ABSTRACT
This study aimed to identify phenotypic plasticity in populations of Jatropha curcas. The experiment was conducted with a completely randomized design with eleven treatments and four replications. Morphophysiological variables were analyzed in the agricultural year, 2014-2015. Positive correlations were only observed between crown diameter and seed production, and canopy diameter and stomatal density in adaxial epidermis, indicating that canopy diameter can be used as a descriptor in plant breeding programs. Cluster analysis confirmed the existence of diversity among populations of J. curcas, with the formation of two groups, demonstrating the narrow genetic basis of Jatropha found in different regions of Brazil. The analysis of phenotypic plasticity demonstrated that morphological variables had a higher coefficient of plasticity in relation to physiological and productive variables. The morphological and physiological variables can be used in J. curcas breeding programs to study diversity and phenotypic plasticity.
Key words: Biofuel, genetic diversity, Jatropha curcas, oleaginous.
Abbreviation:
CAR, Carotenoids; Cl a, chlorophyll a; Cl b, chlorophyll b; CS, the length of the seed; DCL, stem diameter; DCP, canopy diameter; DMSO, dimethyl sulfoxide; DS, diameter seed; EAB, stomatal density in abaxial; EAD, stomatal density in adaxial epidermis; FA, asexual; FBH, the first branch height; FF, feminine flower; FH, hermaphrodites flower; FM, masculine flower; LA, leaf area; LS, width of the seed; NI, number of inflorescences per plant; NR, number of branches; PCD, crown diameter; PH, plant height; PO, oil yield; PROD, productivity; PS, weight of 100 seeds; SLA, specific leaf area; TOS, oil content in the seeds.
The world's dependence on energy sources has compromised natural resources, owing to the increase in gases that enhance the greenhouse effect in the atmosphere as a result of human activities such as burning of fossil fuels and land use. The search for sources of clean and renewable energy becomes necessary to minimize climate change. In this context, biofuels represent a sustainable alternative for partial or total replacement of fossil fuels.
Brazil features a high capacity for biofuel production, owing to its vast territory, climatic diversity, a number of potentially suitable species, and manpower with technical expertise in the field of agricultural science (Santos et al., 2014). The main raw materials used for production of biodiesel in Brazil are soybeans, beef tallow and cotton, with contributions of 69.24, 26.18 and 3.07%, respectively, and other materials accounting for only 1.51% of the production (ANP, 2015). Therefore, the need exists for diversifying the sources of raw material with the introduction of promising species. Jatropha curcas, popularly known as physic nut, stands out as a plant with great potential for oil extraction as well as high physical and chemical quality for biodiesel production (Laviola et al., 2012).
J. curcas is a perennial, monoecious species belonging to the family Euphorbiaceae and has been used by rural communities for various ends, such as soil conservation and as source of decomposed organic matter rich in nitrogen, phosphorus and potassium. The latex extracted from the stem and branches presents healing effect, being used for pharmaceutical purposes (Openshaw, 2000). Its high cultivation potential has attracted the attention of researchers and promoted rapid expansion of the species in the world. The planted area of J. curcas corresponded to 900,000 ha in 2008, rising to 4.7 million ha in 2010 and expected to reach 12.8 million ha in 2016 (Contran et al., 2013).
Although, Jatropha is distributed in different regions of Brazil, the absence of cultivars with improved production stability has limited the expansion of the species. Thus, research aiming to develop improved J. curcas cultivars has been intensified; nevertheless, breeding programs are still rare when compared with other oleaginous species (Laviola et al., 2012). Several genetic diversity studies report that the genetic basis of Jatropha is narrow, probably because of a common ancestry (Kanchanaketu et al., 2012; Reis et al., 2015).
The large edaphoclimatic diversity existing in Brazil, with marked variation of abiotic factors between regions, may favor the selection over generations of genotypes with greater ability to display phenotypic plasticity. Breeding programs of J. curcas have aimed to solve the problematic environment-genotype interaction. Expansion of the commercial exploitation of this culture depends on the clarification of basic agronomic aspects not yet available for the species, as well as on the development of superior materials with uniform maturation and production stability.
In this perspective, it is observed that the species, J. curcas lacks superior materials and basic elucidations to ensure production stability. The degree of improvement of Jatropha is still incipient, and raises concern among investigators that research is in demand as regards genetic improvement, interaction of genotype x environment, phenotypic plasticity and management practices. Thus, this study aimed to identify the diversity and phenotypic plasticity in populations of J. curcas.
