ABSTRACT
Orange (Citrus sinensis) is a non-climacteric fruit that can be stored for long periods. However, the development of physiological and phytopathological disorders limits its postharvest storage. The objective of this study was to evaluate the effects of coating orange with propolis extract on the physicochemical characteristics of the ‘Pera’ orange during storage under ambient temperature. The fruits were selected and submitted to five postharvest treatments, three different forms of dip coating (70% alcohol, hydroalcoholic extract of propolis to 2.5%, hydroalcoholic extract of propolis to 5%), and two controls (one uncoated and one uncoated fruit kept under refrigeration). The variables weight loss, firmness, total soluble solid (TSS), titratable acidity (TA), ratio TSS/TA, and hydrogenic potential (pH) were evaluated at 0, 10, 18, and 25 days of storage. Treatment with propolis extract coating reduced the weight loss until the 18th day of storage. The fruit coated with propolis extract remained firmer up to 25 days of storage; this result is not significantly different from that of other postharvest treatments. The postharvest “refrigerated” treatment showed lesser weight loss and firmness during the storage period as a result of chilling injury. Coating with propolis extracts resulted in significant alterations of small magnitude in the variables TSS, TA, TSS/TA, and pH in oranges at the end of the storage period.
Key words: Citrus sinensis, coating, storage, quality, weight loss.
Orange (Citrus sinensis) is a non-climacteric fruit that features respiratory activity and relatively low ethylene production, which declines slightly after harvest. The physical and chemical parameters in fruits can change during postharvest period (El-Ramady et al., 2015; Volpe et al., 2002). Therefore, some problems related to long storage conditions of oranges, including physiological and biochemical losses are able to improve disorders both in the skin and pulp, besides phytopathological agents that result in deterioration of fruit (Chitarra and Chitarra, 2005).
Postharvest conservation during storage at low temperatures allows reduction on cell metabolism during physiological process during maturation. However, it can cause physiological disorders such as chilling injury if the storage temperature is less than the Security Minimum Temperature (SMT) (Kluge et al., 2007). To orange storage any authors have already described optimal temperature changing of 0 to 9°C at 85 to 90% relative humidity (RH), at which the fruit can be stored by 3 to 13 weeks, depending on the cultivar and climatic conditions (El-Ramady et al., 2015; Chitarra and Chitarra, 2005). However, prolonged exposure to low temperatures than SMT is critical for orange fruit, and symptoms of chilling injuries are characterized by the appearance of necrotic superficial depressions in the bark (Kluge et al., 2006). Under this problematic with fruits, principally with characterized summer fruits, a technique of coating procedure has been used successfully (Aquino et al., 2015; Cissé et al., 2015; Ali et al., 2015a; Daiuto et al., 2012).
The application of coatings is a technique that is commonly practiced in postharvest orange conservation (Alleoni et al., 2006; Contreras-Oliva et al., 2011; Kouassi, et al., 2012; Pereira et al., 2014; Vieites et al., 1996). This procedure provides protection against water loss and allows increase postharvest conditions on shelf life (Pereira et al., 2014). However, thickness and coating type can lead to improper way to improve shelf life (Chitarra and Chitarra, 2005). Normally, these coating are prepared with wax and resins dissolved by apolar solvents. The excess of coating under the tissue can lead to an excessive production of ethanol and acetaldehyde, which are associated with anaerobic conditions (Contreras-Oliva et al., 2011). Overall, these substances when produced change fruit flavor.
The biopolymers used in the coating formulations include polysaccharides, proteins, lipids, and resins (Song and Cheng, 2014; Chitarra and Chitarra, 2005). Coatings with hydroxypropyl methylcellulose and beeswax provided weight loss control and maintenance of firmness and nutritional quality of ‘Valencia’ oranges (Contreras-Oliva et al., 2011). These coating materials containing essential oils (Du Plooy et al., 2009), which have fungistatic effect on oranges. Alleoni et al. (2006) observed a lower weight loss ‘Pera’ orange coated based protein concentrate of whey, associated with two types of plasticizers (glycerol and sorbitol) after 11 days of storage. In a few years, propolis has also been evaluated to improve quality of coatings. The hydrophobic compounds of propolis, as waxes and essential oils, acts as a barrier to water vapor and gas exchange (Ali et al., 2015b, 2014; Carvalho et al., 2013; Zahid et al., 2013) and possess broad spectrum of antimicrobial activity (Ali et al., 2015b, 2014).
