
|
|
|
|
Smela ME, Sophie S, Curier E, Bailey A, John ME (2001). The chemistry and biology of aflatoxin B1. Carcinogenesis 22:535-545
Crossref
|
|
|
|
|
Strosnider H, Azziz-Baumgartner E, Banziger M, Bhat RV, Breiman R, Brune M, DeCock K, Dilley A, Groopman J, Hell K, Henry SH, Jeffers D, Jolly C, Jolly P, Kibata GN, Lewis L, Liu X, Luber G, McCoy L, Mensah P, Miraglia M, Misore A, Njapau H, Ong C, Onsongo MTK, Page SW, Park D, Patel M, Phillips T, Pineiro M, Pronczuk J, Schurz RH, Rubin C, Sabino M, Schaafsma A, Shephard G, Stroka J, Wild C, Williams JT, Wilson D, (2006). Workgroup Report: Public Health Strategies for Reducing Aflatoxin Exposure in Developing Countries. Environ. Health Perspect. 114:1989-1903.
Crossref
|
|
|
|
|
Suhagia BN, Shah SA, Rathod IS, Patel HM, Shah DR, Marolia BP (2006). Determination of gatifloxacin and ornidazole in tablet dosage forms by high-performance thin-layer chromatography. Anal. Sci. 22:743-745.
Crossref
|
|
|
|
|
Taylor SJ, Green NPO, Stout GW (1998). Third Edition Biological Science Cambridge Low Price Editions. pp. 575-582.
|
|
|
|
|
Tucek S, Musílková J, Nedoma J, Proška J, Shelkovnikov S, Vorlícek J (1990). Positive cooperativity in the binding of alcuronium and N-methyl scopolamine to muscarinic acetylcholine receptors. Mol. Pharmacol. 38:674-680.
|
|
|
|
|
Williams JH, Phillips TD, Jolly PE, Stiles JK, Jolly CM, Aggarwal D (2004). Human aflatoxicosis in developing countries: a review of toxicology, exposure, potential health consequences, and interventions. Am. J. Clin. Nutr. 80:1106-1122.
|
|