ABSTRACT
The competitiveness of Brazilian soybean in the international market is highly dependent on biological nitrogen fixation, whose efficiency is related to factors that affect the survival of the bacteria, such as the chemicals used in the treatment of seeds. The study objective was to evaluate Bradyrhizobium pre-inoculation (10 days before sowing) of soybean seeds treated with fungicides and insecticide compared to the standard inoculation performed on the planting day. Four experiments were conducted in two distinct ecosystems, two in Brazilian savanna (Cerrado) and two in Cerrado/forest transition areas of Roraima state. In each ecosystem, the experiments were performed in areas without and with soybean cultivation history. The treatments were in: 1- Control without inoculation; 2- inoculation on sowing day in untreated seeds; 3- treatment with carbendazim; 4- treatment with pyraclostrobin + methyl thiophanate + fipronil; 5- treatment with fludioxonil + metalaxyl-M. Groups 3, 4, and 5 were pre-inoculated 10 days before sowing. Viable cells in the seeds were recovered on the sowing day. Nodule number and dry mass, root, and aerial part dry mass were evaluated 35 days after emergence. Grain yield was evaluated at harvest. The number of viable cells was negatively affected by seed treatment. For all evaluated variables, treatments with pre-inoculation plus fungicides/insecticide were similar to the standard sowing-day inoculation. Pre-inoculation performed 10 days before planting, along with seed treatment with fungicides/insecticides, positively affected soybean crop productivity and could be used without compromising the nitrogen fixation.
Key words: Glycine max (L.) Merr., biological nitrogen fixation, Amazon.
Soybean (Glycine max L. Merrill) is a plant of the Fabaceae family very important to agricultural, with significant economic and social impact and it is the main cultivated crop in Brazil (Cattelan and Dall’Agnol, 2018). Brazil soybean production for 2017/18 is estimated at 112 million metric tons and harvested area is estimated at 35 million hectares, thus, Brazil and United States of America are currently the world’s largest producers (USDA, 2018). Nitrogen is the most required element by soybean and it can be supplied by the use of nitrogen fertilizers and by the biological nitrogen fixation (Hungria et al., 2007). Most of nitrogen requirements to cultivation of soybean in Brazil is provide by inoculation with selected nitrogen-fixing bacteria, Bradyrhizobium japonicum, Bradyrhizobium elkanii and Bradyrhizobium diazoefficiens (Hungria and Mendes, 2015). These bacteria can covert atmospheric nitrogen to ammonia and the inoculation with Bradyrhizobium is an indispensable technology in Brazilian soybean production. This technology is used in practically all soybean-producing regions in Brazil and generates an economy of over 15 billion US dollars that would have to be spent with nitrogen fertilizer each season (Hungria and Mendes, 2015; Araújo et al., 2017). It makes Brazilian soybean highly competitive in the international market (Hungria et al., 2005). The effectiveness of symbiosis between Bradyrhizobium and soybean plants depends on the inoculation process carried out by the producers on the farm. For example, seed treatment with fungicides may kill the bacteria in the moment of inoculation and compromise productivity (Zilli et al., 2009; Costa et al., 2013).
Given this widespread use of inoculation, Brazilian researchers have sought alternatives to maximize the efficiency of biological nitrogen fixation (BNF), through inoculation methods, compatibilization of fungicides, micronutrients, and other methods (Campo and Hungria, 1999). The treatment of seeds with fungicides, in addition to controlling important pathogens that are transmitted via seeds, is an efficient practice to ensure adequate populations of plants under soil-climatic conditions unfavorable to the germination and emergence of soybean (Balardin et al., 2011). Water deficiency, in particular, slows the germination and emergence process and lengthens seed exposure to soil fungi (Rhizoctonia solani, Pythium spp., Fusarium spp., Aspergillus spp., and others), that may cause seedling decay or death (Embrapa, 2011; Henning et al., 1997). However, fungicides can result in mortalities of up to 60% after 2 h in contact with Bradyrhizobium cells and 95% after 24 hr (Campo et al., 2009). Thus, compatibilizing the application of fungicides and inoculants is necessary to guarantee a higher bacterial population in the seeds and consequently increase nodulation in the roots, thereby increasing biological nitrogen fixation efficiency and crop productivity.
