ABSTRACT
Tacinga inamoena (K. Schum.) [N. P. Taylor & Stuppy] is a native Cactaceae from the semiarid region of Northeastern Brazil whose fruit is eaten by the local population. It seems that there is a significant amount of functional compounds such as polyphenols and betalains in this fruit as reported for other well-known cacti. Although there are not enough studies that have been conducted, it has attracted interest regarding nutritional and functional viewpoints. In this sense, changes in bioactive compounds during maturation need to be evaluated as this fruit faces very unstable conditions during its development, which can lead to drastic changes of the constituents in the pulp and peel. Thus, the objective of this study was to evaluate the content of bioactive compounds and antioxidant activity during the maturation of Tacinga inamoena fruit from fruit bearing plants grown under Brazilian semiarid conditions. The contents of total chlorophyll declined parallel to an increase of the total carotenoids, yellow flavonoids, and betalains. However, a sharp difference between the content of these pigments in the peel and pulp was observed which characterized the main changes during fruit maturation. This fruit presented considerable carotenoid content, reaching 348 μg/100 g in the peel and 29 μg/100 g in the pulp when fully ripe. Total antioxidant activity (TAA) was higher in the pulp of more mature fruit. TAA was correlated with the bioactive compounds, with the exception of betacyanins, which were betalains present in smaller amounts in this fruit.
Key words: Cactaceae, quipá, carotenoids, polyphenols, betalains, antioxidant activity.
The Brazilian semiarid region has several species of native fruit with great potential for exploitation, not only due to their
peculiar aroma and flavor, but mainly for the presence of compounds with functional appeal, and which are still unexplored (Silva et al., 2009; Dantas et al., 2013). Tacinga inamoena, known as quipá, cumbeba, or gogóia, is a native Cactaceae in Northeastern Brazil that is found throughout almost all of the semiarid region (Lima, 1989), and is one species whose fruit composition needs to be further studied, although it has been recognized as a po-tential source of functional compounds (Silva et al., 2009).
The differential in quality of cactus fruit is due to the profile of antioxidant compounds, especially those from the Opuntia genus, which present betalain pigments and phenolic compounds such as flavonoids and phenolic acids (Stintzing et al., 2005; Kim et al., 2011; Dhaouadi et al., 2013). How these compounds act in reducing oxi-dative stress may vary according to their physicochemical properties, medium conditions, and inflammatory bio-markers such as COX-2 (cyclooxygenase-2) and iNOS (inducible Nitric Oxide Synthase), whose regulation is dependent upon the extract dose and action time (Tesoriere et al., 2005; Tenore et al., 2012; Kim et al., 2013; Allegra et al., 2014).
Among cactus compounds, betalains stand out as one of the main constituents. Betacyanins are the class that give color ranging from red to pinkish, and betaxanthins correspond to color ranging from yellow to orange (Gandía-Herrero and García-Carmona, 2013). T. inamoena fruit presents a significant amount of the betaxanthin group (Dantas et al., 2015). However, due to their simultaneous occurrence in most fruits, their combination results in the development of a very peculiar color, which usually becomes evident during the maturation of the fruit (Castellar et al., 2012). Because of these events, fruits generally increase their antioxidant status by the preponderant accumulation of one of these classes. Opuntia fruit with yellow-orange pulp or peel exhibit high amounts of indicaxanthin (Stintzing and Carle, 2007). When isolated, betaxanthins have antioxidant and anti-inflammatory properties, which contribute to the development of products that can reduce the development of oxidative stress (Kim et al., 2013; Naselli et al., 2014), showing antigenotoxic and chemoprotective effects (Brahmi et al., 2011, 2012).
Fruit bearing plants of Brazilian biodiversity have been poorly studied, even though the research in the botany, ethnobotany, and floristic fields has brought valuable information that support further studies aiming to add value through sustainable use (Lucena et al., 2013; Oliveira et al., 2012). Furthermore, they have the potential to go beyond these aspects and bring insights toward health promotion, such as the profile and quantification of bioactive compounds to support further studies by performing in vivo evaluation about aspects such as functionality and bioavailability. The Opuntia genus in Brazil presents great exploitation potential, especially its fruits (Silva et al., 2009), which are internationally valued and recognized as important sources of pigments, vitamins, sugars, and gelling materials (pectins). The vast exploitation potential is, above all, due to their antioxidant properties (Cha et al., 2013; Dantas et al., 2015), which can be differentiated according to fruit portion (Osorio-Esquivel et al., 2011) and maturation (Cayupán et al., 2011; Castellar et al., 2012).
