The confirmation of the presence and invasion of Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) in Brazil (Czepak et al., 2013) and in South and Central America (Murúa et al., 2014; Smith, 2014; Arnemann et al., 2016) brought serious implications in terms of the management of this pest for the main agricultural crops cultivated in these areas. Furthermore, Kriticos et al. (2015) alerted about the extraordinary spread potential of this pest to North America and in July 2014, USDA/APHIS and Florida Department of Agriculture and Consumer Services (FDACS) confirmed the first detection of H. armigera in USA.
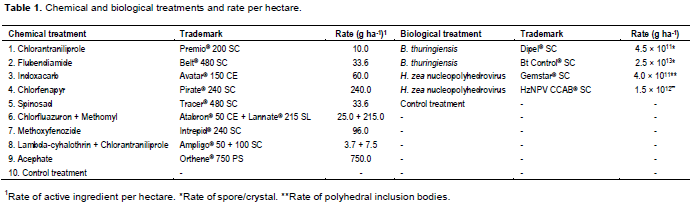
The management of H. armigera populations poses great challenges for the Brazilian soybean farmers, because there is little information available on the chemical and biological control of this pest in Brazil. These difficulties led the Brazilian Ministry of Agriculture and Food Supply to take immediate measures, such as the emergency registration of insecticides for the control of H. armigera. It makes the control recommendations susceptible to doubt and errors.
The lack of regional control results still leaves the technical assistants, industry, farmers, and researchers without information to establish the management of this pest during recent crop production cycles. Therefore, to evaluate the role of chemical and biological insecticides used to control larvae of H. armigera under soybean field conditions, two experiments were accomplished at two locations under different environmental conditions.
Two experiments were performed during the 2013/2014 growing season in Restinga Seca and Santa Maria, State of Rio Grande do Sul (RS), Brazil. In Restinga Seca, treatments were sprayed on the 3rd of February 2014 at the full pod (R4) growth stage and densities of H. armigera were 1.2 and 3.7 small (< 1.5 cm) and large (> 1.5 cm) larvae m-2, respectively. In Santa Maria, treatments were sprayed on the 10th of February 2014 at the beginning seed (R5.1) soybean growth stage, with densities of 2.5 and 1.0 small and large larvae m-2, respectively. The soybean variety on both areas was BMX Potencia RR. The species of Helicoverpa occurring on these experiments were identified at the Laboratory of Integrated Pest Management (LabMIP) of the Federal University of Santa Maria using the identification key of Hardwick (1965) from adults and larvae collected during the experiments’ evaluations. The voucher specimens were deposited at LabMIP.
The experiment was carried out in a randomized complete block design with four replications and plot sizes of 4 × 6 m (24 m2), distributed randomly with 0.5 m between each other on the field. Nine chemical and four biological insecticides were sprayed (Table 1; all insecticides were obtained from commercialized market insecticides). In both experiments, treatment applications were performed after 6:00 PM with a CO2 pressurized backpack sprayer and a flow rate of 150 L ha-1. The evaluations were conducted with a vertical beat sheet (Guedes et al., 2006) in order to count the number of small and large larvae collected on a 1.0 m2 area per plot at 3, 7, 10, and 14 days after spraying (DAS). Control efficiency (E) of treatments was calculated according to the equation (Abbott, 1925):

In the Restinga Seca experiment, grain yield was obtained by harvesting 2 km2 of each plot on the 5th of April 2014. The cost benefit ratio (C:B - how many dollars returned per dollar invested) of each treatment were calculated with additional yield over control treatment, the cost of insecticide application and net income. The Economic Injury Level (EIL) were calculated considering the yield loss (kg/ha) of one H. armigera larvae/m2 of 58 kg/ha (Rogers and Brier, 2010). The number of larvae (x) was transformed to the square root of x + 0.5 and submitted to joint analysis. The means were grouped using the Scott-Knott test (P>0.05).