Although inoculation with nitrogen-fixing bacteria is used in most Brazilian soybean crops, seed inoculation at the time of sowing is often described as an activity that reduces planting efficiency due to time spent on inoculation operations. This makes some producers to not use inoculation (Campo and Hungria, 2006). Pre-inoculation may enable faster sowing and increase the use of inoculation, particularly by producers in areas with limited planting time. Pre-inoculation of seed is an alternative method to increase the planting efficiency and new technologies are being developed to inoculant companies in Brazil (Araújo et al., 2017). Thus, new pre-inoculants that are compatible with fungicide and/or insecticide treatment of seeds can enable faster sowing and increase the use of inoculation. The objective of this study was to compare pre-inoculation of fungicide and insecticide-treated soybean seeds 10 days before sowing with the standard inoculation performed on the sowing day.
Field experiments
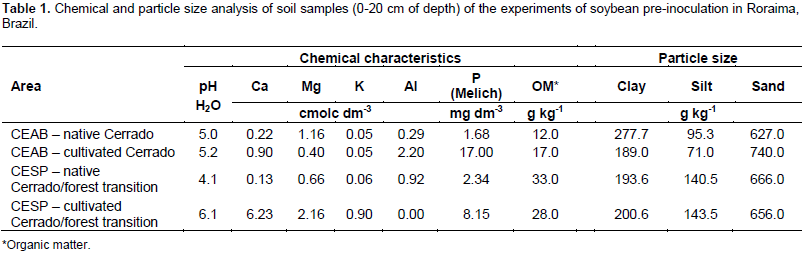
Four experiments were conducted. Two experiments were conducted at the Água Boa Experimental Field (CEAB) (02°40'10.7" N, 60°50'55.8" W) in the municipality of Boa Vista, which is in the Brazilian savanna (Cerrado) ecoregion and has dystrophic Yellow Argisol. One CEAB experiment was conducted in an area without soybean and one in an area with at least two years of soybean cultivation. Two experiments were conducted at the Serra da Prata Experimental Field (CESP) (02°23'25.3" N, 060°58'59.8" W) in the municipality of Mucajaí, which is in a Cerrado/forest transition ecoregion and has dystrophic Yellow Latosol. One CESP experiment was conducted in an area without soybean and the other in an area with at least two years of soybean cultivation. Both experimental fields belonged to Embrapa Roraima. Chemical characterization of the soil at 0 to 20 cm depth was performed for each area before the beginning of the experiments (Table 1), following the procedures described in Embrapa (1999). Soil correction was performed two months before soybean planting. Liming was performed with dolomitic limestone. The CEAB area received 1500 kg ha-1, and the CESP area received 2000 kg ha-1, applied by scattering and incorporated via plowing and two harrows. The soil in undisturbed areas with no soybean history at both CEAB and CESP was corrected with 100 kg ha-1 P2O5 in the form of single superphosphate, 60 kg ha-1 K2O in the form of KCl, and 50 kg ha-1 of FTE BR12 Nutriplant (7.1% Ca, 5.7% S, 1.8% B, 0.8% Cu, 2.0% Mn, 0.1% Mo, and 9.0% Zn) as a source of micronutrients.
In addition to correcting the fertilization, a planting fertilization was conducted in the sowing furrow according to that recommended for the region (Embrapa, 2005). It used 100 kg ha-1 of P2O5 in the form of single superphosphate and 90 kg ha-1 of K2O in the form of potassium chloride, the latter being applied 50% at planting and 50% at 35 days after germination (Embrapa, 2005). The experimental design for the four experiments was in randomized blocks with five treatments and four replicates. The treatments tested were:
(1) Control without inoculation
(2) Inoculation of untreated seeds with standard liquid inoculant on the day of planting
(3) Pre-inoculation of carbendazim (500 g L-1; Derosal® 500 sc, Bayer S.A.,Brazil) fungicide-treated seeds with liquid inoculant 10 days before planting.
