ABSTRACT
The effect of seasonality on abundance of the African weaver ant (AWA) was determined in the cashew fields at Bagamoyo and Kibaha districts, Coast Region of Tanzania. Twenty cashew trees colonized by AWA were randomly selected per site and its abundance was monitored during cashew on-seasons and off-seasons in 2011 and 2012. Results showed that abundance of AWA, which was expressed as mean numbers of leaf nests per tree and colonization of trails on main branches, varied significantly between cashew on-seasons and off-seasons. The mean numbers of leaf nests per tree during cashew on-season and off-season varied between 8.3 and 5.0 and between 7.5 and 4.8 at Bagamoyo and Kibaha, respectively, in 2011. Similarly, in 2012 it varied between 9.5 and 5.6 and between 8.6 and 5.3 at Bagamoyo and Kibaha, respectively. The mean percentage AWA colonization of trails during cashew on-seasons and off-seasons varied between 72.5 and 54.2% and between 73.3 and 50.9% in 2011and 2012; it also varied between 74.3 and 57.0% and between 72.6 and 54.9% at Bagamoyo and Kibaha, respectively. The abundance of AWA in the two parameters studied varies significantly between the two seasons. This suggests the use of conservation strategies during the off-seasons to supplement diets of AWA.
Key words: Colonization, Oecophyllalonginoda, off-season, on-season, trail.
Ants are more abundant and ubiquitous nearly in all types of terrestrial habitats, especially in the tropics (Kaspari, 2000; Fisher, 2010). They are considered as useful tools for biodiversity evaluation, monitoring purposes, quick response to environmental changes and relative ease of sampling (Kaspari and Majer, 2000; Bestelmeyer et al., 2000; Underwood and Fisher, 2006). Ants also play key roles in ecological processes namely nutrient cycling, energy turnover, pollination, seed dispersal and regulating populations of other insects (Hölldobler and Wilson, 1990; Andersen and Majer, 1991; Gomezi and Zamora, 1992). Ants are used as indicators of exposure to environmental stressors (Whitford, 1999; Wang et al., 2000), whereby their distributions are determined by rainfall patterns and seasonal temperatures (Lindsey and Skinner, 2001; El Keroumi et al. 2012). For example, higher abundance and richness values of ants were recorded during the dry season in the Moroccan Argan Forest (El Keroumi et al., 2012). On the other hand, in the semi-arid Karoo of South Africa, ant abundance and diversity were higher during summer than in winter (Lindsey and Skinner, 2001). The effect of seasons was also reported at species level, the common pugnacious ant (CPA), Anoplolepis custodiens Smith (Hymenoptera: Formicidae) was the most abundant species during summer, while Monomorium albopilosum Emery (Hymenoptera: Formicidae) was the most abundant species during winter (Lindsey and Skinner, 2001). Apparently, increase of primary productivity is the most important factor, which determines the abundance of ant species at a given area, followed by the temperature and seasonality (Kaspari et al., 2000).
Arboreal ants are partially herbivorous and they consume nectaries and hemipteran honeydew (Davidson et al., 2003; Blüthgen et al., 2004). More importantly, arboreal ant species of the genus Oecophylla are efficient in controlling insect pests (Hölldobler and Wilson, 1990). Members are commonly known also as the weaver ants, which consist of two species, the African weaver ant (AWA), O. Longinoda and the green tree ant, O. smaragdina. The distribution of these species depends on the vegetation, physical factors such as temperature and rainfall (directly or indirectly) and the abundance of competitor ant species namely Pheidole megacephala Fabricius and Anoplolepis custodiens Smith (Hymenoptera: Formicidae) (Lokkers, 1986). The AWA is widely distributed in Sub-Saharan Africa (SSA), particularly in the equatorial tropical forests (Hölldobler and Wilson, 1990; Van Mele and Cuc, 2007). In East Africa, the AWA is most abundant in the coastal forests of Kenya and Tanzania. More than 80 species of shrubs, cultivated and wild trees are used by AWA as host plants (Varela, 1992). The AWA plays an essential role in regulating populations of sap-sucking pests in East Africa (Seguni, 1997; Olotu et al., 2012) and West Africa (Van Mele et al., 2007; Dwomoh et al., 2009). In Tanzania, AWA colonies are widely distributed in coconut orchards (Varela, 1992; Seguni, 1997) and cashew orchards in Tanzania (Stathers unpublished; Olotu et al., 2012). It forms large polydomous colonies consisting of many leaf nests in the crowns of a wide range of host plant species (Varela, 1992). These host plants supply nectaries that supplement their diets (Way and Khoo, 1991; Blüthgen and Fiedler, 2002). Preliminary information showed that the abundance of AWA in cashew agro-ecosystem rely on availability of food resources mainly honeydew and the sap-sucking pests, which reaches their peak at the onset of cashew flowering periods (Stathers unpublished; Olotu et al., 2012).
