The LERCAFE test consists in immersing coffee seeds in an active chlorine solution, which reacts with the seeds’ endosperm, thus staining the viable parts dark green. The goal was to adapt the LERCAFE methodology to coffee seeds and assess the isoenzymatic profile of seeds subjected to the test. Two experiments were conducted, the first with adequacy of the LERCAFE test methodology, and the second experiment adequacy of the LERCAFE test methodology with the content of active chlorine quantified. A completely randomized design was used, with four replications of 50 seeds in a factorial scheme 4x4 (4 cultivars and 4 treatments of LERCAFE test). In the first experiment, it was possible to sort the cultivars into two quality levels by means of the treatments at 2.5% for 3 h, 3.5% for 2 h and 3 h. In the second experiment, it was found that the test enables determining the coffee seeds’ physiological potential by using 2 and 3% active chlorine for 5 and 3 h, respectively. The coffee seeds subjected to LERCAFE test show changes in the activity of esterase, malate dehydrogenase, superoxide dismutase, catalase and alcohol dehydrogenase enzymes, and the activation or deactivation of these enzyme systems vary with the concentration and immersion time in the solution of active chlorine.
There are several factors that contribute to a successful implementation of a coffee farming, among them, the use of healthy and well developed seedlings, which are the base of support for the establishment of culture, mainly because it is a perennial crop (Carvalho et al., 2012). The time for seedling formation becomes larger, due to the coffee seeds possessing slow and uneven germination (Lima et al., 2012).
Studies have been intensified regarding LERCAFE test, because it allows obtaining results concerning the viability of coffee seeds in a short period of time as well as being easy to perform, but little is known about the mechanisms of action involved between the coffee seeds´ endosperm and the active chlorine presents in the sodium hypochlorite solution.
Zonta et al. (2008) used the LERCAFE test to estimate germination and to characterize damage in coffee seeds. According to the authors, the test is efficient in detecting damage caused by drying at high temperature and by shoot borer attack. The test was also used to evaluate and characterize mechanical damage in coffee seeds, proving to be efficient (Zonta et al., 2011).
As it is a test that combines the reaction of active chlorine with possible killed/injured regions of the seeds´ endosperm, the discovery of possible enzymatic processes involved in the test can support the understanding of the reactions that occur between the seed´s endosperm and the active chlorine. A wide variety of proteins and structural enzymes is responsible for the integrity and cellular metabolism and, so, the activity of certain enzymes, such as superoxide dismutase, esterase, the malate dehydrogenase and alcohol dehydrogenase, associated with reserves breaking or new tissue biosynthesis, can determine the deterioration stadium of seeds (Carvalho et al., 2000).
Thus, this research objective is to standardize the LERCAFE test methodology for assessment of coffee seeds quality (Coffea arabica L.), and to verify the behavior of seeds enzymatic systems after LERCAFE test.
The research was carried out in the Federal University of Jequitinhonha and Mucuri Valleys’ Seed Laboratory and in the Federal University of Lavras’ Seed Central Analysis Laboratory, with coffee seeds originating from the Três Pontas Experimental Farm, provided by Agricultural Research Corporation of the State of Minas Gerais (EPAMIG), in two experiments, as described:
Experiment 1 - Adequacy of the LERCAFE test methodology
The LERCAFE test was performed in coffee seeds without the parchment (manually removed) for all cultivars. Seeds were subjected to immersion treatments in aqueous solution of sodium hypochlorite at concentrations of 2.5, 3.5, 5.0 and 6.0% of active chlorine, during periods of 2, 3 and 6 h at 30°C, with the aim to determine the best concentration and immersion time for the experimental evaluation. The active chlorine concentrations were obtained from the dilution of commercial sodium hypochlorite, with 10% content of active chlorine, in distilled water. The test was performed following the methodology proposed by Reis et al. (2010).
