ABSTRACT
Erythropheleum suaveolens stem bark saponins fractions were investigated for antioxidant and antibacterial activities. In vitro systems such as, 1, 1- diphenyl-2-picrylhydrazyl (DPPH) radical, reductive potential and inhibition of lipid peroxidation were carried out to determine the antioxidant activities. The antimicrobial activity was determined using the hole-in-plate agar diffusion technique and the minimum inhibitory concentration (MIC) of each fraction was determined using the agar dilution method. All the fractions scavenged DPPH radicals but not comparable with ascorbic acid. These fractions also inhibited lipid peroxidation induced with highest percentage inhibition exhibited by 70:30 fraction which compare favourably with tannic acid. The reducing potential decreased with increasing concentration for all the fractions. The result of antibacterial studies revealed that 70:30 fraction inhibited the tested bacteria to a greater extent than the other fractions while the determination of the MIC of each fraction against the organisms showed that 70:30 fraction possessed a broader spectrum of activity against gram positive cocci of medical importance and also inhibited all the gram negative organisms tested. The study indicates saponin fractions of E. suaveolens stem bark to be potential candidates as antioxidant and antimicrobial agents with the 70:30 saponin fractions being the most promising and that these two activities augment each other.
Key words: Erythropheleum suaveolens, saponins, 1, 1- diphenyl-2-picrylhydrazyl (DPPH), lipid peroxidation, reductive potential and antimicrobial.
Saponins are surface-active, high molecular-weight and chemically complex group of compounds that occur naturally in plants, to a lesser extent in lower marine organisms and some bacteria (Riguera, 1997; Yoshiki et al., 1998; Price et al., 1987). Saponins generally are composed of two major parts, one or more sugar or oligosaccharide moieties glycosidically linked to a triterpenic or steroidic liposoluble structure (aglycone or
sapogenin). The aglycone may contain one or more unsaturated C–C bonds. This combination of polar and non-polar structural elements in their molecules explains their soap-like behaviour in aqueous solutions (Tsukamoto et al., 1995; Oleszek and Stochmal, 2002). Reported pharmacological properties elicited by some isolated saponins include hypocholesterolaemic, anticarcenogenic, immunostimulant, hypoglycemic, molluscicidal, antiprotozoa, antioxidant etc (Hostettmann and Marston, 1995). Such findings raise the possibilities of exploiting plant extracts rich in these metabolites as pharmacological and veterinary products, and herbal medicines (Attele et al., 1999; Oakenfull, 1981). Saponins have found wide applications in beverages and confectionery, as well as in cosmetics (Price et al., 1987; Petit et al., 1995; Uematsu et al., 2000).
Erythrophleum suaveolens is an example of plant rich in saponins. It is a perennial tree of about 30 m in height, bole seldom straight, slightly buttressed, often low-branching and producing a dense spreading crown. It is referred to by various names by natives. It is referred to in English as sassy, sasswood, red water tree and ordeal tree. The species are of ethnological interest as ordeal poison as indicated in many of their Europeans and African names. The English ‘red water tree’ is derived from the capacity of the stem bark decoction or infused in water to turn the water red (Burkill, 1985).
The stem bark is the most interesting part of the tree being the source of most notorious ordeal poison in Africa to trial criminals accused of serious misdemeanors, or person suspected or accused of witchcraft.
Ngounou et al. (2005) in their studies on the antimicrobial activity of diterpenoid alkaloids (amide norcassaide and norerythrosua-veolide) from E. suaveolens reported that these compounds showed potent antimicrobial activities against bacteria and yeasts.
The antibacterial potentials of E. suaveolens aqueous and chloroform fractions against some selected bacterial isolates were investigated by Aiyegoro et al. (2007). The two fractions were reported to compare favourably with the standard antibiotics, streptomycin and ampicillin at concentration of 1 mg/ml and 10 μg/ml respectively.
Antioxidants are the body’s natural defense mechanisms against the damaging effects of "free radicals" and oxidation reactions that damage cells and cause disease. The main function of antioxidants is to prevent oxidation in various ways. It has been known for some time that antioxidants play a very important biological role in the body by preventing against oxidative damage (particularly oxidative DNA damage), thus preventing cardiovascular, neurological and carcinogenic diseases and delaying chronic health problems like cataracts (Williamson et al., 2000; Morton et al., 2000).