Chemical control of H. armigera in soybean
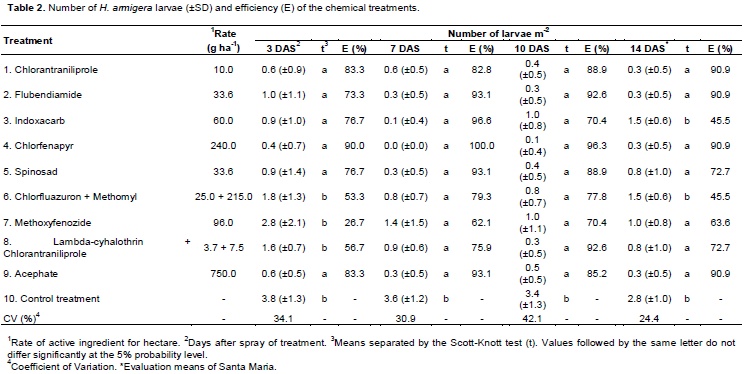
Most chemical treatments significantly reduced density of H. armigera larvae compared to the control treatment. At 3 DAS, control efficiency was the highest for chlorantraniliprole (83.3%), chlorfenapyr (90%), and acephate (83.3%; percent of control efficiency are indicated in parentheses after the treatments names; Table 2). At 7 DAS, control efficiency of larvae was above 90% for flubendiamide (93.1%), indoxacarb (96.6%), chlorfenapyr (100%), spinosad and acephate (93.1%). Chlorantraniliprole reduced the population density of H. armigera by 82.8%. At 10 DAS, chlorantraniliprole, flubendiamide, chlorfenapyr, spinosad, lambda-cyhalothrin + chlorantraniliprole, and acephate controlled the larvae >80%. Chlorantraniliprole, flubendiamide, chlorfenapyr, and acephate maintained >90% mortality of larvae at 14 DAS.
Chlorfenapyr (240 g a.i. ha-1) always had the highest control efficiency (at all evaluations). This insecticide uncouples oxidative phosphorylation in mitochondria, thereby interrupting ATP production (Raghavendra et al., 2011). Thereby, this insecticide can be used as an alternative mode of action during the same crop season, delaying or mitigating development of insecticide resistance on this pest.
Chlorantraniliprole and flubendiamide at tested doses of 10 and 33.6 g a.i. ha-1, respectively, had similar efficiencies against H. armigera consistent with their chemical group (anthranilic diamides). In cotton, flubendiamide 60 g a.i. ha-1 reduced the H. armigera larval population by decreasing crop damage by 96% (Thilagam et al., 2010). Furthermore, doses of chlorantraniliprole (31.5 to 52.5 g a.i. ha-1) effectively controlled H. armigera in cotton in Australia (Leven et al., 2011). Therefore, in the present study, doses of chlorantraniliprole and flubendiamide lower than those recommended elsewhere effectively controlled H. armigera. It demonstrates the importance of local evaluation of insecticides. It was found out that spinosad (33.6 g a.i. ha-1) was effectively >80% at 7 and 10 DAS. Similarly, it has high control efficiency (>90%) in cotton, albeit, at greater doses than those used in the present study (range: 72 to 96 a.i. ha-1) (Leven et al., 2011). The use of higher doses of spinosad was suspected to be related to larval resistance, as reported in Pakistan (Ahmad et al., 2003), India (Kranthi et al., 2000), and Australia (Gunning and Balf, 2002). Improved metabolism by cytochrome P450 oxidase may be predisposed to rapid development of resistance to spinosad (Wang et al., 2006). Indoxacarb had limited residual effects in our results. Vinaykumar et al. (2013) reported reductions within seven DAS on soybean. However, this insecticide had a low residual effect due to its high photodegradation (DT50 = 4.5 days at pH 5 and 25°C; FAO). Therefore, indoxacarb requires applications at 7 to 10 days intervals, due to low persistence, despite high initial efficacy.
Biological control of H. armigera in soybean
The mortality varied according to size of larvae and DAS. Therefore, results of biological treatments are separately shown. At 3 DAS, all treatments had efficiencies <70%, attributed to delayed pathology of Baculovirus or Bacillus thuringiensis (Table 3). Dipel® and Bt Control® had the highest control of small larvae (63.3%). Gemstar® controlled 47.4% of large larvae. At 7 DAS, Bt Control® and HzNPV CCAB® controlled 85.7 and 100%, respectively, of small larvae, whereas Dipel® and Bt Control® had a higher control of large larvae (86.8 and 73.3%, respectively). At 10 DAS, Bt Control® (100%), HzNPV CCAB® (100%), and Gemstar® (87.5%) had the highest control of small larvae. However, for large larvae, Bt Control® and HzNPV CCAB® had control efficiency of 84.2 and 78.9%, respectively. At 14 DAS, Bt Control®, HzNPV CCAB®, and Gemstar® had control efficiency of 100%.
Biological insecticides only had a significantly larvae mortality after 7 DAS, due to its contamination and action mechanism, in which the insecticide needs to be ingested by the larvae to become pathogenic to the insect. Bt Control® had a higher mortality, mainly of small larvae of H. armigera compared to Dipel®. Dipel® and Bt Control® had faster mortality to large larvae than Gemstar® and HzNPV CCAB®, which can be attributed to the median lethal time (LT50). Dipel® has a LT50 of 6.3 h for first instar larvae of H. zea (Junior et al., 2009), whereas for baculovirus it exceeds 3 days (Castro et al., 1999). Even though Dipel® and Bt Control® have the same active ingredient, the commercial products tested had distinct efficiencies due to their amount of B. thuringiensis spores/ml. Thereby, the dose of Dipel® to control H. armigera has to be increased.