(4) Pre-inoculation of pyraclostrobin (25 g L-1) + methyl thiophanate (225 g L-1) fungicide-treated and fipronil (250 g L-1; Standak Top®, BASF, Brazil) insecticide-treated seeds with liquid inoculant 10 days before planting.
(5) Pre-inoculation of fludioxonil (25 g L-1) + metalaxyl-M (10 g L-1; Maxim XL, Syngenta, Brazil) fungicide-treated seeds with liquid inoculant 10 days before planting.
These fungicides and insecticide were chosen because they are widely used in soybean cultivation in Brazil. Each experimental plot measured 5 m x 5 m, with 10 furrows spaced 0.50 m apart, with a spacing of 1.0 m between plots. The soybean cultivar used was BRS Tracajá, recommended for the state of Roraima, Brazil, with a stand of 14 plants per linear meter (Gianluppi et al., 2001).
At 35 days after germination (at flowering), 10 plants were sampled from each experimental plot (from the central area of second line of each plot). First, the shoots parts were collected and stored in Kraft paper bags. Then, with the aid of a straight blade, the roots were removed along with their nodules, which were stored in plastic bags until arrival in the laboratory. The number of nodules (NN), nodule dry mass (NDM), root dry mass (RDM), shoots dry mass (SDM), and total dry mass (TDM) were evaluated in the soil microbial laboratory of Embrapa Roraima. All dry mass variables were determined by drying the material at 65°C for 72 h. At 118 days, when the physiological maturation of the soybean was reached, the grains were harvested. For evaluation purposes, the six central rows of each plot were collected, always leaving one meter of border at the head of the rows. Grain yield was corrected to 13% moisture and is expressed in kg ha-1.
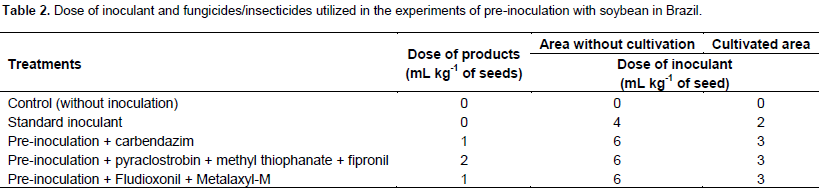
Seed treatment
Ten days prior to planting, seed treatment and inoculation of groups 3, 4, and 5 were performed. Seed treatment and pre-inoculation were performed according to manufacturer recommendations (Table 2). For areas with no history of soybean cultivation, the inoculant dose was twice that recommended for cultivated areas, due to the absence of an population of Bradyrhizobium that could establish symbiosis with soybean, following the manufacturer’s instructions. The seeds were packed in a polyethylene bag with a capacity three times the volume of the seeds. First, the fungicide was added, leaving an equivalent air volume to that occupied by the seeds, closing the bag and mixing with rotary movements to homogenize the distribution of the products. The treated seeds were transferred to a tray to dry for 2 h. The same procedure was used for seed inoculation. After drying, these were packed in a Kraft paper bag and stored at 20 to 30°C for 10 days. The liquid inoculants used in the treatments were Rizoliq® (standard inoculant, lot B01038203) and Rizoliq Top® (pre-inoculant, lot T040239), both supplied by Rizobacter of Brazil. These bacterial inoculants had SEMIA 5079 and SEMIA 5080 (Bradyrhizobium japonicum) with declared concentrations of 5.0 x 109 (standard inoculant) and 6.0 x 109 (pre-inoculant) colony-forming units (CFUs) per mL. On the tenth day after pre-inoculation, field-planting of all treatments was conducted. Inoculation of group 2 (standard inoculant without seed treatment) was conducted on the day of planting according to the manufacturer’s recommendation.