Although knowing that maturation is a complex process defined genetically and mainly controlled by hormones and environmental conditions, major compounds in the peel and pulp of cactus fruits drastically change during this process, modifying the antioxidant status in response to the increase or decrease of antioxidant compounds. For Opuntia megacantha, the increase of ascorbic acid, phenolic compounds, and betalains was highly correlated with the antioxidant properties (Cayupán et al., 2011). In the same way, Opuntia stricta fruit present a remarkable amount of betalains (mainly betacyanin), which are synthetized during fruit development and evolve until completely changing the whole color of the fruit when the highest antioxidant potential is reached (Castellar et al., 2012). Thus, for both cases the screening of non-traditional fruits must start with monitoring major compounds that are mostly responsible for contributing to health promotion.
Considering this, T. inamoena fruit represent an increased interest in the search to identify compounds with health promotion appeal, as well as for application in the composition of food products (Saénz et al., 2009; Fernández-López et al., 2010). Overall, these aspects are set as an important tool to add value that would increase and/or diversify the use of Cactaceae fruits in the semiarid region of Northeastern Brazil. In this sense, this study evaluates the content of bioactive compounds and antioxidant activity during the maturation of Tacinga inamoena fruit from fruit bearing plants grown under Brazilian semiarid conditions.
Plant material
Fruit from T. inamoena plants were collected in two areas of natural occurrence located in the microregion of Curimataú, State of Paraíba, Brazil, where plants are irregularly distributed in communities. After collection early in the morning, fruit that presented several maturation patterns without any visual defect were submitted to classification based on the evolution of the peel color inherent to the fruit’s development. Thus, maturation was classified into six stages: 1 - Entirely green coloration; 2 - Light green coloration; 3 - Predominantly green coloration with yellow nuances; 4 - Yellowish coloration with pink nuances; 5 - Pink coloration with yellow nuances, and 6 - Pink coloration with orange nuances. Forty fruits were used per replicate (≈ 450 g) for each maturity stage. The fruit was processed, making the separation between peel (epicarp) and pulp (mesocarp + pulp with seeds), and seeds were manually separated. Samples were kept at -18 °C until time of assessment.
Bioactive compounds
The total chlorophyll content was spectrophotometrically determined at 652 nm according to Dantas et al. (2013), and the results were expressed in mg/100 g. For total carotenoids, readings were performed at 450 nm and the results were expressed in µg/100 g of fresh matter according to Higby (1962).
Betalain content was performed spectrophotometrically using Nilson’s (1970) equations. Extracts obtained from fruit at each maturity stage were prepared using water as the extractor solution. After the samples were weighed and mixed in distilled water, they were centrifuged at 12,000 rpm for 25 min at 4 °C. The supernatants were stored and the residue was re-extracted twice more. The resulting supernatants were mixed, and the final volume was adjusted to 30 mL with distilled water, and then analyzed immediately afterwards. The absorbance of the extracts was measured at 476, 538 and 600 nm, and the betalain content (mg/100 g) was estimated by the following equations:
x = 1.095(A
538 - A
600) e
y = -0.258 × Ð
538 + A
476 - 0.742 × A
600. Finally, the betacyanin content was obtained by BTC = (
x × R × 100)/1120, and betaxanthin by BTX = (
y × R × 100)/750, where R is the dilution factor, and

and

the extinction coefficients for betanin and vulgaxantina-I, respectively.
The yellow flavonoid content was spectrophotometrically determined at 374 nm according to Dantas et al. (2013). About 1.0 g of fresh weight was added to 10.0 mL of extract solution composed of 95% ethanol and 1.5 N HCl in the ratio of 85:15 (v/v). The results were expressed as mg/100 g fresh weight.