Usually, polyphenols and carotenoid pigments being the major nutritional antioxidants in food attract most of the research in this area. Some saponins have also been found to have anti oxidative or reductive activity (Francis et al., 2002). From our recent investigations (Akinpelu et al., 2012) saponin fractions from the stem bark of E. suaveolens protected red blood cells to some degree and one of the fractions (70:30) compete favourably with standard drug used in protecting the cell membrane integrity. This study is to investigate further the antioxidant and antibacterial activities of E. sauveolens fractions. This is a view to discover and explain possible mechanisms through which saponin exert its antioxidant and antibacterial activities.
Plant materials
Dried stem-barks of E. suaveolens were obtained from the Central Local Market (Oja Tuntun) in Ile-Ife, Osun State, Nigeria. The plant was identified and authenticated by Dr. H .C. Illoh, Department of Botany, Obafemi Awolowo University, Ile-Ife, Nigeria. The voucher specimen copy has been deposited at the IFE Herbarium Obafemi Awolowo University, Ile-Ife, Nigeria.
Sources of microorganisms
The test organisms employed for screening antimicrobial activities of the extracts were gram positive organisms (Bacillus subtilis, Clostridium sporogenes, Staphylococcus aureus, Staphylococcus epidermidis, Staphylococcus capitis, and Staphylococcus xylosus), gram negative organisms (Escherichia coli, Shigella spp., Proteus spp., Klebsiella spp., and Pseudomonas aeruginosa) and yeasts (Candida albicans and Candida pseudotropicalis). All organisms were obtained from the Department of Pharmaceutics Microbiology Laboratory of Obafemi Awolowo University. Ile-Ife, Nigeria. The bacteria and yeasts were sub-cultured into fresh nutrient agar plates and sarbouraud dextrose agar plates respectively 24 h before use for antimicrobial test.
Preparation of alcoholic extract
Ethanolic extract of the stem bark of E. suaveolens was prepared according to the procedure as earlier described by Oyedapo and Amos (1997). Powdered (900 g) dried stem bark was suspended in 4.2 L of 80% (v/v) ethanol for 72 h at room temperature. The suspension was filtered through two layers of cheese-cloth. The extraction process was repeated four times until the extract became clear. The filtrates were combined and concentrated under reduced pressure on Edwards High Vacuum Pump rotatory evaporator (Edward Vacuum Co-operation, Crawley, England) at 35°C to give a coffee brown residue. The total crude ethanolic extract weighed 500 g which was 55.56% of the starting material and was stored in the dessicator until required for further processing.
Isolation of saponins from crude ethanolic extract of E. suaveolens
Isolation of crude saponin was carried out according to a procedure that was based on the methods described by Abdel-Gawad et al. (1999) and Wagner et al. (1984). The ethanolic extract (10 g) was washed twice with chloroform (50 ml x 2) and, also twice with ethylacetate (50 ml x 2). The residue was allowed to dry and then dissolved in 50% (v/v) methanol. The water-methanol solution was extracted three times (100 x 3) with butanol. On evaporation, a syrupy residue was obtained which was taken up in methanol. The residue was dissolved in 50 ml 50% (v/v) methanol followed by the addition of diethylether (100 ml) to precipitate crude saponins. The upper diethylether layer was carefully removed; the residue was dissolved in a little amount (10 ml) of methanol and was poured into diethylether (200 ml). The upper diethylether was again removed and the residue was dissolved as described earlier and precipitated by the addition of diethylether. The precipitate was further purified by repeated dissolution in methanol and precipitation with diethylether until a cream light brown precipitate was obtained. The procedure was carried out until 350 g of crude ethanolic extract was analysed. A total yield of 55.35 g of light brown precipitate termed crude saponins was obtained.