Propolis is a resinous substance produced by Africanized bees Apis mellifera L. by the action of its enzymes on plant exudates (Sforcin, 2007). Its extracts is a good option of coating material with respect to its origin, and it is presumably safer for both the consumers and the environment when used as a substitute to synthetic materials commonly used in postharvest conservation of fruit.
Therefore, this study aimed to evaluate the coating effects with propolis extract on the physicochemical characteristics of ‘Pera’ oranges during storage under ambient temperature condition.
Propolis, brown type, extracted by Africanized bees Apis mellifera L., was picked up from apiaries located in the southern state of Paraná, Brazil, with bee pasture typical mixed Ombrophylous forest ecosystem. The crude propolis was subjected to pre-cleaning, washing with cold water, and drying at 60°C for 10 h. The dried propolis was then packed in polyethylene bags and stored in a freezer at -5°C for 12 h. Then, 100 g of the material was triturated in a blender mix, packaged in an amber glass-bottle container, and the volume was made to 1 L with 70% ethanol (1th dilution). The suspension was kept to stand for 5 days at room temperature (25 ± 1°C). After this period, the homogenate was filtered by quantitative filter paper (JP 42; Quanty®; blue strip). The solution was used as stock solution to be used to obtain final concentrations of 2.5 and 5.0% (2th dilution) (Carvalho et al., 2013). In all dilutions, ethanol was used as diluent. Samples of ‘Pera’ oranges were acquired in a commercial market place and transported until laboratory analyses. The fruits were screened by shape uniformity, color, ripening index maturity and absence of pathogens. For the experimental setup, a group of 200 fruits were separated in five treatments as follows:
Treatment 1: Control - fruit without coating
Treatment 2: Alcohol - fruit coated with 70% (v/v) ethanol
Treatment 3: 2.5% propolis - fruit coated with 2.5% (w/v) of propolis hydroalcoholic extract;
Treatment 4: 5% propolis - fruit coated with 5% (w/v) of propolis hydroalcoholic extract;
Treatment 5: Refrigerated - uncoated fruit refrigerated at 9 ± 1°C.
The coatings were applied by immersion of the fruit in solutions mentioned individually for 5 s. After application, the fruit were placed horizontally on a nylon screen to drain the excess fluid for approximately 5 min. The fruit of the postharvest treatments “control”, “alcohol”, “2.5% propolis”, and “5% propolis” were placed on a workbench in a completely randomized design under the following storage conditions: 25 ± 3°C at 68 ± 10% RH. The fruit of the postharvest treatment “refrigerated” were stored under refrigeration at 9 ± 1°C at 70 ± 5% RH, based on the retail market conditions in Brazil that usually does not control the temperature and relative humidity during storage.
‘Pera’ oranges were obtained from the local market of Rio Paranaíba, Minas Gerais, Brazil and selected based on the of their peel color with no more than 15% of the surface area of the yellow peel. Evaluation of the orange fruit was performed before the application of postharvest treatment (time 0) and at 10; 18; and 25 days of storage, with three different periods of evaluation. The experimental units were subjected to analyses of weight loss (non-destructive group), firmness, total soluble solid (TSS), titratable acidity (TA), ratio TSS/TA, and hydrogen potential (pH) (destructive group), according to methods described by Adolfo Lutz Institute (2008).
For the weight loss analyses (non-destructive group), each fruit was weighed at the beginning of the experiment (time 0) and at 10; 18; and 25 days of storage. The weight loss analysis was arranged in a completely randomized design in a split plot factorial 5 × 3, with the plots postharvest treatments (control, alcohol, 2.5% propolis, 5% propolis, and refrigerated) and in subplots evaluation times (10; 18; and 25 days of storage), with six replications. The analysis was performed by using a semi-analytical electronic balance (BL-320H model; Splabor), with 0.001 g sensitivity. Final weight was subtracted from the initial weight of the fruit and the results were expressed as percentage.