Total extractable polyphenols and antioxidant activity was performed according to Silva et al. (2012). Samples were then mixed with 4 mL of 50% methanol, and the tubes were shaken for 1 minute, followed by 1-h rest. Afterwards, the extract was centrifuged at 4°C and 15,000 rpm for 15 min. The supernatant was kept, 4 mL of 70% acetone was added to the residue, and then it was subjected to the same procedure. The supernatants were put together and the final volume was adjusted to 10 mL by adding distilled water. The extracts were kept at -20°C until analysis.
The total extractable polyphenol content was determined using a spectrophotometer by Folin-Ciocalteu’s method, with modifications (Silva et al., 2012). Based on a previous study, an aliquot of 300 µL of the extract was used for all maturity stages, which was diluted to 1000 µL with distilled water. The oxidation was performed by adding 1 mL of Folin-Ciocalteu’s reagent in distilled water (1:3, v:v), followed by neutralization with 2.0 mL of 20% sodium carbonate, and adding 2.0 mL of distilled water. The reading was performed at 700 nm after being kept in the dark for 30 min at room temperature. The estimated content of phenolic compounds was performed using a standard curve of gallic acid (R2 = 0.99), and the results expressed in mg of gallic acid per 100 g of fresh weight.
Total antioxidant activity
Total antioxidant activity of extracts was determined by α, α- diphenyl-β-picrylhydrazyl (DPPH) free radical scavenging method (Dantas et al., 2013). Three dilutions (2000, 6000, and 8000 mg/L) were prepared in triplicate by previous tests based on the standard curve of DPPH• (0 - 60 µM DPPH; R2=0.99). From each dilution, an aliquot of 0.1 mL added to 3.9 mL of DPPH• radical (60 µM) was used. An amount of 100 µL of the control solution composed of 4 mL 50% methanol, 4 mL 70% acetone, and 2 mL distilled water was used. Pure methyl alcohol was set as blank (GenesysTM 10S UV-VIS), and reads were performed at 515 nm. Results were expressed by the EC50 value, which aims to provide numerical parameters of how much fresh fruit weight is able to provide antioxidants and verify their effectiveness in scavenging DPPH• free radical (g of fruit/g DPPH•).
Statistical analysis
The experiment was designed as completely randomized with factorial arrangement of 2×6 for all variables (two fruit portions and six maturity stages), except for the antioxidant activity and content of phenolic compounds, which were only analyzed in the pulp. All variables were analyzed considering six repetitions. Data were submitted to analysis of variance by F test (p ≤ 0.05) and Pairwise Correlations using the JMP® software (SAS Institute Inc. 2012). Results were expressed as means and respective standard error values.
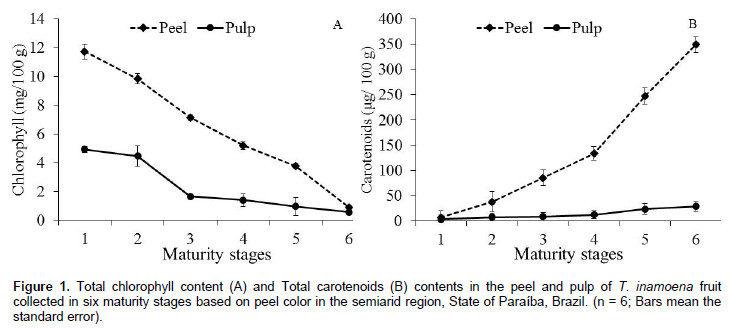
The total chlorophyll content in the T. inamoena fruit peel decreased as its maturity stages progressed, showing a marked decrease from 11.74 to 0.91 mg/100 g in the peel, and from 4.9 to 0.56 mg/100 g in the pulp (Figure 1A). It was observed that the content of total carotenoids of T. inamoena fruit showed significant difference (p < 0.01) between the fruit portions studied. During ripening, a rapid quantitative increase from 7.04 to 348.95 μg/100 g in the peel and from 3.40 to 28.67 μg/100 g in the pulp was observed (Figure 1B). All these changes presented a linear behavior as the green color disappeared and carotenoids together with other compounds became more concentrated in both the pulp and peel.