Mortality also depends of larval stage and dose sprayed. The control efficiency of Bt Control®, Gemstar®, and HzNPV CCAB®, was higher to small larvae. Likewise, for the quantity of spores/ml, the amount of OBs on HzNPV CCAB® is higher than Gemstar®. Because of this, the control efficiency of HzNPV CCAB® reached 100% early for small larvae. These present findings were consistent with previous findings on larval stage and dose sprayed. Increase in the dose of OBs applied per larvae results in faster mortality and shorter survival time (Georgievska et al., 2010).
Soybean yield and benefit cost ratio from chemical and biological treatments
The active ingredients acephate (2,643 kg ha-1), spinosad (2,594 kg ha-1), chlorfenapyr (2,576 kg ha-1), chlorantraniliprole (2,447 kg ha-1), and flubendiamide (2,497 kg ha-1) had the highest soybean yield (Table 4), attributed to larvae control efficiency. In this way, the benefit cost ratio was higher for acephate (1:10.0), chlorantraniliprole (1:6.6), and flubendiamide (1:5.3), because of low cost of insecticide application and high soybean yield. Although, acephate had higher benefit cost ratio, should lead to the highest yield, because it had a similar control efficiency of chlorantraniliprole and flubendiamide. The insecticide acephate has to be retested. Conversely, even with excellent control efficiency and high productivity, the benefit cost ratio of chlorfenapyr and spinosad insecticides was only 1:3.7 and 1:4.3, respectively, because of their high spray cost.
The yield of the biological treatments, Bt Control® (286 kg ha-1), Gemstar® (297 kg ha-1), and HzNPV CCAB® (301 kg ha-1), differed from control treatment and Dipel® (Table 3). The benefit cost ratio was similar between Gemstar® and HzNPV CCAB® (baculovirus treatments), 1:5.0 and 1:5.7, respectively. Bt Control® had the highest benefit cost ratio (1:6.6). These biological insecticides had a similar benefit cost ratio to the chemical treatments acephate, chlorantraniliprole, and flubendiamide.
This result supports that an application of a biological insecticide affect the selectivity to natural enemies which naturally control the
pests.
H. armigera economic injury level (EIL)
Once the density and population distribution of H. armigera have been determined, the next step is to decide whether a control program is required by EIL. The EIL is the population density of an insect that causes economic loss equal to the control cost (Pedigo and Rice, 2006). The EIL depends on cost of insecticide application, value of soybean kilogram, the damage (in kg), and the efficiency of the control method/treatment used.
The insecticide Dipel® had a mean efficiency of 60%, with a spray cost of US$13.20. Considering the soybean bag value of US$15.00, the EIL for Dipel® is much lower (0.5 larvae m-2), mainly because of its low control efficiency (Table 5), moreover, to its low cost of insecticide application ha-1 (C). In general, the biological insecticides (Dipel®, Bt Control®, Gemstar®, and HzNPV CCAB®) had lower EILs compared to chemical insecticides. Therefore, biological insecticides should be applied in the beginning of an infestation by H. armigera. Conversely, the insecticide chlorfenapyr had the highest control efficiency among the evaluated insecticides. It means control efficiency of 94%, with the high cost of insecticide application ha-1 (US$35.90), soybean value of US$15.00 increased the EIL to 2.3 H. armigera larvae m-2. It means that an efficient treatment can support higher pest density and each soybean field has to be sampled to know the density of pest and to decide the correct time to start the control.
These findings support how to manage H. armigera on soybean in Brazil, looking at the control data from chemical and biological insecticides, the cost benefit ratio and the EIL.
Monitoring of H. armigera during all the soybean growth stages are essential to make decisions from these results on when to control, which insecticide, and the dose that will result in a higher benefit cost ratio.
The objectives of this study were supported with efficient alternatives of chemicals and biological insecticides to control H. armigera on soybean. Five chemical treatments are efficient to control H. armigera, from 3 to 14 DAS chlorantraniliprole, flubendiamide, chlorfenapyr, spinosad, and acephate. The biological treatment Bt Control® is efficient to control small and large larvae. Gemstar® and HzNPV CCAB® are efficient to control small larvae. The treatments, chlorantraniliprole, flubendiamide and acephate provided the highest yield and cost benefit ratio, which are similar to Bt Control®, Gemstar®, and HzNPV CCAB®. The EIL is flexible and range from 2.3 larvae m-2 to chlorfenapyr up to 0.2 larvae m-2 to Dipel®.