Experiment 2 - Adequacy of the LERCAFE test methodology with the content of active chlorine quantified
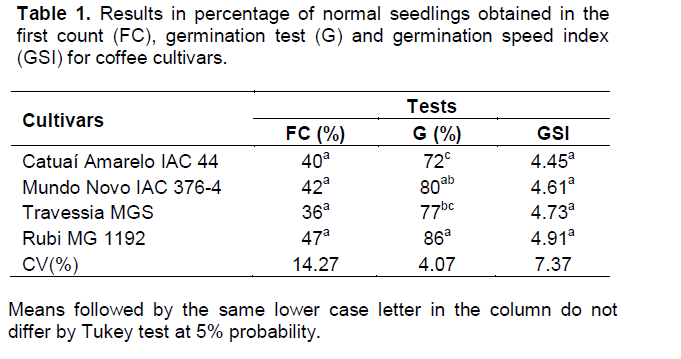
Coffee seeds batch (Coffea arabica L.) of the cultivar Catuaí Vermelho IAC 99, from the 2010/2011 crop was used. The moisture content, the germination test, the first count and the germination speed index (GSI) were performed as described in experiment 1. For the adequacy of the LERCAFE test methodology, sodium hypochlorite solutions were tested with concentrations of 1, 2, 3, 4 and 5% respectively of active chlorine for the periods of 1, 2, 3, 4 and 5 h of immersion in a germination chamber at 30°C, and the contents of active chlorine were obtained from the dilution of commercial sodium hypochlorite (ready for analysis) in distilled water quantified according to Brazil (2005). The coffee seeds were classified as germinable and non-germinable following the classification presented in experiment 1 (Figure 1). For the identification of effective treatments in the estimation of viability by LERCAFE test, the unilateral left Dunnett test was applied, with 5% of probability, finding, thus, significant results compared to the control treatment (germination test). For this, data were installed in a completely randomized design with four replications of 50 seeds and subjected to analysis of variance.
To assess the enzymatic activity, the behavior of seeds subjected to effective treatments in determining the coffee seeds viability was evaluated; for this, four replications of 50 seeds were used, representing each treatment. Into the enzyme assessment, it was also inserted the control treatment (T), in which the seeds were not subjected to the LERCAFE test, but wetted for 3 h on paper substrate (moistened equivalent to 2.5 times the weight of the substrate). The seeds of these treatments were crushed using a TE613/1 model Rebnal mill, cooled at 4°C in the presence of antioxidant PVP (Polyvinylpyrrolidone) and liquid nitrogen in a mortar. After milling, the material was stored at - 86°C, for the isoenzyme analysis. The extraction of the protein was performed by adding 100 mg of the seed powder to 280 µl of extraction buffer (Tris 0.2), homogenized by vortexing and, then, kept in the refrigerator for 1 h. The samples were centrifuged at 14,000 rpm at 4°C for 1 h.
The electrophoresis in polyacrylamide gels was performed in a discontinuous system (7.5% separating gel and 4.5% concentration gel). The gel / electrode buffer system used was Tris-glycine pH 8.9. To perform the eletrophoretic run, 60 µl of the supernatant of the extracted material were applied to the gel channels and the run was performed at 4°C, 150 V, for 6 h. At the end of the run, the gels were revealed for the following enzyme systems: esterase (EST), malate dehydrogenase (MDH), superoxide dismutase (SOD), catalase (CAT) and alcohol dehydrogenase (ADH), according to the methodology described by Alfenas, (2006).
In order to compare the germination potential of the coffee seeds submitted to the LERCAFE test, and to the control treatment (T), Tukey test at 5% probability was used. Data were subjected to analysis of variance and the means were compared by Tukey test. The experiment was installed in a completely randomized design with four replications of 50 seeds. Statistical analyzes were performed with the aid of SISVAR® statistical program (Ferreira, 2010).
Experiment 1: Adequacy of the LERCAFE test methodology
In Table 2, assessments of viability obtained by LERCAFE test are observed (visual evaluation). Treatments of 2.5% of active chlorine for 3 h and 3.5% of active chlorine for 2 and 3 h allowed distinguish cultivars at two levels of quality, being Rubi, Travessia and Mundo Novo superior compared to the cultivar Catuaí Amarelo, with inferior quality.
