ABSTRACT
A cross-sectional study was conducted in the three woredas of Sidama zone, Southern Nations, Nationalities and Peoples' Region (SNNPRS) with the aim of determining the overall prevalence of African horse sickness (AHS) in equines and the effect of putative risk factors on the prevalence of AHS. A total of 230 equines (66 donkeys, 161 horses and 3 mules) were included. Serological examination was conducted using blocking enzyme linked immuno sorbent assay (blocking ELISA) revealing a point prevalence of 46.97, 13.66 and 33.33% AHS in donkeys, horses and mules, respectively. The overall prevalence of AHS in equines in Sidama zone of the three districts was 23.47%. The prevalence at Dale, Hula and Wonsho woreda’ was 52.72, 10.47 and 17.98%, respectively. Statistical analysis of the result showed significance difference in prevalence among donkeys, horses and mules (P< 0.05). The prevalence of 52.72% in midland and 14.28% in highland revealed statistically significant difference (P<0.05). The difference observed between Dale and Hula, and Dale and Wonsho was seen to be statistically different, but no difference was seen between Wonsho and Hula. Among the hypothesized risk factors, agro ecology is the predominant risk for the prevalence of AHS and this was asserted by P-value (P<0.05).
Key words: African horse sickness, enzyme linked immuno sorbent assay (ELISA), equines, sero-epidemiology, Sidama, prevalence.
African horse sickness (Peste equina Africana, Peste equine) is an insect born viral disease characterized by severe pyrexia, wide spread hemorrhage and edematous exudations (OIE, 2008). AHS virus affects all species of Equidae family (horses, mules, donkeys and zebras) and transmitted by a biting midge belonging to the genus Culicoides (Maclachlan and Guthrie, 2010). This devastating disease is endemic in sub- Saharan African (OIE, 2008). In the late 1980s, AHS spread out side its endemic region reaching Spain and Portugal (OIE, 2008; Ferhandey et al., 2012).
African horse sickness virus (AHSV) belongs to the family Reoviridac, and genus Orbivivus (OIE, 2008). Like other Orbiviruses, AHSVvirions are double-stranded with their genomes composed of 10 double stranded RNA (dsRNA) segments (Cetre-Sossah et al., 2008). Nine serologically distinct AHS virus serotypes (AHSV-1 to AHSV-9) have been identified with the evidence of cross-neutralization among them (Cetre- Sossah et al., 2008; OIE, 2004).
The disease confined to sub-Saharan Africa although periodic epizootics have caused severe outbreaks of the disease outside enzootic regions like North Africa, Middle East, and Southern Europe (Rodriguez-Sanchez et al., 2008; Torrecuadrada et al., 2011). The nine known virus serotypes of AHSV have been isolated from clinical cases of the disease in Kenya (Binepal et al., 2013). The most important factor in the epizootology of AHS is the host.
The existence of the reservoir of infection is suggested by the fact that the disease passes from one season to another in a particular area. An outbreak mostly occurs during rainy season and quickly disappears during dry and cool periods before it appears when wet and warm weather returns.
Many factors can contribute to the poor performance of equines, among which viral diseases characterized by high morbidity and mortality rates are the first. Clinically, the disease is characterized by an acute pulmonary form, a cardiac form or sub-acute form, mixed form and a mild form know as horse sickness fever (Upadhyaya, 2011). Hence, African horse sickness is one of the viral diseases characterized by up to 95, 50 and 10% mortality rates in horses, mules and donkeys, respectively (OIE, 2008).
According to the Central Statistical Authority of Ethiopia (2009) there are 5. 42 million donkeys, 1.78 million horses and 373, 519 mules. It has the largest equine population, probably with the highest density per square kilometer in the world (Mulualem et al., 2012). An important but often overlooked aspect is that in most cases, donkeys and mules are reared by land less and marginal farmers and are the means of subsistence for millions in the least privileged parts of the world. These beasts of burden receive little care and are subjected to intensive work throughout their lives. Like most parts of the country, in Sidama zone as well, the attention given to these animals was very poor despite their enormous role in the economy and they are subjected to arrays of management constraints and diseases.
Among the reported diseases, African horse sickness has been known for years (Sidama Zone Agricultural and Rural Department (SZARD), 2011). However, to the authors’ knowledge, in the last decades, no systemically collected information are available pertinent to the disease in the study area. Hence, the research work aimed to estimate the prevalence of African Horse sickness and to identify some of the hypothesized risk factors in three woredas’ of Sidama
zone, Hawassa, Ethiopia.