Analyses of firmness, TSS, TA, TSS/TA, and pH (destructive group) were determined in an entirely randomized design in a factorial arrangement of subdivided plots 5 × 3 + 1, and the plots postharvest treatments (control, alcohol, 2.5% propolis, 5% propolis, and refrigerated) and at subplots evaluation times (10; 18; and 25 days of storage), with the addition of the analyses performed at time zero, with six replications. For the determination of firmness, a digital penetrometer (PTR-300 model; Instrutherm®) with a probe diameter of 5 mm was used. The firmness was measured at two opposite points of the fruit in the equatorial region of the fruit, and a small portion of the peel was removed using a blade. The results were expressed in Newton (N). The TSS was measured directly by using a digital refractometer (PAL-1 model; Atago®), with automatic temperature compensation to 20°C, and the results were expressed as percentage (m/m). The TA was determined by titration of the sample with NaOH 0.01 mol L-1, using 1% phenolphthalein as an indicator and expressed as percentage of citric acid, the dominant acid in orange fruit. The TSS/TA was calculated by dividing the TSS and TA, and the results were expressed by the resultant absolute value. The pH values were obtained by direct reading using the digital pH meter (MPA-210 model; Tecnopon® - Piracicaba, Brazil), calibrated with pH 4.0 and 7.0 buffer solutions, and the results were expressed by the resultant absolute value.
The data were checked by homogeneity of variances (Hartley test) and normality of residuals (Jarque - Bera test). The variables weight loss and TSS/TA were subjected to log and square root transformations, respectively, to suit analysis of variance (ANOVA) assumptions, using the F test at 5% significance. The influence of factors (postharvest treatments and storage period) and their interactions on the responses were submitted to factor analysis of split plot. After the split of analysis of variance (ANOVA), average postharvest treatments were compared by Student-Newman-Keuls (SNK) with p < 0.05. The mean of period analysis were submitted to regression analysis with p < 0.05, and the adjustment of the data was fetched to models with up to two dependent factors. When the interaction between the factors was not significant (p > 0.05), the marginal averages were used to compare treatments. When the interaction was significant, however, it proceeded to the unfolding of the interaction.
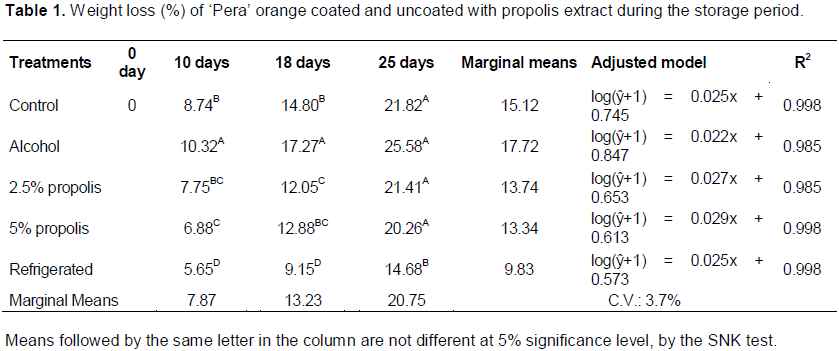
There was influence of storage period (F = 608.29; p < 0.001), the types of postharvest treatment (F = 29.42; p < 0.001), and the interaction between factors (F = 2.43; p = 0.037) on the percentage weight loss (Table 1). A significant increase in weight loss was noted for all postharvest treatments during the storage period. This loss can be attributed to the metabolic reactions such as respiration and transpiration of the product, which reduces the amount of water present in the plant tissue (Chitarra and Chitarra, 2005).
The fruit coated only with alcohol showed greatest loss of weight, which was significantly higher than that for other types of postharvest treatments, until the 18th day of storage. Oranges concerning the postharvest treatment showed wrinkling and dehydration bark visually perceptible. The propolis extract coatings showed lower permeability to water vapor between the fruit and the medium until the 18th day of storage, thereby reducing the weight loss of oranges. Ali et al. (2015b) also observed an highest percentage weight (19.1%) in peppers coated with ethanol under refrigeration conditions at 13°C and RH 90%, while control fruits and coated with 1% and 5% ethanolic extract propolis did not differ (17.2, 18 and 16.85%, respectively). However, pitaya coated with 0.5% ethanol extract of propolis showed a 13% weight loss when stored at 20 ± 2°C and 80 ± 5% RH for 20 days of storage (Zahid et al., 2013).