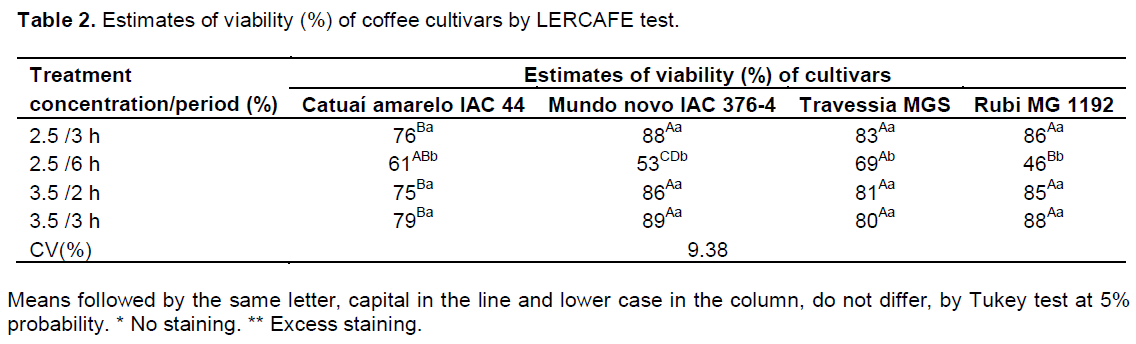
The coffee seeds immersed for 2 h in a sodium hypochlorite solution containing 2.5% of active chlorine, did not have greenish coloration in the endosperm, precluding their assessment (Table 2). Zonta et al. (2010) when testing the efficiency of LERCAFE test, failed to achieve satisfactory results for the treatments in which the coffee seeds were immersed in a sodium hypochlorite solution containing 2.5% of active chlorine
for the periods of 1 and 2 h, because there was absence of staining for these treatments. For treatments of 3.5% of active chlorine for 6 h, 5 and 6% of active chlorine for 2, 3 and 6 h, an intense dark green color was observed, occupying a large part of the seeds´ endosperm, this complicated their assessment by LERCAFE test, and data were not computed.
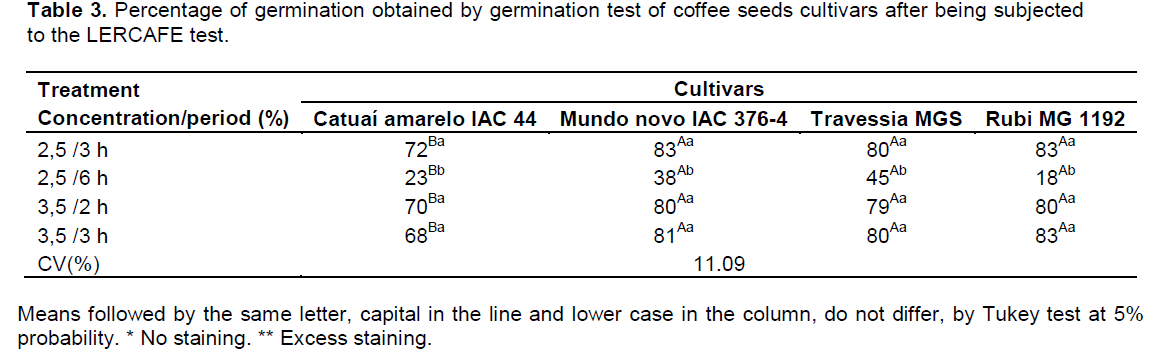
When observing the data in the germination test after the LERCAFE test, only the cultivar Catuaí Amarelo was distinguished as inferior to the other (Table 3). In the treatment of 2.5% for 6 h, it was difficult to distinguish the quality of seeds with excessive staining of the endosperm, preventing the assessment of seeds viability by LERCAFE test. For this treatment, low percentage of germination by the germination test (Table 3) was observed, indicating that the immersion period of 6 h affected negatively seeds´ physiological quality. It is observed, in general, that the LERCAFE test overestimates the results of seed viability. The results of the LERCAFE and germination tests match, however, these results may show considerable discrepancies, due to possible infestation with pathogens in the batch. So, not all abnormalities found in seedlings can be observed in the embryo and, as a result, the LERCAFE test can provide superior results.