Study area
This study was carried out in the Sidama zone of Southern Nations, Nationalities and Peoples Region (SNNPRs). The zones is located in the northern part of SNNPRs, with its capital town at Hawassa, which lies about 275 km south of Addis Ababa. Geographically, the zone lies between 4°27׳and 8°30׳ N latitude and 39°1׳E longitude [Sidama Zone Planning and Economic Development Department (SZPEDD), 2004]. Like most parts of Ethiopia, the relief configuration of Sidama ranges from very high mountains to lowland plains, where the altitude varies between 1001 and 3200 m above sea level (m.a.s.l.). It has an annual rainfall and temperature ranging from 960 to 1100 mm and 18.1 to 20°C, respectively (SZPEDD, 2004).
Study animals
The study animals were equines, namely: horses, donkeys and mules above 4 years of age and non-vaccinated for AHS were considered from two ecological zones of midland (Dale) and highland (Wonsho and Hula) of Sidama zone. A total of 230 equines were selected, of which 66 were females and 164 males. Most of the equines in the area are used for pack, race and cart pulling.
Study design
The study was an observational one made in two distinct agro ecological zones namely midland and highland of the study area. As animals of the same village are usually kept in a communal grazing land, list of pass of respective district was used to represent primary sampling unit (epidemiological unit) and individual animals (secondary unit) using two stage cluster sampling technique. To this effect, a cross sectional study design was employed. Blood samples were collected using plain vacationer tube and information (data) relevant to the epidemiology was collected using semi-structured questionnaire format.
Sample size
A two-stage cluster sampling techniques was used to calculate the actual sample size having the following parameters predetermined: CL= 90%, desired level of precision =5%, expected total clusters prevalence and in between cluster variance (Thrusfield, 2005). Average number of equines per peasant association (n) is estimated to be ten (10), using the formula:
A total of 230 equines in 21 clusters were picked randomly considering 10 clusters from midland and 11 clusters from highland agro ecological zones.
Diagnostic test
Serum was collected from three selected sites and targeted species; to this effect, 10 ml of blood using non-haparinized vacutainer tube were collected and sera were harvested from the clotted blood that was kept for 30 min at 37°C (OIE, 2004). Each serum sample was given an identification code and kept at -20°C until assayed in the laboratory. At the National Veterinary Institute (NVI), ELISA was used to detect the presence of specific antibody against the AHS virus in the sera samples collected.
Test procedure
All reagents were brought to room temperature before use. 100 µl of diluted samples was dispensed into appropriate wells (dilution 1/5). Running the samples in duplicate was recommended. 100 µl of positive control was dispensed into two wells and 100 µl of negative control into two wells. The plate was covered and incubated for 1 h at 37°C and washed 5 times. Then, 100 µl/well of conjugate was added and incubated for 30 min at 37°C.
Again, it was washed 5 times. 100 µl/well of substrate solution was dispensed using a multichannel pipette and incubated for 10 min at room temperature. 100 µl/well of stop solution was dispensed, taking care to avoid dispensing of bubbles. Finally, it was read at 405 nm (OIE, 2004).
Data analysis
The result was analyzed in relation to location, agro ecology, species, sex and mode of service. Data were stored in Microsoft Excel spread sheet. The data were edited, coded and transferred into intercool Stata (2009) version 7.0 (Stata Corporation, College Station, Texas, USA) for descriptive and analytical statistical univariate logistic regression analysis to look for identification of hypothesized risk factors in line with the result.
Overall prevalence
A total of 230 equines sera were examined by blocking ELISA technique. Out of which 54 equines were found to be seropositive which made the overall prevalence to be 23.47%.
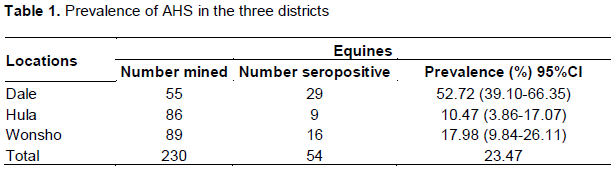
Prevalence of AHS based on districts
An attempt was made to see the influence of location on the prevalence of AHS and accordingly, the prevalence of 52.72, 10.47 and 17.98% were found in Dale, Hula and Wonsho, respectively, in difference between Dale and other districts were found to be statistically significant (P<0.05) between the three locations (Table 1).
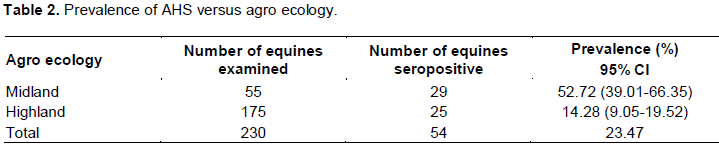
Prevalence of AHS based on agro ecology
AHS seropositivity was also assessed in relation to agro ecology and the prevalence of midland was 52.72% while it was 14.28% in the highland.