Despite the importance of AWA in the control of sap-sucking insects, there is little information on the effect of seasonality on abundance of AWA in cashew agro-ecosystem in Tanzania. The present study therefore investigated the abundance of AWA with respect to cashew seasons (cashew on-seasons and off-seasons) in order to design conservation strategies for this useful natural enemy.
Experimental sites
Experiments were conducted in cashew fields from January to December in 2011 and 2012 at Bagamoyo (S 06° 49.3', E 38° 54.8', 53.43 m.a.s.l) and Kibaha (S 06° 33.4', E 38° 54.7', 150.57 m.a.s.l), Coast region of Tanzania. The mean temperature recorded at Bagamoyo and Kibaha during cashew off-seasons and on-seasons ranged between 30.1-31.2 and 31.4-32.2ËšC and that of rainfall ranged between 600-800 and 100-600 mm in 2011 and 2012. The cashew seasons are categorized as the cashew off-season and on-season. The former was considered as the inactive reproductive phase or period of non-flowering, nut set and fruiting, which usually occurs between January and June. The later was considered as the active cashew reproductive phase which is marked by new flushes of shoots and mass flowering followed by fruit and nut development, this reproductive phase usually occurs between July and December.
Quantification of AWA abundance
Abundance of arboreal ants is usually estimated indirectly by counting leaf nests per treeand ant trails on main branches (Peng and Christian, 2006) or counts of ants on selected plant parts (Blüthgen et al., 2004). Direct methods to count the ants are always disruptive to nest inhabitants, for example the partial opening of nests for enumerative purposes (Peng et al., 1998). AWA abundance can be considered as the total number of AWA leaf nests per trees and mean percentage of AWA trails on the main branches (AWA colonization). Twenty cashew trees were selected randomly per site. AWA abundance on each tree was quantified as follows: (i) all the leaf nests were carefully counted with the aid of binoculars, and (ii) the total number of main branches with AWA trails was recorded. More than ten AWA walking along a main branch was recorded as one AWA trail. Between one and ten AWA along the main branch was recorded as 0.5 AWA trail (Peng and Christian, 2006). The individual percentage of AWA trails on main branches was calculated as (i), the mean percentage of AWA trails on occupied trees in the field was calculated as (ii) and the average number of nests per AWA occupied tree was calculated as (iii).
(i) Number of main branches with a weaver ant trail in a tree)/(Number of main branches in the tree) x 100
(ii) Mean AWA trail colonization based on trails per tree was calculated asthe average of AWA colonization per field
(iii) Mean number of nests on AWA occupied trees per field was calculated as the sum of all nests counted/20 trees
AWA on a tree was treated as ‘abundant’ when more than 50% of the main branches had AWA trails, or as ‘fewer’ when less than 50% of the main branches had AWA trails. Twenty cashew trees with abundant AWA were randomly selected per site. Quantification of AWA abundance (that is, leaf nests and trails) was done monthly for two consecutive years.