In turn, the amount of betalains, which comprises both betacyanins and betaxathins, varied according to fruit portion and maturation (Figure 2A and B). Betacyanins in the fruit peel were superior (p < 0.05) compared to pulp, especially from maturity stage 4, increasing from 0.31 to 0.71 mg/100 g. In the pulp, however, content ranged from 0.08 to 0.17 mg/100 g (Figure 2A). The same behavior was observed for the betaxanthin content, with higher concentration in the peel (Figure 2B). Average betaxanthin content of 0.18 mg/100 g was observed for the pulp and 0.56 mg/100 g for the peel, in which there was a considerable increase until maturity stage 4 (1.14 mg/100 g), where the fruit showed a predominantly yellow color. The following maturity stages presented fruit with less amounts of betaxanthin in the peels.
The content of yellow flavonoids throughout maturation showed a significant difference between the peel and pulp (p < 0.01), especially from maturity stage 2. T. inamoena fruit showed an increased content from 1.51 to 5.21 mg/100 g in pulp, and from 1.14 to 9.11 mg/100 g in the peel. In both portions, it can be noticed that the content tends to stay nearly the same from maturity stage 4 onwards (Figure 3A). On the other hand, the total extractable polyphenol content of T. inamoena was evaluated just in the pulp, in which the amount increased from 11.21 to 29.84 mg GAE/100 g fresh weight (Figure 3B), following the trend of fruit maturation.
The correlation with the content of yellow flavonoids in T. inamoena pulp was positive (Table 2), showing that this metabolite participates positively in the increase of phenolic compounds during fruit maturation.
Total antioxidant activity of T. inamoena pulp showed that the amount of pulp (g) capable of reducing 50% the initial DPPH• concentration continuously decreased over time (Figure 3C). There was a decrease in EC50 from 1916.33 to 529.66 g FW/g DPPH•, indicating that fully ripe fruit (maturity stage 6) exhibits greater antioxidant activity at the end of maturation. The antioxidant activity of the pulp was highly correlated with total extractable polyphenols (R2 = -0.87), followed by total chlorophyll (R2 = 0.95), total carotenoids (R2 = -0.75), and yellow flavonoids (R2 = - 0.68). The changes in the betaxanthin content of pulp during maturation of T. inamoena fruit was correlated with antioxidant activity (Table 1).
The reduction in total chlorophyll content in T. inamoena fruit highlights the changes during its maturation, also characterized by increasing flavonoid, carotenoid, and betalain content as the major compounds present in both the pulp and peel, and to which different antioxidant properties are attributed in Cactaceae fruits (Felker et al., 2008; Castellar et al., 2012). Because of that, these compounds negatively correlate with flavonoids, carotenoids, and betalains in the peel and pulp (Table 1 and Table 2).
Generally, when chlorophylls decrease, carotenoids take the place of providing an attractive appearance to both the peel and pulp. T. inamoena fruit presented this trend probably because mature green fruit contains chloroplasts that differentiate into chromoplasts during maturation, and chlorophylls are degraded by specific enzymes, leading to an accumulation of carotenoids (Tanaka et al., 2008; Paliyath et al., 2009). Cactus fruits are poorly characterized with regard to the biosynthesis of coronoids, as these compounds are not the major constituent in the tissues of these fruits. However, different amounts have been reported for other cacti: 3.23 mg/100 g for pulp and 21.5 mg/100 g for the pericarp of Opuntia elatior (Alvarez et al., 2008), and 0.92 mg/100 g for Opuntia boldinghii (García-Pantaleón et al., 2009), showing that the values reported herein for T. inamoena fruit are quite lower. Souza et al. (2007) reported a content of 0.47 mg/100 g for the pulp and 3.37 mg/100 g for the pericarp of the same species studied herein. In addition to the attractive coloring, carotenoids have high antioxidant capacity with strong health promotion appeal (Cayupán et al., 2011).
