ABSTRACT
The amount of straw of sugarcane needed to remain in field for sustainability of the production system and quantity that could be used in sectors such as cogeneration and production of bioethanol for optimization of power generation by the sector are unclear issues. The aim of this study was to evaluate the effect of different amounts of straw on the development of the sugarcane root system and yield using the variety SP 801816 in a Rhodic Eutrudox in southern Brazil. Six treatments were evaluated: 0 (no straw), 25, 50, 75 and 100% (20 Mg ha-1) of straw and straw burned at 60, 180 and 270 days after planting in 150 m2 plots. Root samples were collected at 0.45 and 0.75 m from the planting line at depths of 0-0.10, 0.10-0.20, 0.20-0.40 and 0.40 to 0.60 m at harvest, and the stems of the plots were weighed to measure yield. In water stress period, the 50, 75 and 100% straw treatments promoted a greater root mass to 0.20 m deep, which was also reflected in the yield. The 50 and 75% straw treatments resulted in 25% greater yield than the 0 and 25% straw and straw burned, resulting in 28 Mg ha-1 more. The amount, 50% straw retention in the field is sufficient to increase the mass of the roots and, in turn, productivity, which is possible to remove the 50% surplus from the field for industrial processes for the production of energy, without the occurrence of damage to the crop.
Key words: Green cane, biomass, mechanized harvesting, agricultural waste, Saccharum spp.
Brazil supplies over 50% of the sugar sold globally and is the largest producer of sugar and sugarcane ethanol. The mechanized harvest of sugarcane, without straw removal by burning, is currently practiced in the main sugarcane production areas in Brazil, and this practice is increasing in current production areas (Viana et al., 2008). According to Oliveira et al. (1999), the straw that remains on the soil after harvest may range from 8-30 t ha-1 due to the variety and age of the plantation. This residue layer generates physical, chemical and biological changes in the agricultural environment which may interfere with rooting and, in turn, influence the final yield (Souza et al., 2005a; Costa et al., 2011).
Changes in the agricultural environment caused by the residue layer include the increase and stabilization of moisture and soil temperature, elevated levels of organic matter, improved fertility, greater efficiency in erosion control, and interference with light interception by the soil surface (Christoffoleti et al., 2007; Cavenaghi et al., 2007; Garcia et al., 2007; Guimarães et al., 2008, Panosso et al., 2010;, Shukla et al., 2013; Awe et al., 2014).
Despite the fact that root system of sugarcane play a vital role in the regeneration of ratoons after harvest, the root system has often been neglected in research, mainly due to the difficulty of observation, especially in field evaluations. The roots directly influence the efficiency of the absorption of nutrients by the plant, which affects drought resistance and tolerance to soil pests. These impacts are reflected in both the development of the culture and the final yield. According to Smith et al. (2005), root growth is dependent on the soil environment, which influences the shape and size of the root system, and the greater the mass of the roots, the higher the water and nutrient holding capacities.
Vasconcelos and Garcia (2005) states that death or renewal of the root system does not follow cane cutting but rather the water conditions to which the culture is exposed in a given period of development. In this way, the microclimate created by surface residue, characterized by high relative humidity, restriction of soil water loss and temperature stability, provides greater water availability than is found in bare soil. This can change the behavior of the roots and help minimize the decline from one production cycle to another, particularly during periods of water deficit.
Ball-Coelho et al. (1993) reported the positive effects of straw on the productivity of sugarcane under low or irregular rainfall, showing an increase of 43% in dry matter production of sugarcane in soil under straw.
Several authors describe the benefits of retaining sugarcane straw on the soil; however, the amount that is sufficient to promote such benefits and the effects of retaining smaller amounts, has not yet been investigated. Determining the minimum amount of straw to leave in the field is paramount to the sustainability of the sugarcane production system. This information also affects the energy generation sector because excess straw can be used for producing bioethanol and/or bioelectricity, sectors that have high demand for this material. (Azad et al., 2014). It is estimated that the use of straw and bagasse could triple Brazilian ethanol production (without increasing the plantation area) and this would produce the equivalent of 15% of the total energy consumed in Brazil by 2020 (Lima and Natalense, 2010).
Given the importance of this information and the lack of current literature, the aim of this study was to evaluate the effect of different amounts of straw on the sugarcane root system and its productivity in the first crop cycle (plant cane) using the variety SP801816 in a Rhodic Eutrudox soil.
This experiment was conducted at the Sugar and Alcohol Plant Bandeirantes, in the municipality of Bandeirantes, PR, at latitude 23° 06 'S and 50° 21' W 440 m. The climate in the region is Cfa (Koeppen climate classification), with annual rainfall of 1300 mm (average of 30 mm in the driest month).
