Two field experiments were conducted during two successive seasons to study the effect of foliar application with Phenylalanine (zero, 50 and 100 ppm) and /or nickel (zero, 50 and 100 ppm) on growth, yield and essential oil components of genoveser basil plant (Ocimum basilicum L. var. Genoveser). Application of the combined treatment (100 ppm Phenylalanine + 50 ppm nickel) resulted in the maximum values of all growth parameters, oil content and estimated yield of herb, leaves and oil. The essential oil was analyzed using a gas liquid chromatography-mass spectrometer (GC-Ms). All treatments proved that linalool was the main compound followed by 1,8 cineol, α-bergamotene, Æ® cadinol and α-cadinol respectively. These compounds representing about 78.64% of the total oil constituents of genoveser basil. Application of 100 ppm Phenylalanine + 50 ppm nickel increased the relative percent of linalool, α-bergamotene, Æ® cadinol α-cadinol except 1,8 cineole which was decreased by this treatment.
Basil has been cultivated for a long time as a medicinal and aromatic plant in many countries. It belongs to the genus Ocimum (Lamiaceae) which contains nearly 150 species of herbs and shrubs from tropical regions of Asia, Africa, central and south America (Baily, 1942). Basil is an important medicinal plant and culinary herb. Genovese basil (Ocimum basilicum L. var. basilicum) is one of the most important varieties of the genus Ocimum that is well known by its diversity as a source of essential oils. Basil leaves can be used fresh or dried as a spice to add aroma and flavor to confectionary, dressings, salads, pizzas, meats and soups. Its extracts are also used in the manufacturing of pharmaceutical preparations, cosmetics and perfumes (Putievsky and Galambosi, 1999).
Basil traditionally has been used to treat headaches, coughs, diarrhea, constipation, warts, worms and kidney malfunction. Fresh leaves and flowers yield an essential oil of distinctive aroma that possesses various beneficial effects for example, antiseptic, carminative, antimicrobial, sedative, anticonvulsant, antitumor and antioxidative properties. It also possesses a wide range of biological activities since it has insect repellent, nematocidal, insecticidal and antifungal activities (Tilebeni, 2011).
Amino acids can directly or indirectly influence the physiological activities in the growth and development of plants. They are particularly important for cell growth stimulation. Also, amino acids might be as important for the metabolic processes as they are components of enzymes, and insufficient synthesis of the essential amino acids, may lead to stress effects on metabolism and growth (Aberg, 1961). Moreover, they protect the plants from ammonia toxicity as well as protect the plants against pathogens (Goss, 1973). Tugnoli and Bettini (2003) concluded that use of amino acids as foliar spraying makes the plant overcome the nutritional deficiencies that arise during growth.
According to several studies, the foliar application of Phenylalanine (Phe) caused an enhancement in plant growth during vegetative and flowering stages (Gamal et al., 1997) on Cymbopgon citrates herb, (El-Sherbiny and Hassan, 1987) on datura plants, (Moursy et al., 1988) on Datura stramonium, (Refaat and Naguib, 1998) on peppermint (Mentha piperita) and (Habba, 2003) on Datura innoxia.
Also, Bálványos et al. (2002), showed that 66 mg·L-1 Phe maximized growth and alkaloid (lobeline) production of hairy root cultures of Lobelia inflata L., meanwhile 250 mg·L-1 Phe increased growth parameters, photosynthetic pigments, capsaicin and dihydrocapsaicin content of pepper (Capsicum annum L.) (Rashad et al., 2002). Other amino acids have been shown to improve growth and yield of many plants. Foliar application of glutamine at 100 to 200 mg·L-1 significantly increased growth parameters as well as yield of onion and quality of bulbs (Amin et al., 2011). Similarly, (Youssef et al., 2004) on lemon basil plant, (Khattab and Helmy, 2003) on fennel plants (Khattab et al., 2011) and on lemon basil. Talaat and Youssef (2002) found a pronounced increase in vegetative growth of plants as a result of lysine and ornithine treatments.