Betacyanin is responsible for the reddish coloration and tends to increase with maturation (Castellar et al., 2012; Gandía-Herrero and García-Carmona, 2013). However, the content of this pigment in T. inamoena fruit is greatly reduced compared to other species of the Opuntia genus due to the prevalence of carotenoids, flavonoids and betalains of the betaxanthin class (Stintzing and Carle, 2007). Stintzing et al. (2005) observed for Opuntia ficus indica clones with an orange color that the betaxanthin content for the fully ripe fruit was around 76.3 mg/L, correlating with the antioxidant capacity. In a study performed by Castellanos-Santiago and Yahia (2008) with several species of the Opuntia genus, it was found that O. robusta showed betaxanthin content of 0.99 mg/100 g, O. streptacantha showed 1.04 mg/100 g, O. ficus-indica 0.14 mg/100 g, and O. megacantha showed 0.16 mg/100 g, thereby setting a wide content variation in the fruit pulp. Additionally, Naselli et al. (2014) have reported that isolated indicaxanthin from O. ficus-indica can be useful for cancer treatment. This indicates that T. inamoena fruit, as a natural source of this class of compound, can be investigated in this purpose.
Although there is information about the identification of betalainic and phenolic compounds and their antioxidant properties, there are few studies on events controlling the ripening in cactus fruits, especially the interrelations with the synthesis of functional compounds. However, some qualitative and quantitative studies have been developed relating the presence of antioxidant substances and maturation. Cayupán et al. (2011) assessed the orange and yellow varieties of Opuntia megacantha fruit in 5 maturity stages, finding that the increase in contents of ascorbic acid, polyphenols, and betalains are related to high antioxidant capacity in both the peel and pulp. The evaluation of fruit quality has to expand quality indexes that may be considered as an additional harvest criterion to those traditionally used, such as soluble solids, titratable acidity, and ratio.
The presence of functional compounds in fruits varies according to several factors. The most distinct pattern is generally observed between the peel and pulp, depending on the group of compounds concerned. With regard to T. inamoena, this is the first report regarding the quantification of bioactive compounds and their ability in capturing DPPH• radical as reported for the most world widely consumed O. ficus-indica varieties as well as for pitaya groups. Kim et al. (2011) reported differentiated content for both the peel and pulp of red and white pitaya with different antioxidant properties mainly due to the presence of phenolic compound groups such as yellow flavonoids, which comprise a diverse group still in demand of research. The determination of flavonoids and other phenolic groups in cactus fruits, especially those of natural occurrence in the semiarid region of Northeastern Brazil, has an important role as a value-adding tool (Silva et al., 2009). In this sense, it has been reported that quercetin and isorhamnetin are the main flavonoid compounds present in Opuntia spp. (Fernández-López et al., 2010; Matias et al., 2014).
Phenolic antioxidants represent most of the profile present in different fruit portions, and the presence of flavonoids and phenolic acids has been reported in flowers and cladodes of Opuntia spp. and Hylocereus spp. as the most significant components in their composition (Osorio-Esquivel et al., 2011; Tenore et al., 2012). The presence of phenolic acids can be mentioned, with the marked presence of protocatechuic, p-coumaric and ferulic acids, as well as flavonoids, especially taxifolin, myricetin, and isorhamnetin (Tenore et al., 2012; Cha et al., 2013; Dhaouadi et al., 2013). Isorhamnetin and its derivatives are the group of compounds with the highest expression in the composition of O. ficus-indica fruit, especially in the form of isorhamnetin 3-O-rutinoside (Matias et al., 2014). Chavez-Santoscoy et al. (2009) studied nine Opuntia cultivars and found a content of 22.63 mg GAE/100 g for Opuntia leucotricha juice (purple color pulp) and a content of 17.21 mg GAE/100 g for Opuntia ficus indica (reddish orange color pulp); values below those found in this study for the pulp of fully ripe T. inamoena fruit.
Sumaya-Martinez et al. (2011) reported that the red and purple varieties of Opuntia fruit have higher antioxidant activity using the DPPH• method, which is highly correlated with the content of vitamin C and phenolic compounds. This correlation has been reported for Opuntia spp. and Hylocereus spp. species (Stintzing et al., 2005; Beltrán-Orozco et al., 2009), where very high correlation between total phenolic content and antioxidant activity was verified. Polyphenols together with ascorbic acid were the most active metabolites among the chemical constituents of these fruits. In O. ficus-indica, the pulp has low antioxidant activity compared to the peel, and this behavior is also related to the high concentration of phenolic compounds in the peel compared to pulp (Dantas et al., 2015). This also can be seen in T. inamoena fruit herein where total extractable polyphenols had a higher correlation with extracts’ antioxidant activity by DPPH• method.