Only the postharvest “refrigerated” treatment showed significant difference, with smaller loss of weight during the 25 days of storage. As at low temperatures, the metabolic respiration processes gets reduced, and consequently, the weight loss of the fruit is also reduced (Chitarra and Chitarra, 2005). However, this procedure may not be effective for long storage periods, as it has risks of cold disorders (Kluge et al., 2007, 2006). Citrus fruit can develop a disorder called “brown stem”, manifesting dryness, discoloration, and wrinkling of the shell around the peduncle. These characteristics could be observed in fruit under refrigeration in the present study from the 18th day of storage onwards. According to Chitarra and Chitarra (2005), this disorder causes the death of epidermal cells and the collapse of oil glands, and it is considered as a transpiration phenomenon associated with the ability of a fruit to control its weight loss. The RH of the refrigerator (70 ± 5%) may have contributed to the development of the disorder in the postharvest treatment “refrigerated”. In general, fruit stored under refrigeration with RH of 85 TO 95% suffer reduced skin dissection, reduced distortion, lower degreening, and cold disorder (Chitarra and Chitarra, 2005).
The percentage of weight loss of ‘Pera’ oranges was higher than the reported data. Pereira et al. (2014) studied ‘Valencia Delta’ oranges coated with carnauba wax and found 26% weight loss for control fruit and 14% for coated fruit stored for 28 days under the ambient temperature condition. Alleoni et al. (2006) reported weight loss of 4.70 to 8.96% for ’Pera’ oranges coated with protein concentrate whey and plasticizers (glycerol and sorbitol) during the 11 days of storage under 22 ± 1°C at 80 ± 2% RH.
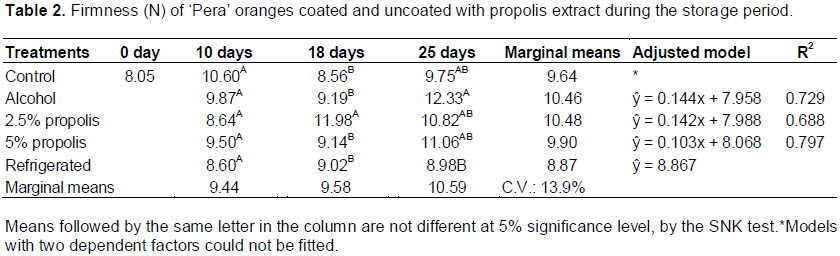
The firmness variable also suffered significant influence of the postharvest treatment (F = 4.03; p = 0.020) and storage period (F = 3.75; p = 0.034) as well as interaction between the factors (F = 2.77; p = 0.019). An increase in the firmness value was noted during the storage period for the postharvest “alcohol”, “2.5% propolis”, and “5% propolis” treatments, as evidenced by the adjusted linear models. The orange fruit subjected to postharvest treatment “refrigerated” showed a relatively constant firmness value during the 25 days of storage (Table 2).
The hardening of oranges during the storage period after the postharvest “alcohol”, “2.5% propolis”, and “5% propolis” treatments can be justified by the presence of water-alcohol solution that can be given to the insolubility pectic material, which inhibits the degradation of pectin by pectinmethylesterase (PME) and polygalacturonase (PG) (enzymes responsible for softening of fruit). The propolis extract coating enhances firmness retention and concurs with the results of Ali et al. (2014) and Zahid et al. (2013). The chilli and pitaya, respectively, appeared firmer than the postharvest treatment control during the duration of storage.