The difference between agro-ecology was statistically significant (P<0.05) (Table 2).
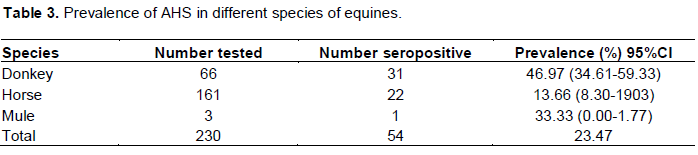
Prevalence of AHS based on species
The prevalence of AHS was 46.97, 13.66 and 33.33% in donkeys, horses and mules, respectively. There was statistically significant difference among donkeys, horses and mules (P<0.05) (Table 3).
Prevalence of AHS based on sex
The prevalence of AHS encountered in males were 28.66% while the correspondence value in female was 10.61% and this difference was seen to be significant statistically (P<0.05) (Table 4).
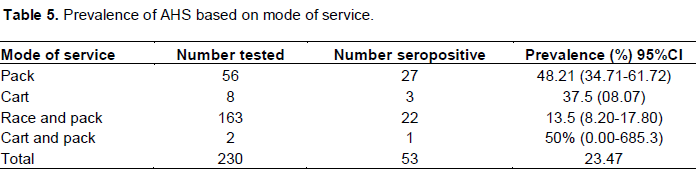
Prevalence of AHS based on mode of service
The prevalence of AHS infection among pack horses were 48.21%, while that of cart and race and pack were found to be 37.50% and that of cart and pack were 50% and statistically significant difference was observed between pack and combination of race and pack. Comparison of cart, and cart and pack need to be reserved as the number is not comparable (Table 5).
This study revealed the overall prevalence of the three woredas’ of Sidama zone, Hawassa, Ethiopia. The present overall prevalence report almost coincides with report of 25% prevalence in selected districts of Jimma zone (Molalegn et al., 2010) but it is not similar to the findings of Demissie (2013) in southern Ethiopia, who reported 33% prevalence. This might be attributed to geographical variation.
In this study, the prevalence difference observed between Dale and other districts (Wossho and Hula) were statistically significant (P<0.05). Similarly, the difference observed between midland and highland were also statistically significant (P<0.05). In this study, Dale was taken to be representative of midland, and Wonsho and Hula for highland. Therefore, the difference observed among districts is confounder, while agro-ecology (midland and highland) is an important factor. Ecology indicates the vector population is possibly manifested by prevalence. Hence, it is suggested that the prevalence in midland could be due to high density of vector population as the ecology favor its breeding (Ende et al., 2011; Tesfaye et al., 2013).
High prevalence was observed in donkeys as compared to horses and mules. The species comparison was made between donkeys and horses alone and the prevalence in donkeys was higher than in horses. The difference was statistically significant (P<0.05). This is contrary to the literature that donkeys and mules are resistant (Alemayehu and Benti, 2009). The deviation could be due to high exposure rate of donkeys which are usually in midland area of Dale.
It is also indicated that there was statistically significant difference (P<0.05) between sex and higher prevalence in male than in female. One of the probable reason was that most of the samples of midland were donkeys and of the male sex. This difference resulted from agro ecological variation rather than sex.
The study further revealed that there was lower prevalence in combined mode of service of race and pack than in pack. Horses are used in combined mode service of race and pack. Donkey is mainly used either as pack or cart. Most of the samples of horses were taken from highland and donkeys from midland land. The difference of the prevalence of AHS in the mode of service is not due to the mode of service they used, but the difference results from the distinct variation between the agro ecology from where the samples were taken. Comparison of cart, and cart and pack was not done because the numbers were not comparable.
The present finding showed that the hypothesized risk factors and agro-ecology is the predominant risk for the prevalence of African Horse sickness in the study area. Therefore, regular vaccination with potent vaccine in line with the disease epidemiology, awareness of the public on the disease epidemiology and further investigations should be done to identify the various risk factors.
The authors have not declared any conflict of interests.
REFERENCES
|
Alemayehu L, Benti D (2009). Study on Reproductive Activity and Evaluation of Breeding Soundness of Jacks (Equis asinus) in and around Debre Zeit, Ethiopia. Livest. Res. Rural Dev. 21:42-45.
|
|
|
|
Binepal VS, Wariru BN, Davies FG, Soi R, Olubayo R (2013). An attempt to define the host range for African Horse Sickness Virus (Orbivirus, Reoviridae) in East Africa, by a serological survey in some Equidae, Camelidae, Loxodontidae and Carnivore. Vet. Microbiol. 31:19-23.