Osorio-Esquivel et al. (2011) found that the different portions of xoconostle fruit (O. joconostle Web.) have different profiles of phenolic compounds, flavonoids, and betacyanins, with a higher concentration of these metabolites in the fruit pericarp. In addition, the antioxidant activity, particularly the methanol extract, was highly correlated with such compounds, especially phenolic compounds. However, the use of the functional potential of cactus fruits depends on the maturity stage at which they are harvested, and this can be presumed from T. inamoena fruit, especially depending on the fruit portion and its stage of maturation.
T. inamoena fruit has a relevant amount of bioactive compounds, mainly phenolic compounds with high antioxidant activity. Changes in the antioxidant activity during maturation of this fruit are correlated to the decrease in pulp chlorophyll content, increased betalains, yellow flavonoids and phenolic compounds. Additionally, more mature fruits present larger amounts of compounds with high antioxidant activity. Finally, further studies are needed to understand the biological behavior of compounds present in T. inamoena fruit using in vivo models and clinical trials to assess the potential health benefits.
The authors have not declared any conflict of interest.
The National Council of Technological and Scientific Development supported this work (Process number 110436/2009-1).
REFERENCES
|
Allegra M, D'Acquisto F, Tesoriere L, Attanzio A, Livrea MA (2014). Pro-oxidant activity of indicaxanthin from Opuntia ficus indica modulates arachidonate metabolism and prostaglandin synthesis through lipid peroxide production in LPS-stimulated RAW 264.7 macrophages. Redox Biol. 2:892-900.
Crossref
|
|
|
|
Alvarez MJM, Pantaleón DG, Camacho DB, Martínez CM, Ojeda NM (2008). Análisis bromatológico de la tuna Opuntia elatior Miller (Cactaceae). Rev. Fac. Agron. 25(1):68-80.
|
|
|
|
Beltrán-Orozco MC, Oliva-Coba TG, Gallardo-Velázquez T, Osorio-Revilla G (2009). Ascorbic acid, Phenolic content, and Antioxidant capacity of red, cherry, yellow and white types of pitaya cactus fruit (Stenocereus stellatus Riccobono). Agrociencia 43(2):153-162.
|
|
|
|
Brahmi D, Ayed Y, Hfaiedh M, Bouaziz C, Mansour HB, Zourgui L, Bacha H (2012). Protective effect of cactus cladode extract against cisplatin induced oxidative stress, genotoxicity and apoptosis in balb/c mice: combination with phytochemical composition. BMC Complement. Altern. Med. 12(1):111.
Crossref
|
|
|
|
Brahmi D, Bouaziz C, Ayed Y, Mansour HB, Zourgui L, Bacha H (2011). Chemopreventive effect of cactus Opuntia ficus indica on oxidative stress and genotoxicity of aflatoxin B1. Nutr. Metab. 8(73):1-16.
Crossref
|
|
|
|
Castellanos-Santiago E, Yahia EM (2008). Identification and quantification of betalains from fruit of 10 Mexican prickly pear cultivars by high-performance liquid chromatography and electrospray ionization mass spectrometry. J. Agric. Food Chem. 56(14):5758-64.
Crossref
|
|
|
|
Castellar MR, Solano F, Obón JM (2012). Betacyanin and other antioxidants production during growth of Opuntia stricta (Haw.) fruits. Plant Foods Hum. Nutr. 67(4):337-43.
Crossref
|
|
|
|
Cayupán YSC, Ochoa MJ, Nazareno MA (2011). Health-promoting substances and antioxidant properties of Opuntia sp. fruits. Changes in bioactive-compound contents during ripening process. Food Chem. 126(2):514-519.
Crossref
|
|
|
|
Cha MN, Jun HI, Lee WJ, Kim MJ, Kim MK, Kim YS (2013). Chemical composition and antioxidant activity of Korean cactus (Opuntia humifusa) fruit. Food Sci. Biotechnol. 22(2):523-529.