On the other hand, Nickel (Ni) is an essential micronutrient for plant growth. It is a constituent of the enzyme urease, and in small quantities (0.01 to 5 μ g/g dry wt) is essential for many plant species to complete their life cycle but higher concentrations of this metal are toxic and may severely interfere with many physiological and biochemical processes of plants (Seregin and Kozhevnikova, 2006). Ni deficiency depressed urease enzyme activity (Eskew et al., 1983) and other enzymes responsible for nitrate reduction (Brown et al., 1990).
Concerning the role of Ni in higher plants nutrition, some researchers reported growth response of plants to Ni fertilization under field conditions (Takishima et al., 1988), or plants grown in nutrient solutions (Gerendas and Sattelmacher, 1999), or in tissue culture media furnished with urea as the sole N source (Gerendas and Sattelmacher, 1997). The earliest report of a growth response to Ni addition under controlled experimental conditions by Brown et al. (1987) indicated that Ni deficiency has a wide range of effects on plant growth, plant senescence, N metabolism and Fe uptake although, excessive Ni inhibits growth and development of plants. Teixeira da silva et al. (2012) reported that Ni has a role in plant growth and nitrogen uptake in crops fertilized with urea in calcareous soils.
As well as Aly (1999) revealed that the main aroma constituent of parsley leaves was 1,3,8-p-menthatriene, which forms about 62% of the essential oil and showed a 10 to 25% increase over that of control with 25 mg per kg soil or higher levels of Ni fertilization. It is suggested that low levels of Ni fertilization strongly improve not parsley leaf yield and quality but also the leaves became safer for human consumption.
Therefore, the objectives of this study were to evaluate the influence of foliar application of different concentrations of Phe and Ni on growth, yield and chemical constituents of genoveser basil under Egyptian environmental conditions.
This investigation was carried out during the two consecutive seasons of 2011/2012 and 2012/2013 at the Experimental Farm of the Faculty of Agriculture, Cairo University, Giza, Egypt. The experimental area was a silt loamy soil of the following characters: sand, 25%; silt, 54%; loam, 21%; organic matter, 0.39%; total N, 1.12% total P, 0.088%; total K, 0.20%; total C, 0.23%; PH, 7.84 and EC (ds/m) 2.35 . The soluble ions (meq/L) were SO4, 8.5; Ca+2, 12.5 and HCO3, 2.5.
Seeds of genoveser basil (Ocimum basilicum L. var. Genoveser) were obtained from Enza Zaden com. Seeds were sown in open seed beds during the second week of February in both seasons. After 40 days from sowing, the seedlings were transplanted into the field at rows (60 cm between rows) 25 cm apart between plants. During soil preparation, 150 kg/fed. superphosphate (15.5 % P2O5) was applied. After transplanting, 200 kg ammonium nitrate (33.5 % N) and 100 kg potassium sulfate (48 % K2O) per fed. were added. The mineral fertilization was divided into two equal portions during the growing season; the first portion was added as mentioned before while the second one was applied after two weeks from the first cut. All agricultural practices were carried out as usually recommended for basil production in Egypt.
Nickel sulphate (as a source of Ni) was added as foliar application at rates of Zero, 50 and 100 ppm, while Phe (as a source of amino acids) was sprayed at levels of Zero, 50 and 100 ppm. Both of them were sprayed qquadrupled during the growing season once a month. The first one was applied after one month of transplanting. All spraying treatments were applied at the early morning and spreading agent “Masrol” was added to the foliar nutrient solution (1mL/L) to reduce surface tension. The experiment was designed as split plot in three replicates with Phe as main plots and Ni as subplots.
Genoveser Basil plants were cut twice during the growing season and then left for seed setting. The first cut was done during the second week of July and the second one in the middle of October in both seasons. For each cutting, 10 plants were randomly selected for determining and recording plant height (cm), plant diameter (cm), fresh and dry weights of plant (g), leaves (g) and flowers (g). The yields of fresh and dry herb (ton/fed; fed: Local area in Egypt = 4200m2), leaves (ton/fed.) and flowers (ton/fed.) were also estimated.