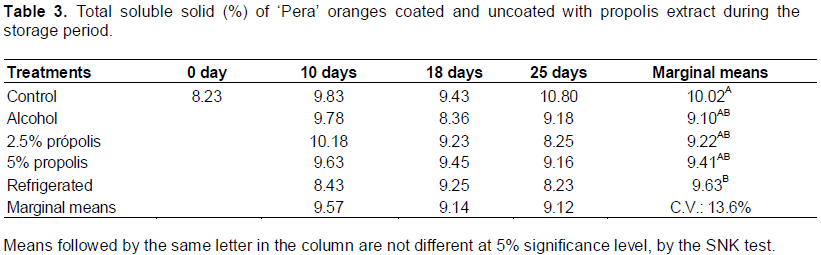
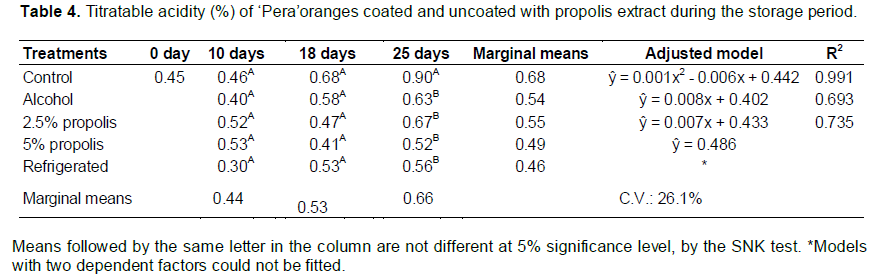
The enzymes PME and PG act on the hydrolysis of glycosidic α-1-4 galacturonic acid when de-esterified, triggering depolymerization and solubilization of pectic (Ali et al., 2004). The inhibitory substances of these enzymes such as sucrose, maltose, and glucose by non-competitive inhibition and some peptides compete for the binding sites of PME, which can explain failure of demethoxylation of pectin chains that decrease the action PG (Wakabayashi, 2000). The TSS of ‘Pera’ oranges were stable during the storage period (F = 0.64; p = 0.532). The different types of postharvest treatments influence the TSS content (F = 3.55, p = 0.031). The interaction of factors on the TSS content was not significant (F = 1.01; p = 0.445) (Table 3). The stability of the TSS values was consistent with that reported by Pereira et al. (2014) and Vieites et al. (1996). Salunkhe and Desai (1984) stated that oranges, being non-climacteric fruit, do not suffer rapid changes in TSS values immediately after harvest. Only the “refrigerated” postharvest treatment showed lower TSS values that those after “control” postharvest treatment, due to the reduction of metabolic process and respiration activity. The TA values varied significantly among the postharvest treatments (F = 3.26; p = 0.041) and during the storage period (F = 14.46; p < 0.01), showing a significant interaction between the factors (F = 2.47; p = 0.031) (Table 4). The TA did not present significant differences between the postharvest treatments until the 18th day of storage. However, after 25 days of storage, the values for postharvest “control” treatment differed significantly from those for other treatments. It was also observed that the acidity was constant over the course of 25 days of storage for the postharvest “5% propolis” treatment.
The fruit of the postharvest “control”, “alcohol”, and “2.5% propolis” treatments showed increased TA content over the 25 days of storage; this effect was much more pronounced in the postharvest “control” treatment. Also noted by Pereira et al. (2014) and Vieites et al. (1996), this increase in acidity can be attributed to the degradation of pectins PME and PG and the possible formation of galacturonic acid, as well as associated with the fermentation process. The TSS/TA value differed significantly for fruit subjected to different postharvest treatments and time of storage (F = 10.70; p < 0.001), as well as presented significant interaction between the factors (F = 3.37; p = 0.021). While the postharvest treatments showed no significant difference (F = 2.31; p = 0.106) (Table 5).
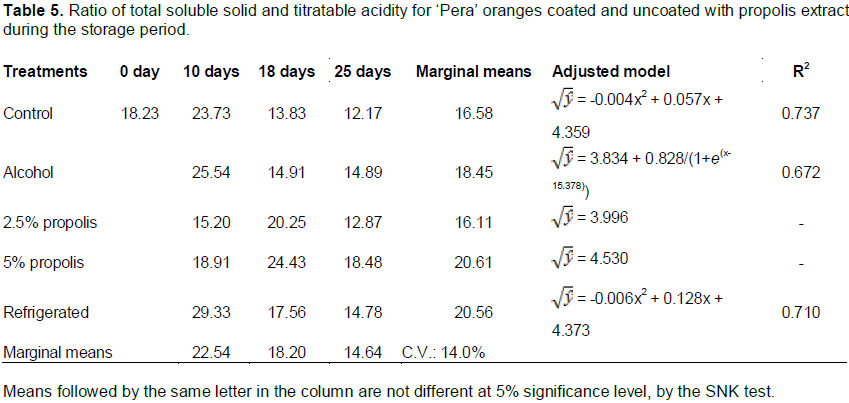
The fruit coated with hydroalcoholic extract of 2.5% propolis and 5% propolis showed no significant differences in the TSS/TA values during storage. For postharvest “control” and “refrigerated” treatments, a decrease in this index was perceived during storage. The variation TSS/TA value is indicative of the fruit ripening stage, which determines the balance of sweet and sour flavors in the fruit (Chitarra and Chitarra, 2005). Values of TSS/TA between 23.50 and 29.68 were found in "Valencia Delta" orange coated with carnauba-based wax stored under ambient conditions (Pereira et al., 2014).