Fractionation of crude saponin
The crude saponin was fractionated on silica gel (Kiesel gel 60; 60-200 mesh) column chromatography using gradient elution mixture of dichloromethane with methanol (DCM: MeOH) according to the modified method of Lin et al. (2008). A clean dry glass column (4 x 40 cm) was carefully packed with silica gel (Kiesel gel 60) to the required height of 30 cm. The silica gel was over layered with alumina powder (aluminium oxide) to a height of 5 cm. The column was washed and equilibrated with dichloromethane (200 ml x 2). Crude saponin (4 g) was thoroughly mixed with dry silica gel powder in a mortar with pestle. The sample was then layered carefully on top of alumina powder and a small amount of clean silica gel was carefully poured on top of packed gel to a height of 2 cm. Elution was carried out with the solvent mixtures (DCM:MeOH; 100:0 – 0:100 ). Fractions were collected for each of the solvent mixtures and evaporated to dryness at 40°C on rotatory evaporator to give various fractions for each solvent system. Each fraction was evaporated to dryness and kept for further analyses.
Tests for saponin
Saponin was tested for according to a procedure that was based on those earlier reported by Evans (2002) and Sofowora (2008) with slight modifications.
Frothing test
Saponin (5 mg) in a test tube was dissolved in distilled water (5 ml). The mixture was shaken vigorously until frothing. The production of honeycomb-like frothing which persisted after warming at 50°C for up to 15 min was indicative of saponin.
Lieberman-Burchard reaction test
Saponin (0.2 g) was dissolved in 1.0 ml acetic anhydride in a test tube, and then concentrated sulphuric acid (2 ml) was added carefully through the side of a test tube by means of a Pasteur pipette. The occurrence of a bluish-green colour in the upper liquid layer on a reddish-brown ring at the interface of the two liquid layers was taken as a positive test for the presence of steroidal nucleus.
Antioxidant assay
DPPH radical assay
The antioxidant activity of the extracts, on the basis of their scavenging activity of the stable 1, 1 – diphenyl-2-picrylhydrazyl (DPPH) free radical, was determined by the method described by
Braca et al. (2001). Aqueous extract (0.1 ml) was added to 3 ml of 0.004% methanol solution of DPPH. Absorbance at 517 nm was taken after 30 min and the percent inhibition activity was calculated using the expression below:
Percentage Inhibition Activity = (Ao– Ae / Ao) x 100
where Ao = Absorbance without extract and Ae = Absorbance with extract.
Lipid peroxidation and thiobarbituric acid reactions
A modified thiobarbituric acid reactive species (TBARS) assay as described by Ohkowa et al. (1979) was used to measure the lipid peroxide formed using egg yolk homogenate lipid rich media. Egg homogenate [0.5 ml of 10% (v/v)] and 0.1 ml of extract were added to a test tube and made up to 1ml with distilled water; 0.05 ml of copper sulphate (0.07 M) was added to induce lipid peroxidation and the mixture was incubated for 30 min. Then 1.5 ml of 20% acetic acid (pH 3.5) and 1.5 ml of 0.8 %( w/v) thiobarbituric acid in 1.1% sodium dodecyl sulphate were added and the resulting mixture was vortexed and then heated at 95°C for 60 min. After cooling, 5.0 ml of butanol were added to each tube and centrifuged for 10 mins. The absorbance of the organic layer was measured at 523 nm. Percentage inhibition of lipid peroxidation by the extract was calculated using the expression [(1-E)/C] x 100, where C is the absorbance value for fully oxidized control and E is the absorbance in the presence of extract.
Reducing power
The reducing power of the extracts was determined according to the method of Oyaizu (1986). Different concentrations of extract (100 – 1000 µg) in distilled water was mixed with phosphate buffer (0.2 M, pH 6.6) and 1% potassium ferricyanide [K3Fe(CN)6]. The mixture was incubated at 50°C for 20 min. To the mixture was added 10% (w/v) trichloroacetic acid, which was then centrifuged at 3000 rpm for 10 min. The upper layer of the solution (2.5 ml) was mixed with distilled water (2.5 ml) and 0.1% FeCl3 (0.5 ml) and the absorbance was measured at 700 nm, increased absorbance of the reaction mixture indicated reducing power. Ascorbic acid was used as a standard to compare the reducing power of the extracts.