Representing samples of air dried herb of each replicate at each cut in both seasons were subjected to hydro-distillation for 3 h using Clevenger apparatus to extract and to determine essential oil percent according to Egyptian Pharmacopoeia (1984). The resulted essential oil was separately dehydrated over anhydrous sodium sulphate and kept in silica vials with Teflon-sealed caps and stored at 2°C in the absence of light till GLC analysis. The percentage of extracted essential oil was determined and recorded on the basis of oil volume to herb dry weight (ml/100g dry herb). Essential oil yield (L/fed.) was also calculated by multiplying oil percent with dry yield.
The essential oil constituents were analyzed and determined in the oil samples of the first cut of the second season. The dehydrated oil of each treatment was subsequently analyzed using a gas liquid chromatography-mass spectrometer (GC-MS) to evaluate oil quality. The gas chromatography–mass spectrometry (GC-MS) analysis of the essential oil samples was carried out using gas chromatography-mass spectrometry instrument stands at the Department of Medicinal and Aromatic Plants Research, National Research Center, Egypt with the following specifications. Instrument: a TRACE GC Ultra Gas Chromatographs (THERMO Scientific Corp., USA), coupled with a THERMO mass spectrometer detector (ISQ Single Quadruple Mass Spectrometer). The GC-MS system was equipped with a TG-WAX MS column (30 m x 0.25 mm i.d., 0.25 μm film thickness). Analyses were carried out using helium as carrier gas at a flow rate of 1.0 ml/min and a split ratio of 1:10 using the following temperature program: 40°C for 1 min; rising at 4.0°C/min to 160°C and held for 6 min; rising at 6°C /min to 210°C and held for 1 min. The injector and detector were held at 210°C. Diluted samples (1:10 hexane, v/v) of 0.2 μL of the mixtures were always injected. Mass spectra were obtained by electron ionization (EI) at 70 eV, using a spectral range of m/z 40 to 450. Most of the compounds were identified using mass spectra (authentic chemicals, Wiley spectral library collection and NIST library).
The collected data were subjected to the analysis of variance of split-plot design in Randomized Complete Block Design (RCBD) arrangement according to Snedecor and Cochran (1990) using MSTAT-C V.2.1 software package (Freed et al., 1989). Differences among means were compared for each trait by least significant difference test (L.S.D.) at 5% level of significance (Steel et al., 1997).
Growth parameters of genoveser basil as affected by foliar application of different levels of Phe and Ni are shown in Tables 1, 2, 3 and 4 at the first and the second cuts of the two seasons. Phe applied at 50 or 100 ppm concentration showed positive effect on all the growth parameters (Table 1). The highest values were obtained as a result of Phe application at 100 ppm followed by 50 ppm for all tested parameters in first and second cuts. The increments in plant height, plant diameter, plant fresh and dry weights, leaves fresh and dry weights compared to control reached 11.8, 17.0, 25.7, 28.7, 25.7 and 25.8% respectively, for the first cut of the first season and 15.7, 14.9, 30.1, 30.1, 30.0 and 30.0% consecutively, for the second cut.
With regard to Ni application it significantly augmented all growth parameters of genoveser basil at both 50 and 100 ppm concentrations. There was a progressive increase in growth values with increasing Ni concentration up to 50 ppm then the values significantly declined but they were still superior to the control for all tested parameters (Table 1). The maximum values were obtained as a result of Ni application at 50 ppm.
The increments in plant height, plant diameter, plant fresh and dry weights, leaves fresh and dry weights over control were 12.8, 7.5, 30.9, 33.9, 30.9 and 30.8% respectively, at the first cut and also 15.1, 9.2, 26.0, 26, 26 and 26.1% consecutively, at the second cut for the first season. The data within hand in Table 2 indicated that foliar application of Phe significantly augmented all growth parameters of genoveser basil in comparison with control in the second season.
All growth parameters in the first cut except plant and leaves fresh weights did not significantly increase by increasing Phe level from 50 to 100 ppm but they were still superior to the control, for the first and second cuts in the second season. The highest values of growth parameters were obtained as a result of the highest Phe of 100 ppm. The increments in aforementioned parameters over control were 13.2, 13.3, 26.4, 28.3, 26.4 and 26.2% consecutively, in the first cut and also 7.7, 17.7, 30.5, 30.7, 30.5 and 30.6% respectively, in the second cut. The increase in growth parameters as a result of Phe application may be related to its acting as building blocks of proteins and serving in number of additional functions in regulation of metabolism, transport and storage nitrogen (Davies, 1982). Amino acids play an important role for cell growth stimulation (Nahed et al., 2010).