Crossref
|
|
|
|
Chavez-Santoscoy RA, Gutierrez-Uribe JA, Serna-Saldívar SO (2009). Phenolic Composition, Antioxidant Capacity and In Vitro Cancer Cell Cytotoxicity of Nine Prickly Pear (Opuntia spp.) Juices. Plant Foods Hum. Nutr. 64(2):146-52.
Crossref
|
|
|
|
Dantas AL, Silva SM, Lima MACD, Dantas RL, Mendonça RMN (2013). Bioactive compounds and antioxidant activity during maturation of strawberry guava fruit. Rev. Ciênc. Agron. 44(4):805-814.
|
|
|
|
Dantas RL, Silva SM, Santos LF, Dantas AL, Lima RP, Soares LG (2015). Betalains and Antioxidant Activity in Fruits of Cactaceae from Brazilian Semiarid. Acta Hortic. 1067:151-157.
Crossref
|
|
|
|
Dhaouadi K, Raboudi F, Funez-Gomez L, Pamies D, Estevan C, Hamdaoui M, Fattouch S (2013). Polyphenolic extract of Barbary-fig (Opuntia ficus-indica) syrup: RP–HPLC– ESI–MS analysis and determination of antioxidant, antimicrobial and cancer-cells cytotoxic potential. Food Anal. Meth. 6(1):45-53.
Crossref
|
|
|
|
Felker P, Stintzing FC, Müssig E, Leitenberger M, Carle R, Vogt T, Bunch R (2008). Colour inheritance in cactus pear (Opuntia ficusâ€indica) fruits. Ann. Appl. Biol. 152(3):307-318.
Crossref
|
|
|
|
Fernández-López JA, Almela L, Obón JM, Castellar R (2010). Determination of Antioxidant Constituents in Cactus Pear Fruits. Plant Foods Hum. Nutr. 65(3):253-9.
Crossref
|
|
|
|
Gandía-Herrero F, García-Carmona F (2013). Biosynthesis of betalains: Yellow and violet plant pigments. Trends Plant Sci. 18(6):334-43.
Crossref
|
|
|
|
García-Pantaleón DM, Flores-Ortiz M, Moreno-Álvarez MJ, Belén-Camacho DR, Medina-Martínez CA, Ojeda-Escalona CE, Padrón-Pereira CA (2009). Chemical, biochemical, and fatty acids composition of seeds of Opuntia boldinghii Britton et Rose. J. Prof. Assoc. Cactus Dev. 11:45-52.
|
|
|
|
Higby WK (1962). A simplified method for determination of some the carotenoid distribution in natural and carotene-fortified orange juice. J. Food Sci. 27(1):42-49.
Crossref
|
|
|
|
Kim H, Choi HK, Moon JY, Kim YS, Mosaddik A, Cho SK (2011). Comparative Antioxidant and Antiproliferative Activities of Red and White Pitayas and Their Correlation with Flavonoid and Polyphenol Content. J. Food Sci. 76(1):38-45.
Crossref
|
|
|
|
Kim J, Jho KH, Choi YH, Nam SY (2013). Chemopreventive effect of cactus (Opuntia humifusa) extracts: Radical scavenging activity, pro-apoptosis, and anti-inflammatory effect in human colon (SW480) and breast cancer (MCF7) cells. Food Funct. 4(5):681-688.
Crossref
|
|
|
|
Lima DA (1989). Plantas das caatingas. Academia Brasileira de Ciências. 243 p.
|
|
|
|
Lucena CM de, de Lucena RFP, Costa GM, Carvalho TKN, da Silva Costa GG, da Nóbrega Alves RR, Pereira DD, da Silva Ribeiro JE, Alves CAB, Quirino ZGM, Nunes EN (2013). Use and knowledge of Cactaceae in Northeastern Brazil. J. Ethnobiol. Ethnomed. 9(1):62.
Crossref
|
|
|
|
Matias A, Nunes SL, Poejo J, Mecha E, Serra AT, Madeira PJ, Bronze MR, Duarte CM (2014). Antioxidant and anti-inflammatory activity of a flavonoid-rich concentrate recovered from Opuntia ficus-indica juice. Food Funct. 5(12):3269-3280.