Antimicrobial assay
Colonies of 20 h old cultures of all test organisms were suspended in sterile normal saline and the density adjusted to 0.5 MacFarland Standard. This was swabbed on fresh Meuller-Hinton agar for the bacteria and sarbouraud dextrose agar for the yeast with the aid of sterile cotton tipped applicator. The water soluble extracts were reconstituted with sterile water to give the different concentrations tested. The different extracts were then introduced into holes bored in the agar plates. Sterile distilled water was used as negative control while cefuroxime and ketoconazone was used as positive control antibiotics for the bacteria and yeast respectively. All plates were left in the fridge for 1 h to allow diffusion of test extracts before incubating at 37°C for the bacteria and 25°C for the yeasts. Zones of inhibition were measured in millimeter (mm) after 24 h of incubation.
Minimal inhibitory concentration (MIC)
The MIC of the extract was determined for each of the test organisms using the agar dilution susceptibility tests. Duplicate oven-dried Meuller-Hinton agar plates containing different concentration of test extracts in agar were inoculated with the test bacteria using a multi-inoculator and incubated at 37°C for up to 72 h. The yeasts were tested in duplicate Sarbouraud dextrose agar containing the different concentrations of the extracts and incubated at 25°C. The presence or absence of growth of each test organism was noted on each plate. The minimum concentration inhibiting the growth of a test organism was recorded as the MIC against the organism.
DPPH-Radical scavenging activity
DPPH free radical scavenging method has been widely applied for evaluating antioxidant activity in a number of studies (Brad-Williams et al., 1995). The principle of DPPH method is based on the reduction of DPPH in the presence of a hydrogen donating antioxidant. Quite a number of naturally occurring antioxidants have been demonstrated to play prominent roles in inhibiting both free radicals and oxidative chain reactions within tissues and membranes (Nsimba et al., 2008).
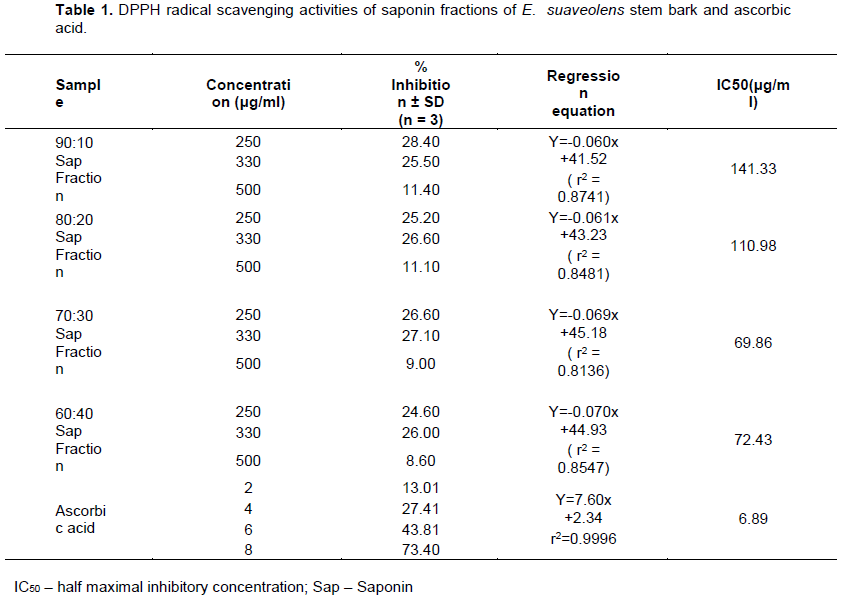
In Table 1 is the result of the DPPH scavenging activity of E. suaveolens stem bark saponins. It was observed that E. suaveolens stem bark saponins inhibited DPPH free radical scavenging activity better at lower concentrations, that is, 250 and 330 µg/ml. Although the DPPH radical scavenging abilities of the saponin fractions were less than that of ascorbic acid but the result showed that E. suaveolens saponin fractions has the proton-donating ability and could serve as free radical inhibitors or scavenger, acting possibly as primary antioxidant.