Furthermore, they can serve as a source of carbon and energy and synthesize other organic compounds, such as protein, amines, purine, alkaloids, vitamins, enzymes, terpenoids and others (Goss, 1973; Abd El-Aziz and Balbaa, 2007). These results are in harmony with those obtained by Moursy et al. (1988) on Datura stramonium, Refaat and Naguib (1998) on peppermint (Mentha piperita), Habba (2003) on Datura innoxia, Balbaa and Talaat (2007) on rosemary plants and Talaat et al. (2013) on ammi visnaga. Also, the results corroborate with the findings of Gamal et al. (1997) on Cymbopgon citrates herb who reported Phe caused an enhancement in plant growth during vegetative and flowering stages.
The data in Table 2 emphasized that all growth parameters in the second season significantly augmented by foliar application of Ni in both cuts in comparison with control. The same tendencies were observed in the second season as that of the first season. The highest values of plant height, plant diameter, and plant fresh and dry weights, leaves fresh dry weights were obtained as a result of Ni application at 50 ppm in both cuts. The increments of all previous parameters over control were13.4, 13.4, 31.3, 28.7, 31.4, and 32.2% respectively, for the first cut and 11.9, 12.5, 27.1, 27, 27.1 and 24.8% consecutively, for the second cut. Increasing Ni concentration to 100 ppm significantly decreased all previous parameters but they were still superior to the control.
Similar finding about Ni effect were reported by Seregin and Kozhevnikova (2006) who mentioned that the small quantities of Ni (0.01 to 5 μg/g dry wt.) is essential for many plants to complete their life cycle but higher concentrations of this metal are toxic and may severely interfere with many physiological and biochemical processes of plants. The earliest report of a growth response to Ni application indicated that Ni deficiency has a wide range of effects on plant growth, plant senescence, N metabolism and Fe uptake (Brown et al., 1987; Teixeira da silva et al., 2012). As well the study of Aly (1999) showed that low levels of Ni fertilization particularly 50 mg/kg soil increased parsley leaf yield and quality without affecting leaf chlorophyll and Fe contents. It was evident from the previous researches that the beneficial effect of Ni on growth could be explained by the fact that Ni (Ni) is a constituent of the enzyme urease and in small quantities is essential for many plant species to complete their life cycle.
Data presented in Table 3 indicated that foliar spray with different levels of Phe significantly augmented some growth and yield parameters in both cuts in the first season. The highest values of flowers fresh and dry weights per plant and dry herb yield were obtained as a result of Phe application at 100 ppm in both cuts. Nevertheless, the differences in earlier mentioned parameters between 50 and 100 ppm did not reach the level of significant. The increments of flowers fresh and dry weights, oil content, dry herb yield and oil yield over control were 25.8, 25.8, 12.7, 28.9 and 44.6% respectively, for the first cut and 30.1, 30.1, 15.5, 30.1 and 50.1% for the second cut. Concerning Ni effect it was found that foliar application of 50 pm Ni resulted in the highest values of flowers fresh and dry weights, oil content, yields of dry herb and oil in both cuts for the first season, except oil content in the first cut. The rate of increments of previous parameters over control were 31, 31.0, 14.1, 34.0, and 51.0% consecutively, for the first cut and 26.0, 31.3, 14, 26.0, and 42.6% respectively, for the second cut.
Data of the second season presented in Table 4 behaved similarly as that of the first season. It showed that foliar application with Phe or Ni significantly increased all tested parameters in comparison with control for both cuts. The highest values of flowers fresh and dry weights were obtained by foliar spray with 100 ppm Phe in both cuts. Meanwhile, the highest values of oil content, dry herb yield and oil yield were produced by foliar spray with 50 ppm Phe in both cuts. The increments in flowers fresh and dry weights, oil content, herb yield and oil yield over control were 25.8, 26.5, 11.8, 28.4 and59.4% respectively for the first cut and also 30.5, 30.3, 9.7, 30.6, and 43.7% consecutively for the second cut.