Viable cell recovery testing
For all treatments, after field planting, pre-inoculated seed samples were taken to the soil microbiology laboratory of Embrapa Roraima for viable cell recovery testing, following the specifications listed in the Regulatory Instructions No. 30, December 12, 2010 (Brazil, 2010). Samples of 100 seeds (considering the weight of 100 seeds of cultivar BRS Tracajá), were placed in sterile Erlenmeyer flasks with a capacity of 250 to 300 mL, containing 100 mL of physiological solution with Tween 80 (polyoxyethylene sorbitan monolaurate) (8.5 g of NaCl in 1.0 L of distilled water, added to 0.4 mL stock solution of Tween 80). This represented the 10° dilution. The Erlenmeyer flask was placed on an orbital shaker for 15 min at 150 rpm. A 1-mL aliquot of dilution 10° was withdrawn, placed in a sterile flask with 9 mL of physiological solution (0.85% NaCl), yielding the 10-1 dilution. This procedure was repeated until the 10-7 dilution was obtained. Samples of the 10-3 to 10-7 dilutions (0.1 mL) were plated in triplicate, by means of the spreading technique, in Petri dishes containing Yeast Extract Mannitol medium with Congo red (0.05%) (Fred and Waksman, 1928). The plates were incubated at 28°C for seven days, and the number of CFUs was estimated (between 30 and 300 colonies). The number of bacteria recovered from the seeds was obtained by the following formula: No. of recovered cells / seed = f x N, where f = dilution factor and N = average number of colonies of the three plates for the selected dilution. The dilution factor is given by the reciprocal of the dilution in the plate multiplied by 10, in the case of inoculation of 0.1 mL (Brazil, 2010).
Statistical analysis
All the evaluated variables were subjected to the tests of normality and homogeneity of the error variance. When these requirements were met and showed significance in the analysis of variance for the F test, the means of the treatments were compared by the Tukey test at the 5% significance level using the Sisvar version 4.3 program (Ferreira, 2011). The data were analyzed jointly to verify the effects of treatments (inoculations), places (Cerrado and Cerrado/forest transition), and interactions between treatments and places in areas with and without a history of soybean cultivation.
No cells were recovered in the control treatment without inoculation, indicating that the seeds were free of Bradyrhizobium cells (Table 3). Seeds treated on the day of planting with the standard inoculant showed a higher number of cells per seed than pre-inoculated seeds treated with fungicides and insecticide, with 4.7 × 106 CFU seeds-1 (seeds intended for areas with no soybean history) and 1.2 × 106 CFU seeds-1 (seeds intended for areas already grown with soybean) recovered, respectively. These values are in accordance with the recommended inoculant dosage for soybean, which should result in 1.2 × 106 cells per seed (Hungria et al., 2007). However, all products used for seed treatment drastically reduced the number of cells recovered 10 days after inoculation. The pre-inoculated group that received the pyraclostrobin + methyl thiophanate + fipronil showed the lowest number of cells recovered per seed for the two ecosystems (Table 3). These results differ from those obtained by Alcântara Neto et al. (2014), in which the product pyraclostrobin + methyl thiophanate + fipronil had the lowest deleterious effect on cell recovery in the seeds. However, those authors only evaluated for a period up to 48 h. Similar results were observed by Costa et al. (2013), they observed a drop in the concentration of cells after four days of inoculation when using seed treatment products, using similar methods, but with different active principles.