Reducing power
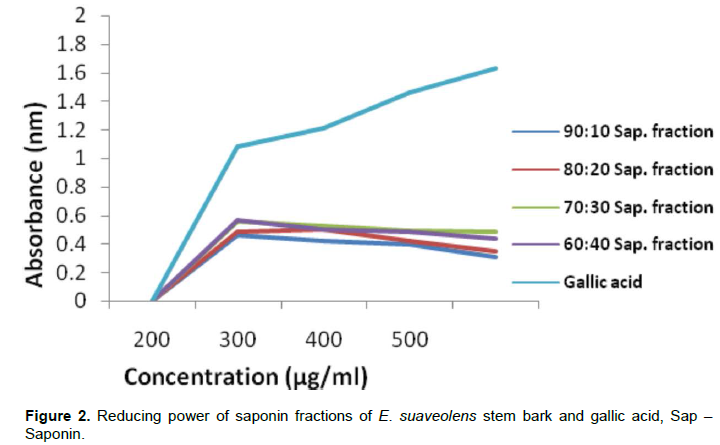
The ability of E. suaveolens stem bark saponin fractions and ascorbic acid (AA) to reduce Fe3+ / ferricyanide complex to ferrous form is represented in Figure 1. Low values indicate high antioxidant activities. The reductive potential of saponin fractions decreased with increase in fraction concentrations. Fraction 60:40 showed highest reductive potential at 200 ug/ml used followed by 70:30, 80:20 and 90:10 fractions respectively, the more polar the fraction the higher the reductive activity. The reductive potential of AA was higher than that of saponin fractions at all concentrations, however it should be noted that the reductive potential of saponin fractions were still appreciable. The reducing capacity of a compound may serve as a significant indicator of its potential antioxidant activity (Meir et al., 1995).
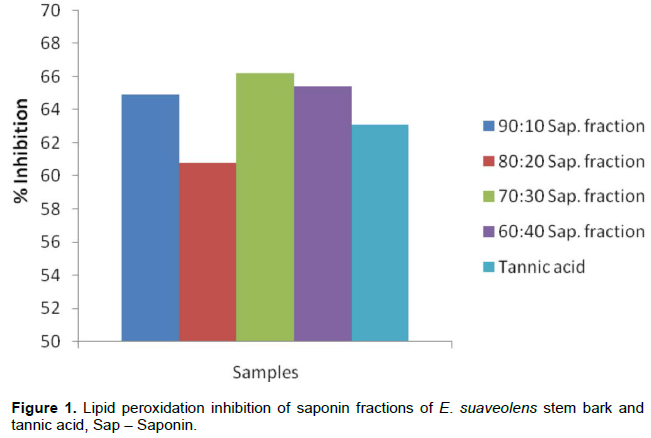
Inhibition of lipid peroxidation
Peroxidation of lipids has been shown to be cumulative effect of reactive oxygen species, which disturb the assembly of the membrane causing changes in fluidity and permeability, alterations of ion transport and inhibition of metabolic processes (Nigam and Schewe, 2000). The extract exhibited strong lipid peroxidation inhibition at 1000 µg/ml in the order 70:30>80:20>60:10>80:20 and compared favourably with tannic acid at the same concentration (Figure 2). This result suggests that E. suaveolen saponins could play a role in protecting the physicochemical properties of membrane bilayer from free radical induced severe cellular dysfunction. The fractions inhibit the oxygen free radicals formation and this may be one of the possible mechanisms of membrane stability activities these fractions as reported by Akinpelu et al. (2012).
Antimicrobial studies
The zones of inhibition of the different saponin fractions of E. suavoelens stem bark to B. subtilis, S. aureus, E. coli, Ps. Aeruginosa, C. albicans and C. pseudotropicalis are presented in Table 2. The results of the antimicrobial studies of the saponin fractions of the bark of E. suaveolens revealed that it had antimicrobial activity which was concentration dependent. The determination of the zones of inhibition of same concentration of each fraction showed that all the fractions were more active against the gram positive bacteria B. subtilis and S. aureus than the gram negative organisms. All the fractions were also found to be active against C. pseudotropicalis with the exception of the 80:20 fraction while C. albicans was inhibited by the 70: 30 and the 60:40 fractions. This shows that the saponin fractions of E.