These results were found to be in harmony with those obtained by Gamal et al. (1997) who reported that foliar application of amino acids (ornithine and Phe) significantly increased plant growth of lemongrass, Khattab and Helmy (2003) also reported that foliar spray with amino acids significantly increased vegetative growth and fruit yield of fennel plants. Furthermore Khattab et al. (2011) found that foliar application of cysteine significantly augmented all growth parameters of lemon basil.
As for Ni, it was obvious from data in Table 4 that Ni application at 50 ppm followed by 100 ppm gave the highest values. Flowers fresh weight, flowers dry weight, oil content and yield of dry herb and oil significantly increased with the application of Ni (50 ppm) by about 30.8, 31.4, 21.8, 28.8, and 54.7% over the control in the first cut respectively and 27.1, 27.2, 17.3, 26.8, and 47.8% in the second cut consecutively. These results were found to be in harmony with those of Helmy et al. (2002) who found that low level of Ni fertilization (40 mg/kg soil) increased coriander leaf yield and quality. Teixeira da silva et al. (2012) reported that excessive Ni inhibits growth and development of plants, induces leaf
chlorosis and wilting and reduce plant yields.
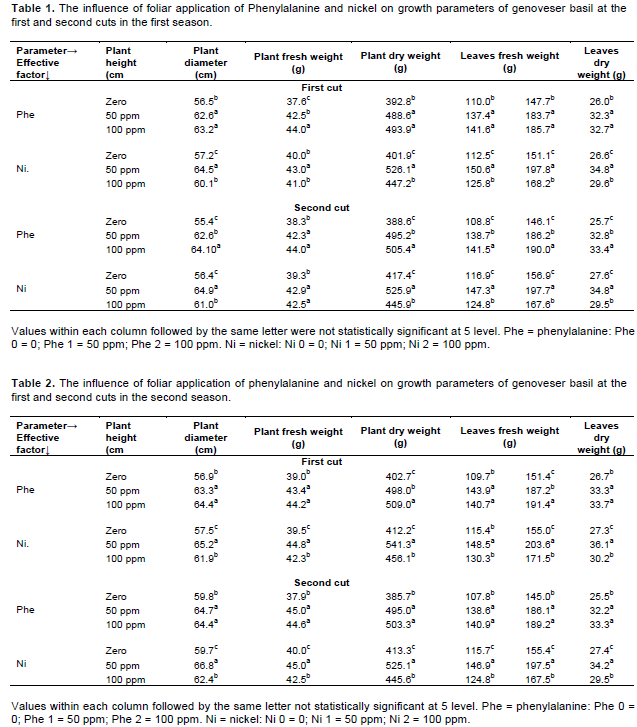
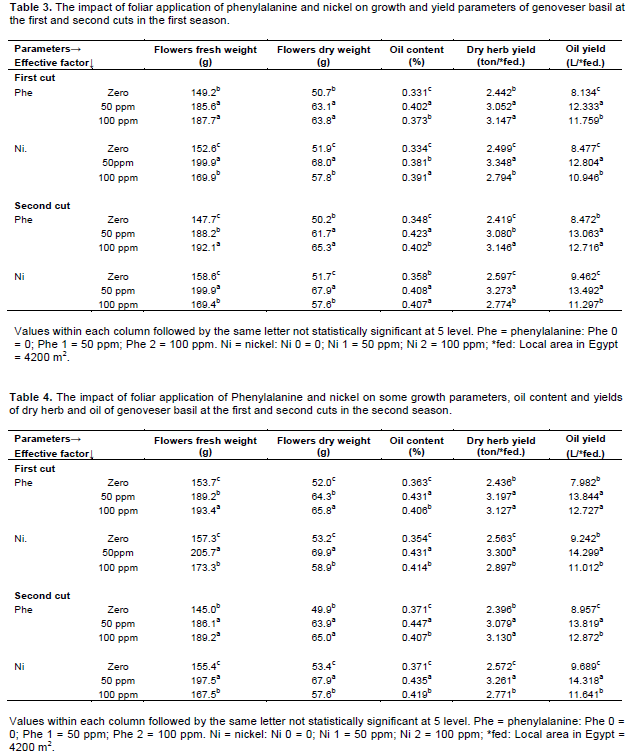
The influence of various concentrations of Phe and Ni on growth parameters in the first season are shown in Table 5 and Figures 1 and 2. Data within hand obviously showed that foliar application of Phe (100 ppm) and Ni (50 ppm) had highly positive effect on plant height, plant diameter, plant fresh and dry weights, leaves fresh and dry weights, yield of fresh and dry leaves followed by 50 ppm Phe and 50 ppm Ni in comparison with control in the first and second cuts except plant diameter in the second cut.