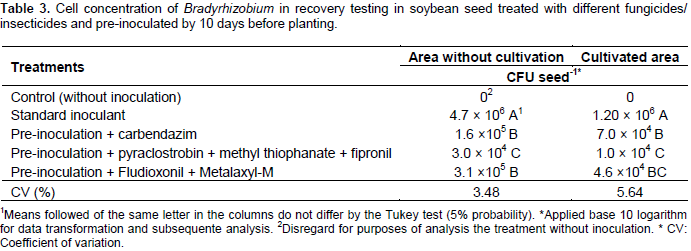
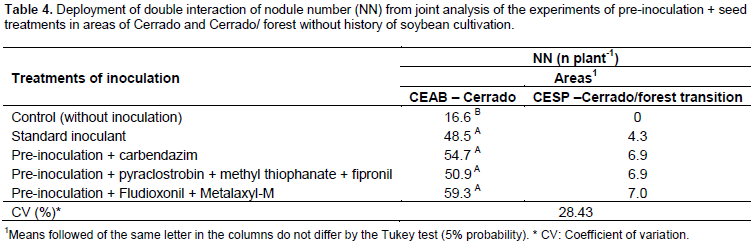
Several studies have demonstrated a reduction in the number of cells recovered in soybean seeds treated with different fungicides and inoculated with standard inoculants (Campo and Hungria, 2000; Campo et al., 2003; Campo et al., 2009; Costa et al., 2013; Alcântara Neto et al. al., 2014). This is the first result demonstrating the recovery of cells in soybean seeds after 10 days of inoculation with three different products to seed treatment used in Brazil, demonstrating that there is a drastic reduction but not total mortality of the cells after this period using an inoculant specific to pre-inoculation. A recent study of soybean pre-inoculation of seeds treated with pyraclostrobin + methyl thiophanate + fipronil and using a bacterial protector showed that after 35 days of inoculation it was possible to recover 1.13 × 10-3 CFU seed-1 (Araújo et al., 2017). Therefore in pre-inoculation of soybean seeds treated with fungicides/insecticides, specific inoculants with a kind of cell protection must be used. As for the results of the four experiments under field conditions, the combined data analysis showed a significant interaction between inoculation treatments X places only for the number of nodules in areas with no history soybean cultivation (Table 4). For the experiment in the Cerrado area in the CEAB, the treatment of seeds even with a drastic reduction in the number of cells was sufficient to induce nodulation, with results similar to the standard inoculant on the day of planting. For the Cerrado/forest transition area, the environment was a determining factor for the reduction of nodulation, since all the treatments showed a low number of nodules (Table 4).
For the other variables evaluated, the pre-inoculated and seed treatments did not differ from the standard inoculant treatment performed on the day of planting, and both were superior to the group that did not receive inoculation (Table 5). Although there were no significant differences, all the pre-inoculated treatments showed a trend of grain yield higher than the treatment using the standard inoculant. On the other hand, the control, which did not receive seed treatment or standard inoculation, was significantly lower in the number of nodules, nodule dry mass, root dry mass, shoot dry mass, total dry mass, and grain yield (Table 5). These results reinforce the importance of soybean inoculation in areas that were not previously cultivated in Brazil. We also observed a statistically significant difference between the places (Cerrado and Cerrado/forest transition) (Table 5). The Cerrado area (CEAB) showed more than three times the nodule dry mass as the Cerrado/forest transition areas. The nodule dry mass has a direct correlation with N content (Döbereiner, 1966) and consequently with shoot dry mass and grain yield. The shoots dry mass of plants cultivated in the Cerrado showed an average of 1.9 grams plant-1 more than the Cerrado/forest transition area and a yield of grain of 1461.9 kg ha-1 higher (Table 5).