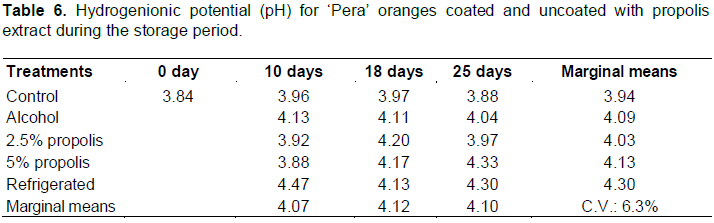
The evolution of TSS/TA oranges can be explained by the relationship rootstock/eating area, age of trees, flowering, and productivity, as well as variation in the climate over the years (Volpe et al., 2002). The author also reported differences in the TSS/TA values within the fruit of the same variety grown in terms of producing region, climate, time of harvest, and soil, among others. The postharvest treatment (F = 2.30; p = 0.052) and storage time (F = 0.16; p = 0.850) did not influence the pH values (Table 6).
According to Chitarra and Chitarra (2005), in a concentration range of acids between 2.5 and 0.5%, the pH may increase and the acidity may decrease, indicating the use of organic acids in the cell vacuole during the breathing process, since they constitute an excellent energy reserves to support the maturation of the fruit. However, in this study, an increase in acidity was noted for postharvest “control”, “alcohol”, and “2.5% propolis” treatments along with maintenance of pH for all postharvest treatments, regardless of whether or not there was variation in TA (Tables 4 and 6). The acid formed during storage are weak acids that are not deprotonated at low pH values, not contributing to change this.The pH value obtained in this study was lower than that found by Pereira et al. (2014) in “Delta Valencia” orange. The authors verified pH between 4.51 and 4.41 for fruits control and 4.51 to 4.37 for the fruits coated with carnauba-based wax stored under ambient conditions.
Coating with hydroalcoholic extract of propolis is effective in reducing weight loss in ‘Pera’ oranges until the 18th day of storage. Fruit coated with propolis hydroalcoholic extract showed, in general, similar behavior to the uncoated fruit kept at room temperature. Oranges coated with ethanol-water solutions maintained the firmness during the 25 days of storage. Changes in the TSS content, TA, TSS/TA, and pH promoted by coatings with hydroalcoholic extract of propolis were small in magnitude during the storage period. The values of TSS, TA, TSS/TA, and pH for “Pera” orange do not change with refrigeration conditions and with coated with hydro-alcoholic extract of propolis.
The authors have not declared any conflict of interest.
The authors thank CAPES-Brazil for granting the research fellowship.
REFERENCES
|
Adolfo L (2008). Métodos físico-químicos para análise de alimentos. 4. ed. 1. ed. digital. São Paulo: Instituto Adolfo Lutz. 1020 p.
|
|
|
|
Ali A, Chow WL, Zahid N, Ong MK (2014). Efficacy of propolis and cinnamon oil coating in controlling post-harvest anthracnose and quality of chilli (Capsicum annuum L.) during cold storage. Food Bioproc Tech. 7(9):2742-2748.
Crossref
|
|
|
|
|
Ali A, Wee Pheng T, Mustafa MA (2015b). Application of lemongrass oil in vapour phase for the effective control of anthracnose of 'Sekaki' papaya. J. Appl. Microbiol. 118(6):1456-1464.
Crossref
|
|
|
|
|
Ali A, Wei YZ, Mustafa MA (2015a). Exploiting propolis as an antimicrobial edible coating to control postharvest anthracnose of bell pepper. Packaging Technol. Sci. 28(2):173-179.
Crossref
|
|
|
|
|
Ali ZM, Chin LH, Lazan H (2004). A comparative study on wall degrading enzymes, pectin modifications and softening during ripening of selected tropical fruits. Plant Sci. 167(2):317-327.
Crossref
|
|
|
|
|
Alleoni ACC, Jacomino AP, Rosa AS (2006). 'Pêra' orange coating with whey protein concentrate film associated to plasticizers. Pesqui. Agropecu. Bras. 41(8):1221-1226.
Crossref2
|
|
|
|
|
Aquino AB, Blank AF, Santana LCLA (2015). Impact of edible chitosan–cassava starch coatings enriched with Lippia gracilis Schauer genotype mixtures on the shelf life of guavas (Psidium guajava L.) during storage at room temperature. Food Chem. 171(1):108-116.