Meanwhile the combined treatment of 100 ppm Phe and 100 ppm Ni reduced aforementioned parameters, except plant diameter in the second cut. Foliar application of 100 ppm Phe + 50 ppm Ni increased plant height, plant diameter, plant fresh and dry weights, leaves fresh and dry weights over control by about 30.96, 33.71, 76.33, 87.26, 75.6 and 74.88% in the first cut respectively, while in the second cut the increments were 39.71, 31.41, 78.85, 78.87, 78.93 and 78.89% respectively.
Data reported in Table 6 emphasized that the same trend was observed in the second season, wherein foliar fertilization with Phe and Ni significantly increased all tested parameters in comparison with control in both cuts. Once again foliar application with 100 ppm Phe + 50 ppm Ni resulted in the highest values of growth parameters followed by 50 ppm Phe + 50 ppm Ni for both cuts. The increases in height, plant diameter, plant fresh and dry weights, leaves fresh and dry weights when applying 100 ppm Phe + 50 ppm in comparison with control were estimated at 35.3, 36.6, 78.1, 78.1, 78.2 and 77.6% for the first cut respectively, and also 25.3, 38.0, 80.0, 80.4, 80.3, and 80.4% for the second cut consecutively. Furthermore, the amino acids are not only building blocks of proteins but also precursors for a myriad of other molecules that serve important functions in plants. Amino acids are involved in the synthesis of other organic compounds, such as protein, amines, alkaloids, vitamins, enzymes, terpenoids and plant hormones that control various plant processes (Glawischnig et al., 2000; Ibrahim et al., 2010). The result within hand are in agreement with those obtained by Gamal et al. (1997) who reported that foliar spraying of Phe (100 ppm) or ornithine (50 ppm) led to significant increases in the number of leaves and tillers per plant, fresh and dry weights of Cymbopgon citrates herb as well as El-Sherbiny and Hassan (1987) on datura, Khattab et al. (2011) on lemon basil where different amino acids enhanced plant growth and yield. Similarly, Talaat and Youssef (2002) found a pronounced increased in vegetative growth of basil plant as a result of lysine and ornithine treatments.
Concerning the effect of Ni it was reported by Chand et al. (2012) that Ni and Pb applied at 25:25 produced 40% higher fresh herbage and root of Mentha arvensis L. while 50:50 ppm exhibited negative effect on the herb and yield. On the other hand, Teixeira da silva et al. (2012) proved that Ni deficiency affects plant growth, plant senescence, nitrogen metabolism and iron uptake and it may play a role in disease resistance, but also excessive Ni inhibits and development of plants.
Results in Table 7 showed that foliar application of Phe and Ni significantly augmented all tested parameters of genoveser basil and yield of herb and oil in the first season. Except oil content, the combined treatment between 100 ppm Phe and 50 ppm Ni induced the maximum values of all tested parameters in both cuts. Plants received combined foliar spray of 100 ppm Phe and 50 ppm Ni surpassed control plants in the flowers fresh and dry weights, oil content, yield of dry herb and yield of oil by 76.6, 76.4, 30.9, 87.7 and 145.9% for the first cut respectively and by 78.1, 78.9, 42.8, 79.0 and 155.5% for the second cut. On the other hand, the combined treatment of 50 ppm Phe and 50 ppm Ni induced the maximum values of oil content in both cuts.