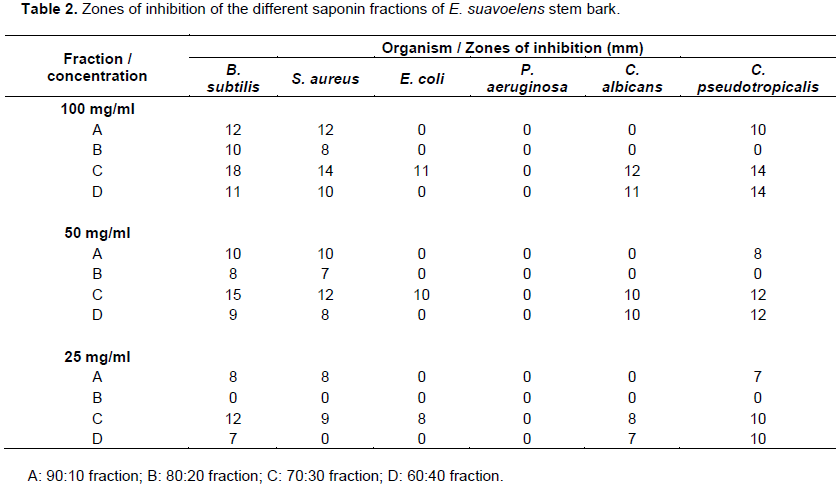
suaveolens had more activity against the gram positive organisms than the gram negative bacteria. This is expected and it suggests that the mechanism of action of the saponins in the tested fractions of the plant is related more to cell wall integrity and functions since the difference in the two groups of bacteria is in the cell wall. The cell wall of gram-positive bacteria is less chemically complex than that of the gram negative bacteria (Lamikanra, 2010) and gram-negative bacteria are known to be resistant to the action of many antimicrobial agents including antimicrobial plant extracts (Kambezi and Afolayan, 2008; El-Mahmood, 2009). The anti-staphylococcal activity of this saponin fraction is significant due to the fact that the spectrum of activity included the methicillin-resistant S. aureus (MRSA) strains which are important causes of many hospital and community acquired infections (CDC, 2012; Taiwo et al., 2004).
The results of the study also showed that the 70:30 fraction inhibited the tested bacterial to a greater extent than the other fractions (Table 3). The determination of the minimum inhibitory concentration of each fraction against a wide range of organisms which included both the gram negative and gram positive bacteria as well as Candida species showed that not only was the 70:30 fraction more active, it also possessed a broader spectrum of activity against gram positive cocci of medical importance and also inhibited all the gram negative organisms tested. This included the notorious multi-resistant organism Pseudomonas aeruginosa (Bousseimi et al., 2005; Olayinka et al., 2004; Bradford, 2001). This activity was not observed with any of the other fractions. This result suggests that the antimicrobial principles in these fractions are likely to be more than one and the 70:30 fraction probably contains the most potent principles than any of the other fractions.
.
Hence it could be surmised that the E. suaveolens saponins could be used to prevent damage caused by free radicals and infections caused by pathogenic bacteria. In addition, 70:30 E. suaveolens saponin fraction stem bark could be a useful starting point if the active saponin principles are to be exploited for development into antimicrobial chemotherapeutic agents in line with the ongoing search for substances to replace the antibiotics in current clinical use which because of the emergence and spread of resistant organisms are less useful than before (Iwata, 1992; Chopra et al., 1997).
The authors have not declared any conflict of interest.
REFERENCES
|
Abdel-Gawad MM, El-Sayed MM, Abdel-Hameed ES (1999). Molluscicidal steroidal saponins and lipid content of Agave decipiens. Fitoterapia, 70:371-381.
Crossref
|
|
|
|
Aiyegoro OA, Akinpelu DA, Okoh AI (2007). (Correct presentation of the reference as per journal's guidelines). In vitro antibacterial potentials of the stem bark of Red water Tree (Erythrophleum suaveolens) . J. Biol. Sci. 7(7):1233-1238.