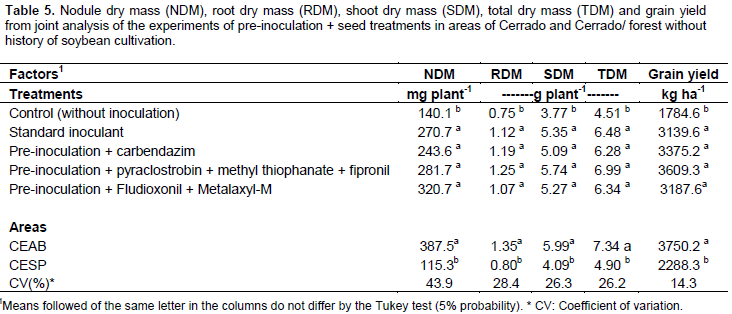
In areas where soybean had already been cultivated, there was no significant interaction between treatments and places. In these areas, shoot dry mass, total dry mass, and grain yield differed between treatments (Table 6). All the treatments inoculated showed superior results to the control that received neither inoculation nor addition of nitrogen. These results reinforce the need to carry out re-inoculation in areas that have previously been cultivated with soybeans, especially in the Roraima Cerrado, where the Bradyrhizobium population in the dry season is drastically reduced (Zilli et al., 2013). In the areas without soybean cultivation, the pre-inoculation with seed treatment did not affect the evaluated variables, as they were similar to the treatment with standard inoculant applied on the day of planting (Table 6). Regarding the places, there was also a significant effect on shoot dry mass, total dry mass, and grain yield, being also higher in the Cerrado areas (Table 6). The deleterious effects of fungicide/insecticide application on nodulation and soybean yield have been inconsistent. Some studies have demonstrated a reduction in nodulation in inoculated and fungicide-treated plants in areas with no soybean cultivation history where there is no established Bradyrhizobium population (Zilli et al., 2009; Costa et al., 2013).
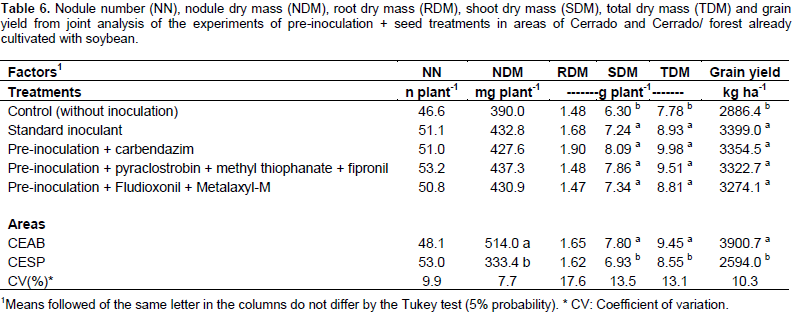
In soil without a history of soybean cultivation, Costa et al. (2013) demonstrated a reduction of up to 50% in nodulation of plants inoculated and treated with different fungicides. When cultivated in an area where there was an established population of bacteria, that is, areas that had already been cultivated with soybeans, there was no reduction in nodulation in soybean plants grown under greenhouse conditions (Bueno et al., 2003; Costa et al., 2013). Campo et al. (2009) observed a decrease in nodulation and productivity of plants that were treated with fungicides, and these effects were higher in sandy soils. Treatment with pyraclostrobin + methyl thiophanate + fipronil did not affect nodulation compared to control without seed treatment (Alcântara Neto et al., 2014). Concerning pre-inoculation, there were no negative effects on the development of soybean for up to five days in the Cerrado of Roraima. However, pre-inoculation together with the treatment of seeds with fungicides (carboxin + thiram) reduced the nodulation, nodule dry matter, shoot dry matter, and grain yield (Zilli et al., 2010). Analogously, Zilli et al. (2009) observed a negative effect of the application of the fungicide based on carbendazim + thiram, with a significant reduction (approximately 50%) of the nodulation and more than 20% (approximately 700 kg ha-1) of grain yield in the group inoculated with the strain SEMIA 587.
The negative results of the treatment of inoculated seeds on the day of planting (Bueno et al., 2003; Campo and Hungria, 2000; Campo et al., 2009; Zilli et al., 2009) with inoculation plus seed treatments (Zilli et al., 2010) were both obtained with standard inoculants. However, in this study, a new inoculant developed for the pre-inoculation was evaluated, and despite the reduction in the number of cells recovered in the seeds, these were sufficient to promote the symbiotic efficiency without affecting the productivity. A recent study of pre-inoculation using a bacterial protector in seeds also found a reduction of Bradyrhizobium cells, but the nitrogen fixation and productivity in the field were not affected in areas with and without history of soybean cultivation (Araújo et al., 2017). It is a new technology will be a valuable tool to soybean producers in Brazil and worldwide. Pre-inoculation along with the fungicides and insecticide promoted nodulation, plant development, and grain yield similar to the standard inoculant applied on the day of planting without seed treatment. In the two environments where the evaluations were conducted (Cerrado and Cerrado/forest transition), and in both native and soybean-cultivated areas, a similar response was observed both for the treatments inoculated with the control treatment and for the treatments where standard inoculant and pre-inoculant were applied.