The experimental area of the present study has Aw climate according to Köppen classification, with annual rainfall of 1,447 mm, average temperature of 21.9°C, and average air relative humidity ranging from 58 to 81%. The region has two distinct seasons: a rain season from October to April, and drought from May to September. The soil of the experimental area is classified as dystrophic red-yellow latosol (EMBRAPA, 2006).
Seeds of J. curcas from different geographical regions of Brazil were planted in November 2011 with spacing of 4 × 2 m (Table 1). The work was conducted following a randomized complete block design with eleven treatments and four replications, between August 2014 and August 2015. The treatments were defined as J. curcas populations.
To obtain the stomatal density, two replicas of adaxial and abaxial leaf surfaces, depicting the middle third region of hydrated leaves, were made with colorless enamel. The count was carried out using an optical microscope equipped with a camera lucida, following the recommendations of Jadrná et al. (2009).
To obtain the specific leaf area (SLA), six leaf disks of 1.2 cm diameter were taken from fully expanded leaves and subsequently dried at 70°C for 72 h for determination of the dry weight. The SLA was obtained via equation proposed by Radford (1967). The leaf area was determined following equation proposed by Severino et al. (2006). The amount of inflorescences was established by counting their number as they emerged in the plant. The number of female, male, hermaphrodite and asexual flowers was obtained by the respective number of flowers counted in each inflorescence upon their opening in the plant, according to Pereira et al. (2011).
For determination of photosynthetic pigments, leaf discs with 1.2 cm of diameter were removed and placed in glasses containing dimethyl sulfoxide (DMSO). Next, extraction was performed in water bath at 65°C for one hour. Samples were taken for spectrophotometric reading at 480, 649 and 665 nm. The content of chlorophyll a (Cl a), chlorophyll b (Cl b), carotenoids (Car) and ratio of chlorophyll a to b were determined following the equations proposed by Wellburn (1994).
The number of branches was obtained by counting all the ramifications from the base of the main stem. Plant height was determined using a measuring tape graduated in meters, covering the stem length up to the apex of the main branch. The stem diameter was established with a digital caliper at the height of sample collection. The crown diameter was measured between the two lateral limits of the plant.
The length, width and diameter of the seeds were determined using a digital caliper, using 25 random seeds as samples. The weight of 100 seeds was obtained using a precision scale (0.001 g). The yield per plant was measured by weighing the seeds. Subsequently, analysis of the oil content of the seeds was performed by nuclear magnetic resonance (NMR). Oil productivity was determined by total seed productivity (kg ha-1) divided by the average density of Jatropha oil (0.910), expressed in L h-1.
The differences in plasticity index associated with morphological, physiological and productive variables were analyzed by Scott-Knott test (p < 0.05). The phenotypic plasticity index, ranging from 0 to 1, was calculated based on the relative distance (DR) between the values of treatments (RDPI), according to Valladares et al. (2006).
The genotypic correlation test between variables as well as the cluster analysis by Mahalanobis distances (D2), using the unweighted average link method (UPGMA), was accomplished using the GENES software (Cruz, 2013). To perform the principal component analysis, created if a correlation matrix and the selection criteria of the axes followed the Broken Stick model in multiple regression analysis to assess productivity, the forward stepwise model (Sokal and Rolf, 1969) was used and both analyses were carried out using the R software (R CORE TEAM, 2015).
The results revealed differences between J. curcas populations, corroborating those found by Reis et al. (2015) when evaluating the genetic diversity in different accessions of this species, based on agronomic descriptors. In addition, this paper reports the existence of phenotypic plasticity in populations of J. curcas.
The descriptive analysis of variables is shown in Table 2. The number of inflorescences and female, male, hermaphrodite and asexual flowers did not follow a normal distribution according to the Shapiro-Wilk test. Variables showing normal standard with greater variations were: oil production (PO), seed productivity (PROD), height of 1st branch (FBH) and specific leaf area (SLA). The PO found in the seeds presented variation from 55.67 to 508.40 L ha-1, with an average of 242.21 L ha-1. The FBH ranged from 4 to 12 cm, with an average of 6.7 cm. The AFE had an average of 71.20 m2 kg-1, varying between 37 and 109 m2 kg-1. The obtained TOS was 33.20%, ranging from 28.40 to 35.80%.
In this regression model, the crown diameter, number of inflorescences, stomatal density in adaxial epidermis and seed length were the variables with greatest contribution to the productivity of J. curcas.