Data analysis
Count and proportion data were transformed to Log (n+1) before being subjected to statistical analysis. Total number of AWA leaf nests during cashew on- and off seasons was analysed by means of the Behrens-Fisher t-test. Analysis of variance (ANOVA) was used to compare the mean difference between treatments, day and time and their interactions using STATISTICA version 11 (Stasoft, Inc., Tulsa, Oklahoma, USA). Bonferroni correction was used to adjust for multi means comparisons. The Bonferroni correction has been frequently considered as the most common way to control the family-wise error rate (McDonald, 2009).
AWA leaf nests
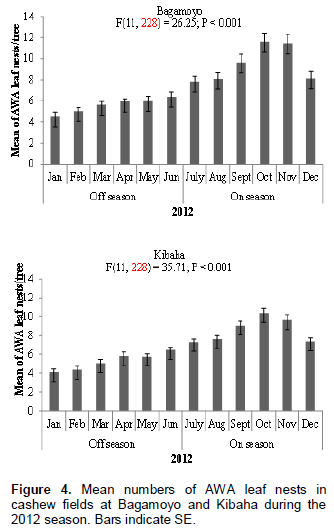
The nest of AWA is illustrated in Figure 1; it is constructed by gluing a single cashew leaf or multiple leaves with larval silk. Numbers of AWA leaf nests per tree in the cashew fields at Bagamoyo and Kibaha varied significantly at P < 0.05 between cashew on-seasons and off-seasons. At both sites more AWA leaf nests were recorded during cashew on-season than during off-season in both 2011 and 2012 (Figure 2). For example in 2011, 933 and 903 leaf nests were recorded during cashew on-seasons as compared to 593 and 577 leaf nests during off-seasons at Bagamoyo and Kibaha, respectively (Figure 2). The mean numbers of AWA leaf nests per tree varied according to month of the year at both sites: Bagamoyo (F(11,228) = 12.74; P < 0.001) and (F(11,228) = 26.25; P < 0.001) during 2011 and 2012, respectively; Kibaha (F(11,228)= 23.66; P < 0.001) and (F(11,228) = 35.71;P < 0.001) in 2011 and 2012, respectively (Figures 3 and 4).
AWA trails colonization
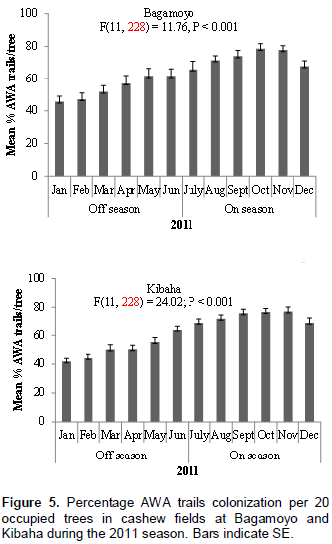
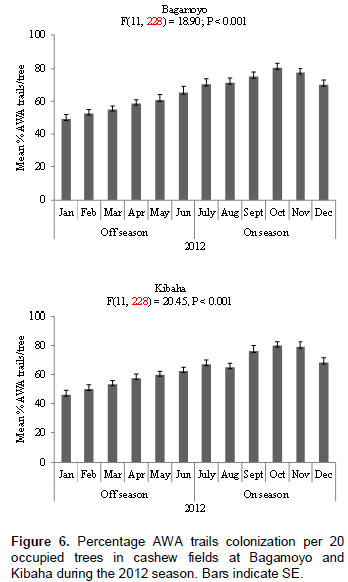
Similar to AWA leaf nests, AWA trails colonization was higher during cashew on-seasons than during off-seasons in the two cashew fields at Bagamoyo and Kibaha during 2011 and 2012 monitoring periods (Figures 5 and 6). For example in 2011, 72.5 and 73.3% trail colonization levels were recorded during cashew on-seasons as compared to 54.2 and 50.9% during off- seasons at Bagamoyo and Kibaha, respectively (Figure 5). The AWA trails colonization also varied according to season, with higher mean percentage during cashew on-seasons in both sites: Bagamoyo (F(11,228) = 11.76; P < 0.001 and F(11,228) = 18.90; P < 0.001) during 2011 and 2012 respectively; Kibaha (F(11,228) = 24.02; P < 0.001 and F(11,228) = 20.45; P < 0.001) in 2011 and 2012, respectively (Figures 5 and 6).