Application of the inoculant to pre-inoculation in seeds treated with fungicides and insecticides reduces the number of cells recovered per seed. Pre-inoculation performed 10 days before planting, along with seed treatment, positively affects soybean crop productivity. The two places tested, a without soybean cultivation and an area already cultivated with soybean, presented highly similar responses.
The authors have not declared any conflict of interests.
The sincere thanks go to Brazilian Agricultural Research Corporation (Embrapa) and Rizobacter of Brazil for providing financial support.
REFERENCES
|
Alcântara Neto F, Pacheco LP, Araújo ASF, Petter FA, Almeida FA, Albuquerque JAAA (2014). Tempo de contato e de combinações de fungicidas, aditivo e inoculante sobre a sobrevivência de rizóbios e nodulação da soja. Ver. Rev. Agro@mbiente On-line 8(1):149-154.
|
|
|
|
Araújo RS, Cruz SP, Souchie EL, Martin TN, Nakatani AS, Nogueira MN, Hungria M (2017). Preinoculation of soybean seeds treated with agrichemicals up to 30 days before sowing: technological innovation for large-scale agriculture. Int. J. Microbiol. 11 p.
Crossref
|
|
|
|
|
Balardin RS, Silva FD, Debona D, Corte GD, Favera DD, Tormen NR (2011). Tratamento de sementes com fungicidas e inseticidas como redutores dos efeitos do estresse hídrico em plantas de soja. Ciênc. Rural 41(7):1120-1126.
Crossref
|
|
|
|
|
Brazil (2010). Ministry of Agriculture, Livestock and Food Supply, Normative Instruction nº 30, November 12, 2010. Brasília, DF.
|
|
|
|
|
Bueno CJ, Meyer MC, Souza NL (2003). Efeito de fungicidas na sobrevivência de Bradyrhizobium japonicum (SEMIA 5019 e SEMIA 5079) e na nodulação da soja. Acta Sci. Agron. 25(1):231-235.
Crossref
|
|
|
|
|
Campo RJ, Hungria M (2000). Compatibilidade de uso de inoculantes e fungicidas no tratamento de sementes de soja. Londrina, Embrapa Soja. (Circular Técnica, 26) 32 p.
|
|
|
|
|
Campo RJ, Araujo RS, Hungria M (2009). Nitrogen fixation with the soybean crop in Brazil: Compatibility between seed treatment with fungicides and Bradyrhizobial inoculant. Symbiosis 48(1-3):154-163.
Crossref
|
|
|
|
|
Campo RJ, Hungria M (1999) Efeito do tratamento de sementes de soja com fungicidas na nodulação e fixação simbiótica do N2. Londrina, Embrapa Soja. (Pesquisa em Andamento,21) 7 p.
|
|
|
|
|
Campo RJ, Hungria M, Laureio E, Miura LM, Sibaldelli RNR, Morais JZ, Souza MP (2003). Compatibilidade de aplicação de inoculantes com defensivos agrícolas e micronutrientes. In: Campo, C. B. H.; Saraiva, O. F. (Org.). Resultados de pesquisa da Embrapa Soja-2002. Londrina, Embrapa Soja. (Documentos, 216) pp. 20-38.
|
|
|
|
|
Campo RJ, Hungria M (2006). Protocolo para análise da qualidade e da eficiência agronômica de inoculantes, estirpes e outras tecnologias relacionadas ao processo de fixação biológica do nitrogênio em leguminosas. In: Anais da 13ª Reunião da Rede de Laboratórios para Recomendação, Padronização e Difusão de Tecnologias de Inoculantes de Interesse Agrícola, Londrina. Anais, Embrapa Soja pp. 89-123.
|
|
|
|
|
Cattelan AJ, Dall'Agnol A (2018). The rapid soybean growth in Brazil. OCL 2018, 25(1):D102.