The choice of the appropriate methodology for the employment of LERCAFE test should be based on the ease of differentiation of viable and non-viable tissue, and on the ability to differentiate batches with different physiological qualities. Another factor that must be taken into account in the assessment of seed viability is the execution time of the test, since a rapid evaluation provides advantages such as the possibility of dropping batches with inadequate quality, also requiring adjusting the concentrations of active chlorine solution.
For this experiment, commercial sodium hypochlorite solutions were used, containing 10% of active chlorine. According to Brazil (2005), the sodium hypochlorite in concentrated solutions degrades under the influence of light and heat; so, the quantification of this solution is essential for standardization of the test. To standardize the LERCAFE test, it is essential to standardize the sodium hypochlorite solution. In order to find a treatment that allows the distinction of batches in different levels of physiological quality, making the test reproducible is important. Thus, it was the second experiment that was performed in which the content of active chlorine present in the sodium hypochlorite solution was quantified. For this, the efficiency of the LERCAFE test with seeds immersed in sodium hypochlorite solution with contents of 1, 2, 3, 4 and 5% respectively of active chlorine for the periods 1, 2, 3, 4 and 5 h at 30°C were assessed.
Experiment 2: Adequacy of the LERCAFE test methodology with the content of active chlorine quantified
The moisture content of the coffee seeds of the cultivar Catuaí Vermelho IAC 99 was 16%, the germination percentage was 82%, and the germination speed index equal to 4.31. Treatments in which seeds were immersed in solution containing 2 and 3% of active chlorine for the periods of 5 and 3 h, respectively, were effective in the estimation of viability by LERCAFE test, because they had means statistically equal to the control (Table 4).
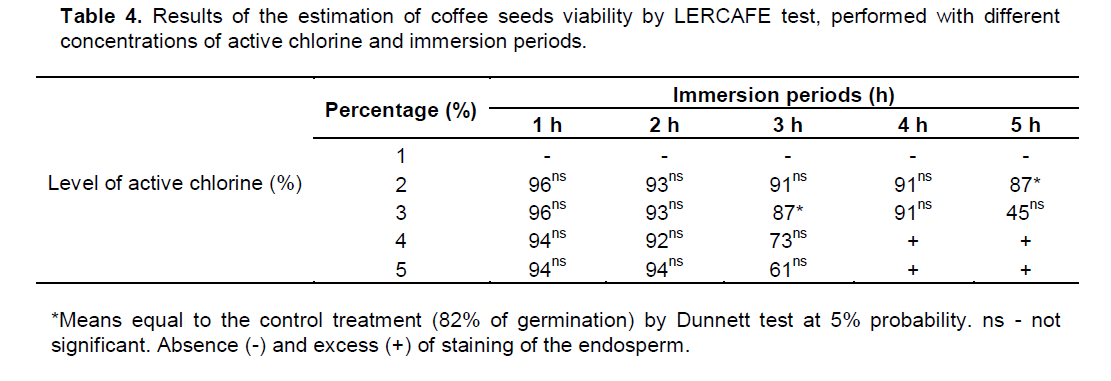
For the other immersion periods, effective treatments were not observed in the estimation of the viability by LERCAFE test. The treatments in which seeds were immersed in a sodium hypochlorite solution with levels of 4 and 5% for periods of 4 and 5 h did not allow the evaluation by LERCAFE test, due to excessive coloration of the endosperm (Table 4). As for all immersion periods in sodium hypochlorite solution with level of 1% of active chlorine, it was not possible to assess seeds by LERCAFE test, because these treatments were not enough to color the coffee seeds’ endosperm, precluding their evaluation.