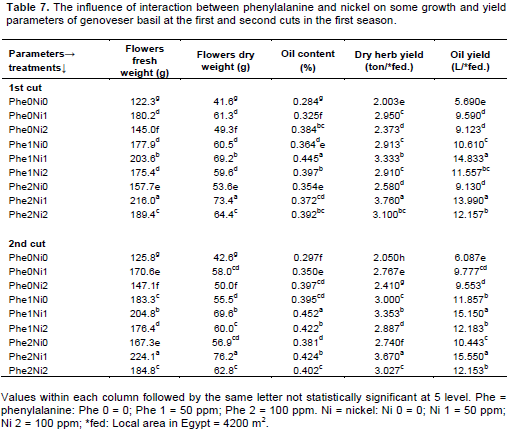
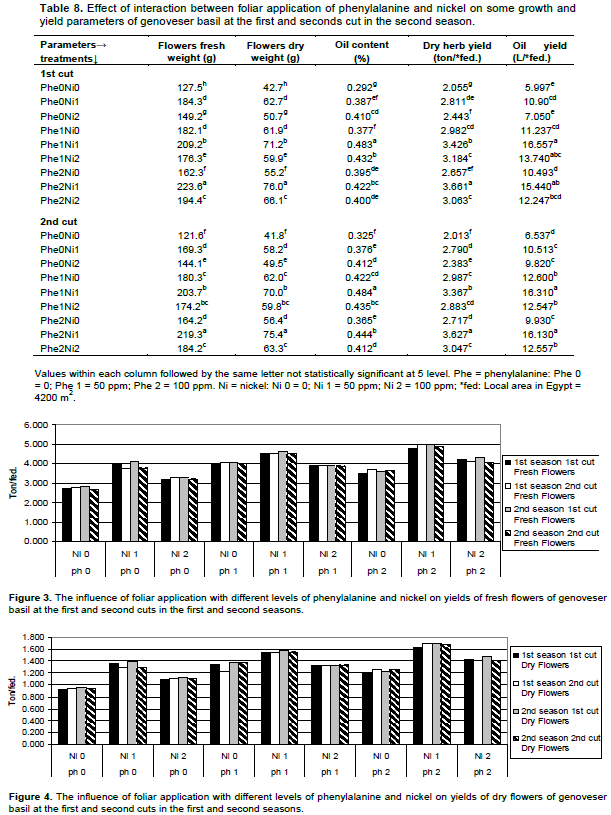
Similar trend was observed in data presented in Table 8 and Figures 3 and 4. It was found that foliar fertilization with combined Phe and Ni significantly increased plant growth, flowers fresh and dry weights, oil content, dry herb yield, oil yield, yields of fresh and dry flowers. The highest values of oil content and oil yield were noticed with the application of 50 ppm Phe + 50 ppm Ni in both cuts of the second season. The combined foliar application of 100 ppm Phe and 50 ppm Ni enlarged the flowers fresh and dry weights, oil content, yield of dry herb and yield of oil over control by 75.4, 78.0, 44.5, 78.2, and 157.5% for the first cut respectively and by 80.3, 80.4, 36.6, 80.2, and 146.7% consecutively, for the second cut.
Amino acids may be playing an important role in plant metabolism and protein assimilation which is necessary for cell formation and consequently increase fresh and dry matter. In addition, Fowden (1973) reported that amino acids acting as the building blocks of proteins can serve in number of additional functions in regulation of metabolism, transport and storage nitrogen. On the other hand, Waller and Nowacki (1978) suggested that the regulatory effects of certain amino acids, like Phe andornithine on plant development are through their influence on gibberellins. The results within hand are in agreement with those obtained by Youssef et al. (2004) on lemon basil, Talaat and Youssef (2002) on basil
where they found that foliar application with different amino acids significantly enhanced plant growth, oil content and yields of herb and oil.
Similar findings were obtained in tobacco (Darwish and Reda, 1975), Datura (El- Bahr et al., 1990), Hyoscyamus muticus L. (Reda et al., 1999), Iberis amara L. (Attoa et al., 2002) and chrysanthemum (El-Fawakhry and El-Tayab, 2003) where different amino acids augmented plant growth during vegetative and flowering stages, and produced a high quality of inflorescences. Studies have proved that amino acids can directly or indirectly influence the physiological activities in the growth and development of plants.