Crossref
|
|
|
|
|
Central Statistical Authority (2009). Federal Democratic Republic of Ethiopia. Central Statistical Authority (CSA), Agricultural sample survey 2008/2009 [2001E.C], Report on Livestock and Livestock characteristics (private peasant holding), Addis Ababa.
|
|
|
|
|
Cêtre-Sossah C, Mathieu B, Setier-Rio ML, Grillet C, Baldet T, Delécolle JC, Albina E (2008). Development and evaluation of a real-time quantitative PCR assay for Culicoides imicola, one of the main vectors of bluetongue (BT) and African horse sickness (AHS) in Africa and Europe. Res. vet. sci. 85(2):372-382.
Crossref
|
|
|
|
|
Demissie GH (2013). Seroepidemiological Study of African Horse Sickness in Southern Ethiopia. Open Science Repository Veterinary Medicine, 2013:e70081919.
|
|
|
|
|
Ende H, Tassew H, Gurmu EB, Amsalu K, Gizaw D (2011). Seroprevalence of African Horse Sickness at Central Highland of Ethiopia. IJAVMS. 9(4):139-148.
|
|
|
|
|
Ferhandey J, Ferhandey P, Rodriguey B, Sotelo E, Robles A, Arias M, Sanchey-vichiano J (2012). New advances in the molecular diagnosis of African Horse sickness (AHS). Proceedings of the 13th international symposium for the world association of veterinary laboratory Diagnosticians, 11-14 November, Melbourne, Australia.
|
|
|
|
|
Maclachlan NJ, Guthrie AJ (2010). Re-emergence of Bluetongue, African horse sickness and other Orbivirus diseases. Vet. Res. 41:35.
Crossref
|
|
|
|
|
Molalegne B, Ashenafi A, Mihreteab B, Shiferaw J, Gelagay A and Esayas G (2010). Serological survey of African horse sickness in selected district of Jimma zone, southwestern Ethiopia. Trop. Anim. Health Prod. 43:1543-1547.
|
|
|
|
|
Mulualem T, Mekonnen A, Wudu T (2012). Seroprevalence and risk factors of African horse sickness in mules and donkeys in selected sites of West Amhara Region, Ethiopia African. J. Microbiol. Res. 6(19):4146-4151.
|
|
|
|
|
Office International Des Epizootics (OIE) (2004): Manual of Standards for Diagnostic Tests and Vaccines, African Horse sicknes. pp. 73-80.
|
|
|
|
|
Office international epizootics (OIE) (2008). African horse sicknes, in manual of diagnostic tests and vaccine for terrestrial animals. 5th ed. New York, W.B. Saunders Company Ltd. pp. 582-583.
|
|
|
|
|
Rodriguez-Sanchez B, Fernandez-Pinero J, Sailleau C, Zientara S, Belak S, Arias M, Sanchez-Vizcaino JM (2008). Novel gel-based and real-time PCR assays for the improved detection of African Horse Sickness Virus. J. Virol. Method 151(1):87-94.
Crossref
|
|
|
|
|
Sidama Zone of Agricultural and Rural Developments (SZARD), (2011). Personal Communication office data, Hawassa, Ethiopia.
|
|
|
|
|
Sidama Zone Planning and Economic Development Department (SZPEDD), (2004). Hawassa, Ethiopia.
|
|
|
|
|
STATA (2009). Stata Statistical Software, Release 7.0 Stata Corporation, College Station, Texas, USA.
|
|
|
|
|
Tesfaye T, Tadesse G, Tewodros F, Mersha C (2013). Seroprevalence and Associated Risk Factors of African Horse Sickness in Arsi and Bale Zones, Southeastern Ethiopia.
|
|
|
|
|
Thrusfield M (2005). Veterinary Epidemiology. 3rd ed., UK, Blackwell science Ltd, pp. 233-250.
|
|
|
|
|
Torrecuadrada M, Jorge L, Langeveld J, Venteo A, Sanz, A, Dalsgaard K, Hamilton W, Meloen R, Casal J (2011). Antigenic profile of African Horse Sickness virus serotype 4 VP5 and Identification of a Neutralizing Epitope shared with Blutongue virus and Epizootic Hemorrhagic Disease virus. Virology 257:449- 459.
Crossref
|
|
|
|
|
Upadhyaya KA (2011). Text Book of Preventive Veterinary Medicine 1st ed. International Book Distributing Company, India. pp. 274-277.
|
|