Genotypic correlation and multiple regression analysis indicate that the crown diameter, stomata density in the adaxial epidermis and length of the seeds can be used as a descriptor in plant breeding programs in order to increase the yield of seeds (Laviola et al. 2011). To assess the genetic diversity of plants J. curcas, Reis et al. (2015) concluded that the morphophysiological descriptors are of paramount importance to the improvement of the species.
The correlation between canopy diameter and stomatal density in adaxial epidermis is associated with self-shadowing in plants with large crown diameter and leaf area index. The high shading creates local conditions (lower incidence of direct radiation, lower temperature, high humidity and high boundary layer) is appropriate for the influx of CO2 and transpiration. Under these conditions, the leaves are larger and have high stomatal density in the adaxial epidermis to maximize gas exchange and thereby increase productivity (Castro et al., 2009).
In J. curcas, inflorescences are located at the apex of the branches and are correlated with the seed yield, interfering decisively with the amount of fruit (Drummond et al., 2010). According to Rocha et al. (2012), crown diameter, number of inflorescences, stomatal density in adaxial epidermis and seed length are quantitative attributes that suffer strong environmental influence, being able to generate differential genotypes over the years.
In principal component analysis (Figure 1), it is observed that only the first two components were necessary to explain 70% of the variation of the data. PC1 explains 50% of the variation; the variables contributing the most to the ordination of Jatropha populations were crown diameter; number of inflorescences, female, male and hermaphrodite flowers; and seed and oil yield. The populations of GO, RO, RN, MT and CE showed the highest values for these variables. PC2 explains 20% of the variation in the data, and only the variable ‘total chlorophyll’ contributed significantly to the formation of the second axis; the populations of GO, RO, MT and MG showed the highest values for this variable.
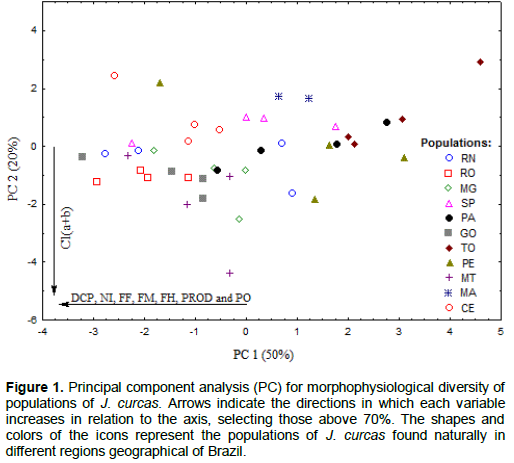
However, the principal component analysis failed to identify the formation of different groups among the populations of J. curcas based on physiological, morphological and productive variables. This proves that the materials under study, although derived from different geographic regions of Brazil, present intra- and inter- population variations. Contradictory results were obtained by One et al. (2014), studying the phenotypic and genotypic diversity in Jatropha, while Osorio et al. (2014) found great phenotypic variations in J. curcas in Central America as compared to Africa, Asia and South America.
Cluster analysis (Figure 2) based on the Mahalanobis distance ranked the populations of J. curcas in two groups, observing similarity between the groups generated at the point 60 of the connection distance. Group one, which included most J. curcas materials, included the states of MT, CE, PE, TO, MA, PA, GO and SP. Group two comprised the states of RO, MG and RN.
Cluster analysis confirmed the existence of genetic diversity among populations of J. curcas, showing that the genetic basis of Jatropha found in different regions of Brazil is narrow, possibly because the analyzed materials were derived from few populations or exchange of seeds occurred after introduction in Brazil (Kanchanaketu et al., 2012). According to Reis et al. (2015), the introduction of materials derived from other countries is necessary to generate greater diversity.
The analysis of phenotypic plasticity (Table 5) showed that the morphological variables had greater plasticity coefficient (0.37) in relation to physiological (0.09) and productive (0.10) variables. Among the morphological and productive variables with higher values of phenotypic plasticity were: asexual (0.67), hermaphrodite (0.59), female (0.53) and male (0.52) flowers, number of inflorescences per plant (0.48), oil yield (0.32) and seed (0.31). The physiological variables had reduced phenotypic plasticity index for the studied characteristics.