The abundance of AWA, expressed total number of AWA leaf nests per tree and their trails on the main branches, was high during cashew on-seasons than during off-seasons in the two sites and both years (2011 and 2012). This was probably due to cashew flowering, which occurs during the dry season of the year (Wait and Jamieson, 1986). The cashew reproductive season in the studied sites was also reported to prevail between August and December (Olotu et al., 2012). This could be due to fluctuations in food resource availability between cashew on-seasons and off-seasons. For example, nectar which is considered as an important source of food to arboreal ants is only available during new shoot flushing and flowering periods (Gottlieb et al., 2005; Stone et al., 1999).
Besides this, during the mass flowering, cashew trees provide nectaries for other purposes such as pollination attraction. However, these nectaries have also been reported to attract other insect fauna such as homopteran insects, Coccus hesperidum Linnaeus (Homoptera: Coccidae) and Hilda patruelis Stål (Homoptera: Tettigometridae) (Stathers unpublished). As a result, AWA also tended the homopteran insects for honeydew in cashew crops (Dwomoh et al., 2009; Olotu et al., 2012).
High abundance of AWA during cashew on-seasons could also be attributed to the occurrence of sap-sucking pests, Helopeltis anacardii Miller, and H. schoutedeni Reuter (Hemiptera: Miridae), and Pseudotheraptus wayi Brown (Hemiptera: Coreidae) during on-seasons. A similar observation was reported in coconut orchards, where an increase in Helopeltis spp. and P. wayi populations coincides with the main growing period of the crop, which begins shortly after the end of the rainy season in July or August, resulting in high abundances of AWA (Seguni, 1997). AWA abundance could also be associated with more nest building activities for establishment of colonies. Apart from nest building, the major workers are also responsible for foraging and defending the colony (Varela, 1992). This could be associated to their higher abundance during the cashew on-seasons to meet the demand for expansion of the colonies.
In conclusion, the abundance of AWA varies significantly between cashew on-seasons and off-seasons at the different sites of the Coast region of Tanzania. High numbers of AWA leaf nests per tree and high AWA trail colonization were recorded during cashew on-seasons compared to off-seasons. Therefore, conservation of AWA during cashew off-seasons is needed for high AWA abundance throughout the year. Provision of diet supplement especially by the use of fish-based bait and suppression of inimical and competitor ant species such as P. megacephala by sprinkling the granules of hydramethylon bait around the bases of the cashew trees are recommended for strengthening AWA colonies during cashew off-seasons. The use of fish based bait has been reported to be effective in supplementing their diets during seasons when food is scarce (Van Mele and Cuc, 2007). The suppression of the inimical ant using Hydramethylon ant bait (Amdro®) has been successfully used to control P. megacephala in coconut (Zerhusen and Rashid, 1992; Seguni, 1997).
The authors have not declared any conflict of interests.
The author acknowledges the Federal Ministry for Economic Cooperation and Development and the German Academic Exchange Service (DAAD), Germany, for providing financial support. He thanks International Centre of Insect Physiology and Ecology (icipe) in collaboration with African Insect Science for Food and Health, the African Regional Postgraduate Programme in Insect Science of icipe and the Mikocheni Agricultural Research Institute for overall assistance during this study and grateful to Messieurs J. Ambrosy, M. George, V. Nyange, B. Mruma and G. Mwingira for technical assistance. He is also grateful to Mr and Mrs Ponzi and M. Chimela for providing access to field sites and farm facilities.