In order to associate the enzyme activity with what was observed by LERCAFE test, it the enzymatic behavior of the treatments which were effective in estimating the viability by LERCAFE test in experiment 2 (Table 4) was assessed. For better interpretation of the results, treatments were added with immersion periods above and below those found for effective treatments. So, the enzymatic behavior of the following treatments was assessed: control (seeds not subjected to LERCAFE test) treatments in which seeds were immersed in with levels of 2 and 3% of active chlorine for the periods 1, 3 and 5 h.
Comparing the germination percentage of the control treatment (T) with germination results found for the treatments in which seeds were subjected to LERCAFE test, it was observed that only the treatment in which seeds were immersed in a sodium hypochlorite solution, containing 3% of active chlorine for a period of 5 h, showed germination statistically lower than the other treatments (Table 5).
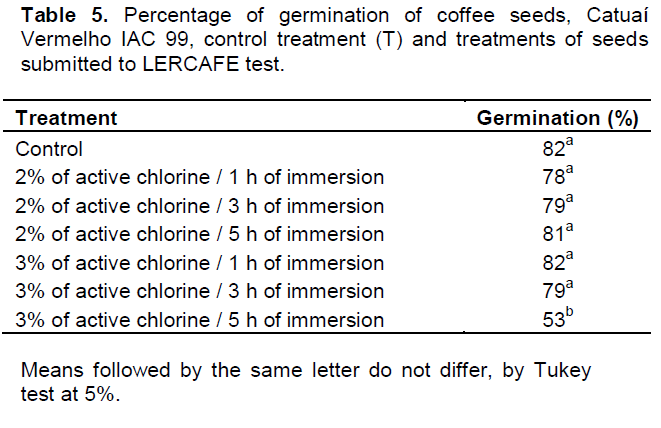
Figure 2 shows the zymogram for the esterase enzyme (EST). According to Santos et al. (2004), this enzyme participates in the hydrolysis of esters reactions, and is directly linked to the lipid metabolism and to the degenerative process of membranes. For treatments with 5 h of immersion in concentrations of 2 and 3%, there was no activity of the EST. This absence may be due to the effect of prolonged immersion period of seeds in sodium hypochlorite solution. The seeds under treatment of 3% for 5 h showed low germination, indicating that the degradation processes were possibly activated (Baker, 1962). However, the seeds under treatment of 2% for 5 h did not show low germination percentage, indicating the effect of exposure of seeds to treatments with high levels of active chlorine, as well as prolonged immersion periods in the activity of EST (Table 1).
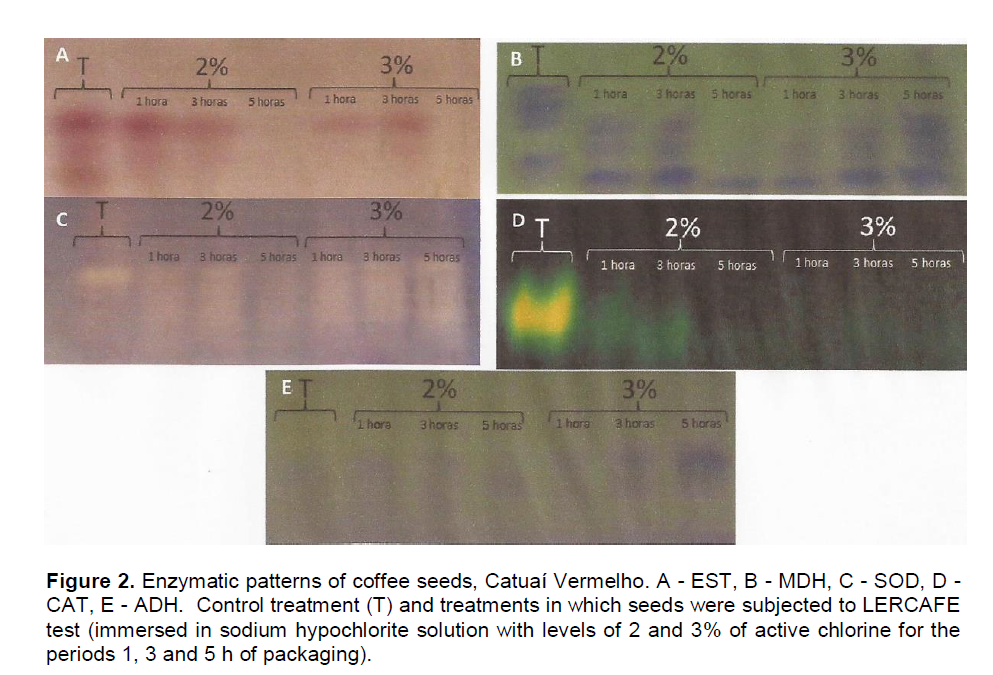
The reduction of the esterase isoenzymes activity can be related to the self-oxidation of fatty acids (Flood and Sinclair, 1981) and loss of integrity of the membrane system and release of lipid or denaturing of the enzyme (Machado, 2000). The malate dehydrogenase enzyme (MDH) catalyzes the last reaction of the Krebs cycle (Tunes et al., 2011). This is an important enzyme for cellular respiratory process, the increase of its activity may be due to the increase of its expression in different cell compartments and / or to the induction of enzyme activity expressed by a higher intensity of the bands. This may have occurred due to the increase of respiration in seeds that were in deteriorating process, since the enzymes involved in respiration