Crossref
|
|
|
|
|
Costa MR, Cavalheiro JCT, Goulart ACP, Mercante FM (2013). Sobrevivência de Bradyrhizobium japonicum em sementes de soja tratadas com fungicidas e os efeitos sobre a nodulação e a produtividade da cultura. Summa Phytopathol. 39(3):186-192.
Crossref
|
|
|
|
|
Döbereiner J (1966). Evaluation of nitrogen fixation in legumes by the regression of total plant nitrogen with nodule weight. Nature 21:850-852.
Crossref
|
|
|
|
|
Embrapa (1999). Manual de análises químicas de solos, plantas e fertilizantes. Rio de Janeiro, Embrapa Solos 370 p.
|
|
|
|
|
Embrapa (2005). Cultivo de soja no cerrado de Roraima. Boa Vista, Embrapa Roraima 135p. (Sistemas de Produção, 1).
|
|
|
|
|
Embrapa (2011). Tecnologias de produção de soja – região central do Brasil 2012 e 2013. Londrina, Embrapa Soja. 261 p. (Sistemas de Produção, 15).
|
|
|
|
|
Ferreira DF (2011). SISVAR: A computer statistical analysis system. Rev. Ciênc. Agrotec. 35(6):1039-1042.
Crossref
|
|
|
|
|
Fred EB, Waksman S (1928). Manual of general microbiology. New York, McGraw Hill 145 p.
|
|
|
|
|
Gianluppi V, Smiderle OJ, Gianluppi D, Nascimento Junior A (2001). Cultivares de soja recomendadas para as áreas de Cerrado de Roraima. Boa Vista, Embrapa Roraima (Comunicado Técnico, 01) 5 p.
|
|
|
|
|
Henning AA, Campo R, Sfredo GJ (1997). Tratamento com fungicidas, aplicação de micronutrientes e inoculação de sementes de soja. Londrina, Embrapa Soja 6p. (Comunicado Técnico, 58).
|
|
|
|
|
Hungria M, Campo RJ, Mendes IC (2007). A importância do processo de fixação biológica de nitrogênio para a cultura da soja: Componente essencial para a competitividade do produto brasileiro. Londrina, Embrapa Soja (Documentos, 283) 80 p.
|
|
|
|
|
Hungria M, Franchini JC, Campo RJ, Graham PH (2005). The importance of nitrogen fixation to soybean cropping in South America. In: Werner D & Newton WE (Eds.) Nitrogen fixation in agriculture: forestry ecology and environment. Dordrecht, Kluwer Academic Publishers pp. 25-42.
Crossref
|
|
|
|
|
Hungria M, Mendes IC (2015). Nitrogen fixation with soybean: the perfect symbiosis? In: Biological Nitrogen Fixation, Brujin FJ (Ed.), Wiley & Sons, Inc, NJ, USA, pp. 1005-1019.
Crossref
|
|
|
|
|
United States Deparment of Agriculture (USDA) (2018). United States Deparment of Agriculture.
|
|
|
|
|
Zilli JE, Campo RJ, Hungria M (2010). Eficácia da inoculação de Bradyrhizobium em pré-semeadura da soja. Pesqui. Agropecu. Bras. 45(3):335-338.
Crossref
|
|
|
|
|
Zilli JE, Pereira GMD, França Júnior I, Silva K, Hungria M, Rouws JRC (2013). Dinâmica de rizóbios em solo do cerrado de Roraima durante o período de estiagem. Acta Amaz. 43(2):153-160.
Crossref
|
|
|
|
|
Zilli JE, Ribeiro KH, Campo RJ, Hungria M (2009). Influence of fungicide seed treatment on soybean nodulation and grain yield. Rev. Bras. Ciênc.Solo 33(4):917-923.
Crossref
|
|