REFERENCES
|
Andersen AN, Majer JD(1991).The structure and biogeography of rainforest ant communities in the Kimberley region of north-western Australia. In: McKenzie NL, Johnston RB, Kendrick PJ (eds), Kimberley rainforest of Australia. Surrey Beatty and Sons, Sydney. pp. 333-346.
|
|
|
|
Bestelmeyer BT, Agosti D, Alonso LE, Brandão CRF, Brown WLJ, Delabie JHC, Silvestre R (2000). Field techniques for the study of ground-dwelling ants: An overview, description, and evaluation. In: Agosti D, Majer JD, Alonso LE, Schultz TR (eds.), Ants standard methods for measuring and monitoring biodiversity. Smithsonian Institution, Washington D.C., USA, pp. 122-144.
|
|
|
|
|
Blüthgen N, Fiedler K (2002). Interactions between weaver ants Oecophyllasmaragdina, homopterans, trees and lianas in an Australian rain forest canopy.J. Anim. Ecol. 71:793-801.
Crossref
|
|
|
|
|
Blüthgen N, Stork NE, Fiedler K (2004). Bottom-up control and co-occurrence in complex communities: honeydew and nectar determine a rainforest ant mosaic. Oikos 106:344-358.
Crossref
|
|
|
|
|
Davidson DW, Cook SC, Snelling RR, Chua TH (2003). Explaining the abundance of ants in lowland tropical rainforest canopies. Sci. 300:969-972.
Crossref
|
|
|
|
|
Dwomoh EA, Afun JVK, Acknor JB, Agene VN (2009). Investigations on Oecophyllalonginoda (Latreille) (Hymenoptera: Formicidae) as a bio-control agent in the protection of cashew plantations. Pest Manage. Sci. 65:41-46.
Crossref
|
|
|
|
|
El Keroumi A, Naamani K, Soummane H, Dahbi A (2012). Seasonal dynamics of ant community structure in the Moroccan Argan forest.. J. Insect Sci. 94:172-181.
Crossref
|
|
|
|
|
Fisher BL (2010). Biogeography.In: L. Lach, C.L. Parr and K.L. Abbott (eds.), Ant ecology. Oxford University, New York, USA. pp. 18-37.
|
|
|
|
|
Gomezi JM, Zamora R (1992).Pollination by ants: consequences of the quantitative effects on a mutualistic system. Oecologia 91:410-418.
Crossref
|
|
|
|
|
Gottlieb D, Keasar T, Motro U (2005). Possible foraging benefits of bimodal daily activity in Proxylocopaolievieri (Lepeletier) (Hymenoptera: Anthophoridae). Environ. Entomol. 34:417-424.
Crossref
|
|
|
|
|
Hölldobler B, Wilson E (1990).The ants, Berlin: Springer Verlag. P 732.
Crossref
|
|
|
|
|
Kaspari M, Majer JD (2000). Using ants to monitor environmental change. In: Agosti D, Majer JD, Alonso LE, Schultz TR (eds.), Ants standard methods for measuring and monitoring biodiversity. Smithsonian Institution, Washington D.C., USA. pp. 89-98.
|
|
|
|
|
Kaspari M (2000). A primer on ant ecology. In: Agosti D, Majer JD, Alonso LE, Schultz TR (eds.), Ants standard methods for measuring and monitoring biodiversity. Smithsonian Institution, Washington D.C., USA. pp. 9-24.
|
|
|
|
|
Kaspari M, Alonso L, O'donnell S (2000).Three energy variables predict ant abundance at a geographical scale. Proc. R. Soc. Lond. Ser. B: Biol. Sci. 267:485-489.
Crossref
|
|
|
|
|
Lindsey PA, Skinner JD (2001). Ant composition and activity patterns as determined by pitfall trapping and other methods in three habitats in the semi-arid Karoo. J. Arid Environ. 48:551-568.
Crossref
|
|
|
|
|
Lokkers C (1986). The distribution of weaver ant, Oecophyllasmaragdina (Fabricius) (Hymenoptera, Formicidae) in Northern Australia. Aust J. Zool. 34:683-687.