may be activated in seeds with reduced quality as in the study of Shatters et al. (1994). It was noted that treatments with higher germination had a stable or decreased enzyme activity. As the treatment of 3% for 5 h had a greater influence of active chlorine (Figure 2). The superoxide dismutase (SOD) and catalase (CAT) enzymes are efficient mechanisms in cellular detoxification process, participating in removing process of free radicals (Taveira et al., 2012).
Among the enzymes responsible for the defense system, the SOD is one of the most important (Corte et al., 2010). This group of metalloenzymes catalyzes the formation of hydrogen peroxide from superoxide radicals, protecting the cell from oxidative processes (Taveira et al., 2012). However, the accumulation of peroxide can also be toxic to the cell, and can kill it, especially in the presence of iron (Eanton, 1991). Looking at Figure 2, it is noted that there was intense activity of SOD for all treatments, with low activity of this enzyme for the control treatment (T), showing the effect of treatment of LERCAFE test on SOD activity, that possibly activated the antioxidant system, as consequence to the stress generated by immersing the seeds in sodium hypochlorite solution. The treatment of 3% for 5 h showed the most intense band for this enzyme, and the seeds subjected to this treatment were the ones that showed the greatest drop in germination potential (Table 1).
Through the oxidation-reduction cycle, the CAT acts as a key enzyme in the removal process of hydrogen peroxide, participating in the control of these endogenous peroxides (Ataíde et al., 2012). Thus, the reduction of CAT activity reduces the ability to prevent against oxidative damage. As the CAT is an enzyme being able to perform the detoxification of O2- and H2O2 (Taveira et al., 2012). But, looking at Figure 2, it is noted that there were no changes in the enzyme profile for this enzyme, being that the CAT activity wasn’t visible for the control treatment (T). Probably the stress caused by immersing seeds in sodium hypochlorite solution caused the inhibition of this antioxidant system (Figure 2).
In Figure 2, the progressive increase in the alcohol dehydrogenase enzyme (ADH) activity was observed, as it increased the content of active chlorine of the sodium hypochlorite solution, as well as the immersion period. This enzyme acts on anaerobic metabolism, reducing acetaldehyde to ethanol and oxidizing NADH to NAD + ADH (Buchanan et al., 2005). Tunes et al. (2011) describe that acetaldehyde is responsible for the acceleration of deteriorating process in seeds, which coincides with what was observed in the treatment of 3% for 5 h.