Crossref
|
|
|
|
|
Akinpelu BA, Oyedapo OO, Iwalewa EO, Shode F (2012). Biochemical and histopathological profile of toxicity induced by saponin fraction of Erythrophleum suaveolens (Guill. & Perri.) bark extract Phytopharmacol. 3(1):38-53.
|
|
|
|
|
Attele AS, Wu JA, Yuan CS (1999). Ginseng Pharmacology: Multiple based bisglycosides from Anemone raddeana Regel. Phytochemistry 45:1031–1033 [Ranunculales –Anemone raddeana].
|
|
|
|
|
Bousseimi K, Thabet L, Ouesiat S, Jaber OB, Ouchtati A, Cherif S, Redjeb SB, Messadi A (2005). Epidemiological profile and antibiotic susceptibility of Pseudomonas aeruginosa isolates within the hospitalized burned patient. Crit. Care 9(Suppl. 1):10-11.
|
|
|
|
|
Braca A, Tommasi ND, Bari LDP, Cosimo PM, Morelli I (2001). Antioxidant principles from Bautiniaterapotensis. J. Nat. Prod. 64:892-895.
Crossref
|
|
|
|
|
Bradford PA (2001). Extended-spectrum {beta}-Lactamases in the 21st century: Characterization, epidemiology, and detection of this important resistant threat. Clin. Microbiol. Rev. 14:933- 951.
Crossref
|
|
|
|
|
Brad–Williams W, Cuvelier ME, Berset C (1995). Use of a radical method to evaluate antioxidant activity, Lebensm-Wiss A Technol. Food Sci. Technol. 28:25-30.
|
|
|
|
|
Burkill H (1985). The useful plants of West Tropical Africa. 3(857):116-120. Centers for Disease Control and Prevention. Methicillin-resistant Staphylococcus aureus (MRSA) infections. Accessed April 17, 2012.
|
|
|
|
|
Centers for Disease Control and Prevention (2012). Methicillin-resistant Staphylococcus aureus (MRSA) infections. http://www.cdc.gov/mrsa/
|
|
|
|
|
Chopra I, Hodgson J, Metcalf B, Poste G (1997). The search for antimicrobial agents effective against bacteria resistant to multiple antibiotics. Antimicrob. Agent Chemother. 41:497-503.
PMid:9055982 PMCid:PMC163740
|
|
|
|
|
Evans WC (2002). Saponins, Cardioactive drugs and other steroids. In: Trease and Evans Pharmacognosy, 15th Edition.
|
|
|
|
|
El-Mahmood AM (2009). Antibacterial activity of crude extracts of Euphorbia hirta Against some bacteria associated with enteric infections. J. Med. Plants Res. 3(7): 498-505.
|
|
|
|
|
Francis G, Kerem Z, Makkar HPS, Becker K (2002). The biological action of saponins in animal system. A review. Brit. J. Nutr. 88:587-605.
Crossref
|
|
|
|
|
Hostettmann K, Marston A (1995). Chemistry and pharmacology of Natural products. Cambridge University Press, Cambridge, isbn-10: 0521020174.
|
|
|
|
|
Iwata K (1992). Drug resistance in human pathogenic fungi. Eur. J. Epidemiol. 8:407-421.
Crossref
|
|
|
|
|
Kambezi L, Afolayan AJ (2008). Extracts from Aloe ferox and Withania somnifera inhibit Candida albicans and Neisseria gonorrhea. Afr. J. Biotechnol. 7(1):012-015.
|
|
|
|
|
Lamikanra A (2010). Essential Microbiology for students and practitioner of Pharmacy, Medicine and microbiology. 2nd ed. Amkra books.
|
|
|
|
|
Lin CN, Chen HL, Yen MH (2008). Flavonoids with DNA strand Scissors activity from Rhus javanica var Roxburghana. Fitoterapia 79:32-36.
Crossref
|
|
|
|
|
Meir S, Kanner J, Akiri B, Hadas SP (1995). Determination and involvement of aqueous reducing compounds in oxidative defence systems of various senescing leaves. J. Agric. Food Chem. 43:1813-1815.
Crossref
|
|
|
|
|
Morton LW, Cacceta RA, Puddey IB, Croft KD (2000). Chemistry and biological effects of dietary phenolic compounds: Relevance to cardiovascular Disease. Clin. Exp. Pharmacol. Physiol. 27:152-159.
Crossref
|
|
|
|
|
Nigam S, Schewe T (2000). Phopholipase A2s and lipid peroxidation. Biochim. Biophy. Acta. 1488:167-181.