Crossref
|
|
|
|
|
McDonald JH (2009). Handbook of Biological Statistics, 2nd Ed, University of Delaware. pp. 262-266.
|
|
|
|
|
Olotu MI, Du Plessis H, Seguni ZS, Maniania NK (2012). Efficacy of the African weaver ant Oecophyllalonginoda (Hymenoptera: Formicidae) in the control of Helopeltis spp. (Hemiptera: Miridae) and Pseudotheraptuswayi (Hemiptera: Coreidae) in cashew crop in Tanzania. Pest Manage. Sci. 69:911-918.
Crossref
|
|
|
|
|
Peng RK, Christian K (2006). Effective control of Jarvis's fruit fly, Bactrocerajarvisi (Diptera: Tephritidae), by the weaver ant, Oecophyllasmaragdina(Hymenoptera: Formicidae), in mango orchards in the Northern Territory of Australia. Int. J. Pest Manage. 52:275-282.
Crossref
|
|
|
|
|
Peng RK, Christian K, Gibb K (1998). Locating queen ant nests in the green ant, Oecophyllasmaragdina (Hymenoptera, Formicidae). Insectes Soc. 45:477-480.
Crossref
|
|
|
|
|
Seguni ZSK (1997). Biology and control of Pheidole megacephala (Hymenoptera: Formicidae, Myrmicinae) especially in relation to use of Oecophyllalonginoda (Formicidae, Formicinae) for biological control of Pseudotheraptuswayi (Heteroptera: Coreidae) in Tanzanian coconut cropping systems. PhD Dissertation, University of London.
|
|
|
|
|
StatSoft Inc.(2012). STATISTICA (data analysis software system), version 11. Available at:
View.
|
|
|
|
|
Stone GN, Gilbert F, Willmer PG, Potts S, Semida F, Zalat S (1999). Windows of opportunity in temporal structuring of foraging activity in desert solitary bee. Ecol. Entomol. 24:208-221.
Crossref
|
|
|
|
|
Underwood EC, Fisher BL (2006). The role of ants in conservation monitoring: if, when, and how. Biol. Conserv. 132:166-182.
Crossref
|
|
|
|
|
Van Mele P, Cuc NTT (2007). Ants as friends: Improving your Tree Crops with Weaver Ants, 2nd edition, Africa Rice Centre (WARDA), Cotonou, Benin, and CABI, Egham, UK. pp. 37-38.
|
|
|
|
|
Van Mele P, Vayssières JF, Van Tellingen E, Vrolijks J (2007). Effects of the African weaver ant Oecophyllalonginodain controlling mango fruit flies (Diptera: Tephritidae) in Benin. J. Econ. Entomol.100:695-701.
Crossref
|
|
|
|
|
Varela AM (1992). Role of Oecophylla longinoda (Formicidae) in control of Pseudotheraptus wayi (Coreidae) on coconut in Tanzania. PhD dissertation, University of London.
|
|
|
|
|
Wait AJ, Jamieson GI (1986).The cashew, its botany and cultivation. Queensl. Agric. J. 112:253-25.
|
|
|
|
|
Wang C, Strazanac J, Butler L (2000). Abundance, diversity, and activity of ants (Hymenoptera: Formicidae) in oak-dominated mixed Appalachian forests treated with microbial pesticides. Environ. Entomol. 29:579-586.
Crossref
|
|
|
|
|
Way MJ, Khoo KC (1991). Colony dispersion and nesting habits of the ants, Delichoderus thoracicus and Oecophylla smaragdina (Hymenoptera: Formicidae), in relation to their success as biological control agents on cocoa. Bull. Entomol. Res. 81:341-350.
Crossref
|
|
|
|
|
Whitford WG (1999). Seasonal and diurnal activity patterns in ant communities in a vegetation transition region of south-eastern New Mexico (Hymenoptera: Formicidae). Sociobiol. 34:477-491.
|
|
|
|
|
Zerhusen D, Rashid M (1992) Control of the big-headed ant Pheidole megacephala Fabricius (Hymenoptera: Formicidae) with the fire ant bait 'AMDRO' and its secondary effect on the population of the African weaver ant Oecophyllal onginoda Latreille (Hymenoptera: Formicidae). J. Appl. Entomol. 113:258-262.
Crossref
|
|