Crossref
|
|
|
|
Naselli F, Tesoriere L, Caradonna F, Bellavia D, Attanzio A, Gentile C, Livrea MA (2014). Anti-proliferative and pro-apoptotic activity of whole extract and isolated indicaxanthin from Opuntia ficus-indica associated with re-activation of the onco-suppressor p16 INK4a gene in human colorectal carcinoma (Caco-2) cells. Biochem. Biophys. Res. Commun. 450(1):652-658.
Crossref
|
|
|
|
Nilson T (1970). Studies into the pigments in beetroot (Beta vulgaris L. ssp. vulgaris var. rubra L.). Lantbrukhogskolans Ann. 36(1):179-219.
|
|
|
|
Oliveira VB, Yamada LT, Fagg CW, Brando MGL (2012). Native foods from Brazilian biodiversity as a source of bioactive compounds. Food Res. Int. 48(1):170-179.
Crossref
|
|
|
|
Osorio-Esquivel O, Álvarez VB, Dorantes-Álvarez L, Giusti MM (2011). Phenolics, betacyanins and antioxidant activity in Opuntia joconostle fruits. Food Res. Int. 44(7):2160-2168.
Crossref
|
|
|
|
Paliyath G, Murr DP, Handa AK, Lurie S (2009). Postharvest biology and technology of fruits, vegetables, and flowers. John Wiley & Sons, 476 p.
|
|
|
|
Saénz C, Tapia S, Chávez J, Robert P (2009). Microencapsulation by spray drying of bioactive compounds from cactus pear (Opuntia ficus-indica). Food Chem. 114(2):616-622.
Crossref
|
|
|
|
Silva FVG, Silva SM, Silva GC, Mendonça RMN, Alves RE, Dantas AL (2012). Bioactive compounds and antioxidant activity in fruits of clone and ungrafted genotypes of yellow mombin tree. Food Sci Technol. 32(4):685–691.
Crossref
|
|
|
|
Souza ACMD, Gamarra-Rojas G, Andrade SAC, Guerra NB (2007). Características físicas, químicas e organolépticas de quipá (Tacinga inamoena, Cactaceae). Rev. Bras. Frutic. 29(2):292-295.
Crossref
|
|
|
|
Stintzing FC, Carle R (2007). Betalains – Emerging prospects for food scientists. Trends Food Sci. Technol. 18(10):514-525.
Crossref
|
|
|
|
Stintzing FC, Herbach KM, Mosshammer MR, Carle R, Yi W, Sellappan S, Akoh CC, Bunch R, Felker P (2005). Color, Betalain Pattern, and Antioxidant Properties of Cactus Pear (Opuntia spp.) Clones. J. Agric. Food Chem. 53(2):442-51.
Crossref
|
|
|
|
Sumaya-Martínez MT, Cruz-Jaime S, Madrigal-Santillán E, García-Paredes JD, Cari-o-Cortés R, Cruz-Cansino N, Valadez-Vega C, Martinez-Cardenas L, Alanís-García E (2011). Betalain, Acid Ascorbic, Phenolic Contents and Antioxidant Properties of Purple, Red, Yellow and White Cactus Pears. Int. J. Mol. Sci. 12(10):6452-68.
Crossref
|
|
|
|
Tanaka Y, Sasaki N, Ohmiya A (2008). Biosynthesis of plant pigments: anthocyanins, betalains and carotenoids. Plant J. 54(4):733-749.
Crossref
|
|
|
|
Tenore GC, Novellino E, Basile A (2012). Nutraceutical potential and antioxidant benefits of red pitaya (Hylocereus polyrhizus) extracts. J. Funct. Foods. 4(1):129-136.
Crossref
|
|
|
|
Tesoriere L, Butera D, Allegra M, Fazzari M, Livrea MA (2005). Distribution of betalain pigments in red blood cells after consumption of cactus pear fruits and increased resistance of the cells to ex vivo induced oxidative hemolysis in humans. J. Agric. Food Chem. 53(4):1266-1270.
Crossref
|