Crossref
|
|
|
|
|
Carvalho JX, Suárez RO, Mendes FQ, Fernandes RVB, Cunha MC, Carvalho AMX (2013). Increased shelf life of eggs through the use of propolis. Semina: Cienc. Agrárias 34(5):2287-2296.
|
|
|
|
|
Chitarra MIF, Chitarra AB (2005). Pós-colheita de frutas e hortaliças: fisiologia e manuseio. 2. ed. Lavras: Universidade Federal de Lavras, 785 p.
|
|
|
|
|
Cissé M, Polidori J, Montet D, Loiseau G, Ducamp-Collin MN (2015). Preservation of mango quality by using functional chitosanlactoperoxidase systems coatings. Postharvest Biol. Technol. 101(1):10-14.
Crossref
|
|
|
|
|
Contreras-Oliva A, Rojas-Argudo C, Pérez-Gago MB (2011). Effect of solid content and composition of hydroxypropyl methylcellulose–lipid edible coatings on physicochemical, sensory and nutritional quality of 'Valencia' oranges. Int. J. Food Sci. Technol. 46(11):2437-2445.
Crossref
|
|
|
|
|
Daiuto ER, Minarelli PH, Vieites RL, Orsi RO (2012). Própolis e cera vegetal na conservação de abacate 'Hass'. Semina: Cienc. Agrárias 33(4):1463-1474.
|
|
|
|
|
Du Plooy W, Regnier T, Combrinck S (2009). Essential oil amended coatings as alternatives to synthetic fungicides in citrus postharvest management. Postharvest Biol. Technol. 53(3):117-122.
Crossref
|
|
|
|
|
El-Ramady HR, Domokos-Szabolcsy E, Abdalla NA, Taha HS, Fári M (2015). Postharvest management of fruits and vegetables storage. In: Lichtfouse E, ed. Sustainable agriculture reviews, v.15. New York: Springer International Publishing. pp. 65-152.
Crossref
|
|
|
|
|
Kluge RA, Azevedo RA, Jomori, LL, Edagi FK, Jacomino AP, Gaziola SA, Aguila JS (2006). Effects of thermal treatments applied on citric fruits under cold storage. Cienc. Rural 36(5):1388-1396.
Crossref
|
|
|
|
|
Kluge RA, Jamori MLL, Edagi FK, Jacomino AP, Aquila JSD (2007). Chilling injury and quality of citric fruits submitted to thermal treatments and cold storage. Rev. Bras. Frutic. 29(2):233-238.
Crossref
|
|
|
|
|
Kouassi KHS, Bajji M, Jijakli H (2012). The control of postharvest blue and green molds of citrus in relation with essential oil-wax formulations, adherence and viscosity. Postharvest Biol. Technol. 73(1):122-128.
Crossref
|
|
|
|
|
Pereira GS, Machado FLC, Costa JMC (2014). Application of coating extends postharvest quality in the 'Valencia Delta' orange during ambient storage. Rev. Ciênc. Agron. 45(3):520-527.
|
|
|
|
|
Salunkhe DK, Desai BB (1984). Postharvest biotechnology of fruits, 1. CRC Press, Boca Raton, FL, 168 p.
|
|
|
|
|
Sforcin JM (2007). Propolis and the immune system: A review. J. Ethnopharmacol. 113(1):1-14.
Crossref
|
|
|
|
|
Song X, Cheng L (2014). Chitosan/kudzu starch/ascorbic acid films: Rheological, wetting, release, and antibacterial properties. Afr. J. Agric. Res. 9(52):3816-3824.
|
|
|
|
|
Vieites RL, Arruda MC, Godoy LJG (1996). Use wax and starch film on 'Pêra' orange refrigerated storage. Semina: Cienc. Agrárias 17(1):83-87.
|
|
|
|
|
Volpe AC, Shoffele R, Barbosa JC (2002). Influence of the accumulated heat unit and rainfall on the ratio and technological index of sweet oranges 'Valência' and 'Natal'. Rev. Bras. Frutic. 24(2):436-441.
Crossref
|
|
|
|
|
Wakabayashi K (2000). Changes in cell wall polysaccharides during fruit ripening. J. Plant Res. 113(1):231-237.
Crossref
|
|
|
|
|
Zahid N, Ali A, Siddiqui Y, Maqbool M (2013). Efficacy of ethanolic extract of propolis in maintaining postharvest quality of dragon fruit during storage. Postharv. Biol. Technol. 79(1):69-72.
Crossref
|
|