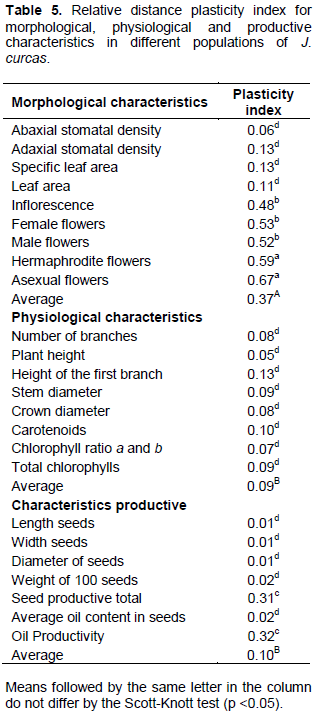
According to Fuzeto and Lomônaco (2000), populations occupying heterogeneous environments have large plastic potential in their external characteristics (phenotype) without genotypic changes being necessary. The detected variations confirm the performance of plasticity as generating mechanism of phenotypic variability and point out the latter’s importance in adaptive and evolutionary processes of species, as the produced changes facilitate the exploration of new niches, resulting in increased environmental tolerance (Via, 1993).
The morphological plasticity seen in populations of J. curcas occurred mainly due to environmental factors, suffering genetic influences. According to Gilbert (2016), inflorescences as well as male, female and hermaphrodite flowers are structures sensitive to changes in the external environment. Productive plasticity was also observed in the present study, where interactions between seed and oil production and the environment could be verified. Therefore, J. curcas exhibits high plasticity, which may vary from higher to lower intensity according to cultivation conditions. These results corroborate those obtained by Drummond et al. (2010) when evaluating the agronomic performance of J. curcas genotypes, finding wide variations in the study variables.
J. curcas populations present phenotypic and genotypic variability, and morphological and physiological variables may be used in breeding programs. Plasticity analysis confirmed the performance of morphological, physiological and productive variables as generating mechanism of phenotypic variability.
Canopy diametercontributes to increased productivity in J. curcas, and can be used as a descriptor for breeding programs of the species.
CAR, Carotenoids; Cl a, chlorophyll a; Cl b, chlorophyll b; CS, the length of the seed; DCL, stem diameter; DCP, canopy diameter; DMSO, dimethyl sulfoxide; DS, diameter seed; EAB, stomatal density in abaxial; EAD, stomatal density in adaxial epidermis; FA, asexual; FBH, the first branch height; FF, feminine flower; FH, hermaphrodites flower; FM, masculine flower; LA, leaf area; LS, width of the seed; NI, number of inflorescences per plant; NR, number of branches; PCD, crown diameter; PH, plant height; PO, oil yield; PROD, productivity; PS, weight of 100 seeds; SLA, specific leaf area; TOS, oil content in the seeds.
The authors have not declared any conflict of interests.
The authors are grateful to CAPES for the scholarship granted and Graduation Program in Plant Production and State University of Goiás for the incentive grant for research to the corresponding author.
REFERENCES
|
ANP (2015). Agência Nacional do Petróleo, Gás Natural e Biocombustíveis. Available at:
View. Accessed: 16/10/2015.
|
|
|
|
Castro ED, Pereira FJ, Paiva R (2009). Histologia vegetal: estrutura e função de órgãos vegetativos. Lavras: UFLA. P 234.
|
|
|
|
|
Contran N, Chessa L, Lubino M, Bellavite D, Roggero PP, Enne G (2013). State of the art of the Jatropha curcas productive chain, from sowing to biodiesel and by-products. Ind. Crop Prod. 42(1):202-215.
Crossref
|
|
|
|
|
Cruz CD (2013). Genes – a software package for analysis in experimental statistics and quantitative genetics. Acta Sci. Agron. 35(3):271-276.
Crossref
|
|
|
|
|
Drumond MA, Santos CAF, Oliveira VR, Martins JC, Anjos JB, Evangelista MRV (2010). Desempenho agronômico de genótipos de pinhão manso no Semiárido pernambucano. Ciênc. Rural. 40(1):44-47.
Crossref
|
|
|
|
|
EMBRAPA–Centro nacional de pesquisa de solos (2006). Sistema brasileiro de classificação de solos. Rio de Janeiro: Embrapa Solos.
|
|
|
|
|
Fuzeto AP, Lomônaco C (2000). Potencial plástico de Cabralea canjerana subsp. polytricha (Adr. Juss.) Penn. (Meliaceae) e seu papel na formação de ecótipos em área de cerrado e vereda, Uberlândia, MG. Rev. Bras. Bot. 23:169-176.