These results could be explained by the fact that amino acids can serve as a source of carbon and energy when carbohydrates become deficient in the plant; amino acids are determinate, releasing the ammonia and organic acid from which the amino acid was originally formed. The organic acids then enter Kreb’s cycle, to be broken down to release energy through respiration (Goss, 1973).
Concerning the effect of Ni, the illustrated results are in agreement with what was obtained by Prasad et al. (2008) who reported that Ni2SO4·7H2O caused a significant increase in herb yield by 21.6% more than the control. Also Aly (1999) suggested that low levels of Ni fertilization particularly 50 mg/kg clay soil, strongly improve not parsley leaf yield and quality but also the leaves became safer for human consumption. Also, Helmy et al. (2002) found that low levels of Ni fertilization 40 mg/kg soil, increased coriander leaf yield, oil content and quality. The latter effect of Ni on growth may be due to the fact that Ni is a constituent of the enzyme urease and in small quantities is essential for many plant species to complete their life cycle.
In terms of essential oil constituents of genoveser basil, thirty compounds were identified. These compounds were listed in Table 9 in an ascending order. All treatments showed that the major oil constituent was linalool with mean relative percent (37.18%). The second main compound was 1,8 cineole with mean relative percent (16.4%) followed by α-bergamotene (14.71%). As well as the fourth and fifth compounds, Æ® cadinol and α-cadinol had mean relative percent of 6.82 and 3.53% respectively. These compounds represented 78.64% of the oil constituents of genoveser basil.
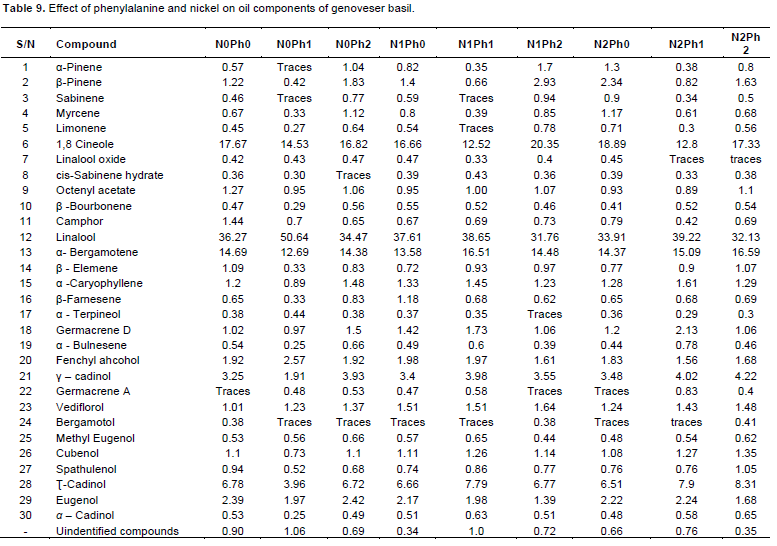
A major differences between the oil constituents from plants treated with Phe were observed in the contents of linalool, 1,8 cineole, α-bergamotene, Æ® cadinol and α-cadinol. Plants sprayed with 50 ppm Phe without Ni showed an increase in linalool content with a corresponding decrease in the rest of aforementioned compounds. While those sprayed with 100 ppm Phe decreased linalool content compared to control. The rest of the other components in the oil were unaffected by treatments. On the other hand, application of 100 ppm Ni + 50 ppm Phe increased the relative percent of linalool, α-Bergamotene, Æ® cadinol and α-cadinol, except 1,8 cineole which decreased by this treatment. Meanwhile foliar spraying with 100 ppm Ni +100 ppm Phe decreased linalool and 1,8 cineol in comparison with control, except α-bergamotene, Æ® cadinol and α-cadinol which they increased by aforementioned treatment.
Similarly, some other authors showed that amino acid treatments resulted in numerous differences in the components of the essential oil (Khattab et al., 2011) who reported that increasing cysteine from 50 to 100 ppm increased hydrocarbons on the cost of oxygenated compounds in the lemon basil oil. Also, Khattab and Helmy (2003) pointed out that almost all concentrations of ornithine or cysteine increased the relative percent of anethole in the fennel oil.