Crossref
|
|
|
|
|
Ngounou FN, Maifouo RN, Tapondjou LA, Lontsi V, Kuete V, Penlap V, Etoa FX, Duboisaid MA, Sondengam BL (2005). Antimicrobial diterpenoid alkaloids from Erythrophleum suaveolens (Guill. and Perr.) Brenan. Bull. Chem. Soc. Ethiopia 19:221-226.
|
|
|
|
|
Nsimba RY, Kikuzaki H, Konishi Y (2008). Antioxidant activity of various extract fractions of Chenopodium quinoa and Amaraathus sp. seeds. Food Chem. 106:760-766.
Crossref
|
|
|
|
|
Ohkowa M, Ohisi N, Yagi K (1979). Assay for lipid peroxides in animal tissue by thiobarbituric acid reaction. Anal. Biochem. 95:351-358.
Crossref
|
|
|
|
|
Oakenfull D (1981). Saponins in food. Food Chem. 6:19-40
Crossref
|
|
|
|
|
Oleszek W, Stochmal A (2002). Triterpene saponins and flavonoids in the seeds of Trifolium species. Phytochem. 61:165-170.
Crossref
|
|
|
|
|
Olayinka AT, Onile BA, Olayinka BO (2004). Prevalence of multi-drug resistance (MDR) Pseudomonas aeruginosa isolates in surgical units of Ahmadu Bello University Teaching Hospital, Zaria, Nigeria: An indication for effective control measures. Ann. Afri. Med. 3:13-16.
|
|
|
|
|
Oyaizu M (1986). Studies on product of browning reaction prepared from glucosem mine. Japan J. Nutr. 44:307-315.
Crossref
|
|
|
|
|
Oyedapo OO, Amos S (1997). Further investigation into the activities of the root extract of Plumbago zaylanica. Phytother. Res. (London). 11:62-63.
Crossref
|
|
|
|
|
Petit PR, Sauvaire YD, Hillaire-Buys DM, Leconte OM, Baissac YG, Posin GR., Ribes GR (1995). Steroid saponins from fenugreek seeds: Extraction, purification, and pharmacological investigation on feeding behaviour and plasma cholepieces of the puzzle. Angew. Chem. Int. Ed. 44:3966-3971.
|
|
|
|
|
Price KK, Johnson LI, Feriwick D (1987). The Chemical and biological significance of saponins in food and feeding stuffs. Food Sci. Nutr. 26:27-135.
|
|
|
|
|
Riguera R (1997). Isolating bioactive compounds from marine organisms. J. Mar. Biotechnol. 5:187-193.
|
|
|
|
|
Sofowora A (2008). Screening Plants for Bioactive Agents. In: Medicinal Plants and Traditional Medicine in Africa. Third edition,Spectrum Books Limited, Ibadan, Nigeria. pp. 181-207.
|
|
|
|
|
Taiwo SS, Onile BA, Akanbi AA (2004). Methicillin-Resistant staphylococcus aureus (MRSA) isolates in Ilorin, Nigeria. Afr. J. Clin. Exp. Microbiol. 5 (2):189-197.
|
|
|
|
|
Tsukamoto S, Shuimada IS, Kudou MK, Okubo M, Kitamura K (1995). Factors affecting isoflavones content in soybean seeds: changes in isoflavone, saponins, and composition of fatty acids at different temperature during the seed development. J. Agric. Food Chem. 43:1184-1192.
Crossref
|
|
|
|
|
Uematsu Y, Hirata K, Saito K (2000). Spectrophotometric determination of saponin in Yucca extract used as food additive. J. AOAC Int. 83:14511454.
|
|
|
|
|
Wagner HH, Nickel H, Aynehchi Y (1984). Molluscicidal Saponins from Gundella tournefortii. Phytochem. 23(11):2505-2508.
Crossref
|
|
|
|
|
Williamson G, Day AJ, Plumb GW, Couteau D (2000). Human metabolic pathways of dietary flavonoids and cinnamates. Biochem. Soc. Trans. 28:16-22.
|
|
|
|
|
Yoshiki Y, Kudou S, Okubo K (1998). Relationship between chemical structures and biological activities of triterpenoid saponins from soybean (Review). Biosci. Biotechnol. Biochem. 62:2291-2299.
Crossref
|
|