Crossref
|
|
|
|
|
Gilbert SF (2016). Chapter Twenty-Two: Developmental plasticity and developmental symbiosis: the return of eco-devo. Curr. Top. Dev. Biol. 116:415-433.
Crossref
|
|
|
|
|
Jadrná P, Kobza F, Plavcová O (2009). Polyploidization of Pelargonium x hortorum L. H. Bailey in greenhouse conditions. Hortic. Sci. 36(1):31-37.
|
|
|
|
|
Kanchanaketu T, Sangduen N, Toojinda T, Hongtrakuli V (2012). Genetic diversity analysis of Jatropha curcas L. (Euphorbiaceae) based on methylation-sensitive amplification polymorphism. Genet. Mol. Res. 11(2):944-955.
Crossref
|
|
|
|
|
Laviola BG, Alves AA, Gurgel FD, Rosado TB, Rocha RB, Albrecht JC (2012). Estimates of genetic parameters for physic nut traits based in the gemplasm two years evaluation. Ciênc. Rural. 42(3):429-435.
Crossref
|
|
|
|
|
One KT, Tanya P, Muakrong N, Laosatit K, Srinives P (2014). Phenotypic and genotypic variability of F2 plants derived from Jatropha curcas x integerrima hybrid. Biomass Bioenergy. 67(1):137-144.
Crossref
|
|
|
|
|
Openshaw K (2000). A review of Jatropha curcas: an oil plant of unfulfilled promise. Biomass Bioenergy. 19:1-15.
Crossref
|
|
|
|
|
Osorio MLR, Salvador AFT, Jongschaap REE, Perez CAA, Sandoval JEB, Trindade LM, Visser RGF, Loo EN (2014). High level of molecular and phenotypic biodiversity in Jatropha curcas from Central America compared to Africa, Asia and South America. BMC Plant Biol. 14:77.
Crossref
|
|
|
|
|
Pereira JCS, Fidelis RR, Erasmo EAL, Santos PM, Barros HB, Carvalho GL (2011). Florescimento e frutificação de genótipos de pinhão manso sob doses de fósforo no cerrado da Região Sul do Tocantins. J. BiotecH. Biodivers. 2(2):28-36.
|
|
|
|
|
R CORE TEAM (2015). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at:
View. Accessed: 19/08/2015.
|
|
|
|
|
Radford PJ (1967). Growth analysis formulae: their use and abuse. Crop Sci. 7(3):171-175.
Crossref
|
|
|
|
|
Reis MVM, Damasceno Junior PC, Campos TO, Diegues IP, Freitas SC (2015). Variabilidade genética e associação entre caracteres em germoplasma de pinhão-manso (Jatropha curcas L.). Rev. Ciênc. Agron. 6(2):412-420.
|
|
|
|
|
Rocha RB, Ramalho AR, Teixeira AL, Laviola BG, Silva FCG, Militão JSLT (2012). Eficiência da seleção para incremento do teor de óleo do pinhão-manso. Pesqui. Agropecu. Bras. 47(1):44-50.
Crossref
|
|
|
|
|
Santos MD, Tejedor M, Jiménez C, Hernández-Moreno JM, Palacios-Díaz MP, Díaz FJ (2014). Recycled urban wastewater for irrigation of Jatropha curcas L. in abandoned agricultural arid land. Sustain. 6(10):6902-6924.
Crossref
|
|
|
|
|
Severino LS, Vale LS, Beltrão NEM (2006). Método para medição da área foliar do pinhão manso. Rev. Bras. Oleaginosas Fibrosas. 14(1):73-77.
|
|
|
|
|
Sokal RR, Rohlf FJ (1969). Single classification analysis of variance. Biometry. The Principles and Practice of Statistics in Biological Research (Emerson R, Kennedy D, Park R, eds). San Francisco: WH Freeman. pp. 204:249.
|
|
|
|
|
Valladares F, Sanchezâ€Gomez D, Zavala MA (2006). Quantitative estimation of phenotypic plasticity: bridging the gap between the evolutionary concept and its ecological applications. J. Ecol. 94(6):1103-1116.
Crossref
|
|
|
|
|
Via S (1993). Adaptive phenotypic plasticity: target or byproduct of selection in a variable environment?. Am. Nat. 142(1):352-365.
Crossref
|
|
|
|
|
Wellburn AR (1994). The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 144(3):307-313.
Crossref
|
|