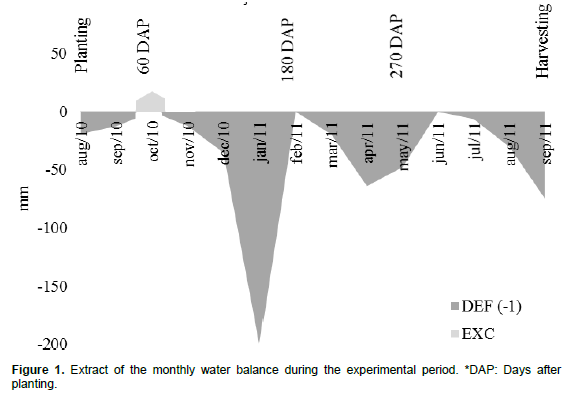
The climatic water balance (Figure 1) was calculated according to Thornthwaite and Mather (1955). Monthly average temperature and total monthly rainfall data from the weather station network of the Agronomic Institute of Paraná (IAPAR), located in Bandeirantes-PR, three kilometers from the experimental site, were used. For the available water capacity (CAD), 100 mm was used. The soil is classified as Rhodic Eutrudox (Santos et al., 2013) with a clayey texture. A particle size analysis showed that this soil was 61% clay, 2% silt and 37% sand.
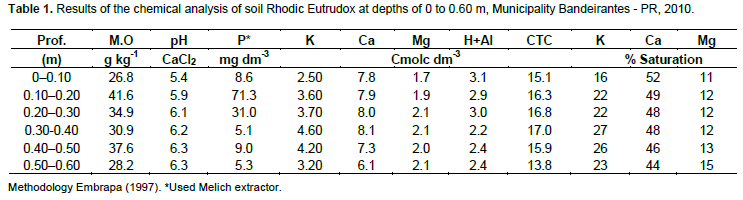
The apparent density and the soil chemical analyzes were performed in layers from 0-0.10, 0.10-0.20, 0.20-0.40 and 0.40-0.60 m deep in the soil profile before the initiation of the experiment, both according to methodology described by the EMBRAPA (1997).
The apparent density of soil was evaluated using the volumetric ring method (internal volume of 50 cm3) to verify the physical existence impediment to the development of the roots. The values of apparent density of soil in the layers were respectively, 1.33, 1.30, 1.30, 1.29 (g cm-3 of the soil).
The soil chemical analyzes results are described in Table 1. There was no need for chemical fertilizers. Prior to planting, 70 Mg ha-1 of filter cake was applied across the entire area after harvest to replenish the potassium extracted by the crop. The soil was prepared with a light harrowing, using a disk harrow.
In the experimental area, sugar cane has been grown for 65 years. During this period, a manual harvesting method with straw removal by burning was used, and in 2010, the plant adopted a mechanized harvesting system and an on-site test was conducted. The experiment was installed in August 2010 and conducted over a crop cycle (plant cane) in a randomized block design with four replications. Each plot consisted of 10 rows of sugarcane (variety SP 80-1816) that were 10 m long (10 x 10 m, 100 linear meters) with a 1.5 m spacing between rows. The data were collected on the six central lines, subtracting 0.50 m from each end. The harvest occurred in September 2011. The authors evaluated six treatments: 0 (no straw), 25 (5 Mg ha-1), 50 (10 Mg ha-1), 75 (15 Mg ha-1), 100% (20 Mg ha-1) and straw burned (20 Mg ha-1 of the straw was burned) on the root system of sugarcane at 60, 180, 270 days after planting (DAP) and on yield at 390 DAP.
The straw used was collected after mechanical harvesting in a cultivation area with the same variety of cane, which were demarcated in plots with the same measures as the experimental area. The amount of residue produced by this variety was estimated from the weight of the dry matter collected at each site. After weighing, the straw was evenly distributed over the experimental site, immediately after planting, according to the percentage required for each treatment.
To evaluate the root system, small trenches were opened between the rows (dimensions of 0.80 × 1.00 × 0.80 m, width, length and height, respectively) and metal cylinders (0.074 m diameter and 0.10 m height) were used for sampling, resulting in a volume of 0.00043 m3 ring (Azevedo et al., 2011). These were spiked with the aid of a hydraulic tensioner in the trench perpendicular to the seed row.
The volume of roots was evaluated horizontally, at a distance of 0.45 and 0.75 m from the planting line, at depths from 0 to 0.10, 0.10 to 0.20, 0.20 to 0.40 and 0.40 to 0.60 m, with four replicates per treatment (Figure 2). Each repetition consisted of three surveys conducted in each plot, to provide representation results. Subsequently, samples were taken to the Roots Study Laboratory, Department of Agriculture, Universidade Estadual de Londrina. Each sample was placed in a plastic bucket with water and stirred manually, then the water and roots in suspension were poured through a 1.0 mm mesh sieve until no soil remained.
All roots retained in the sieves were collected and held in a greenhouse at 65°C until they reached a constant weight. After drying, the roots were weighed on a precision balance, and the results are reported as roots mg per cm3 of soil at each depth.
The fresh mass of stems in milligrams of cane per hectare (Mg ha-1) was evaluated at 390 DAP (September 2011) by collecting all stems contained in the useful area of the plots. Green leaves were dried, tip clipped and then weighed.
The data were subjected to an analysis of variance (ANOVA) and means were compared using Tukey’s test at 1 and 5% significance levels. The software Sisvar 5.3 (Ferreira, 2010) was used for the analyses.
According to Tomé Jr. (1997), fertile soil with a good nutritional status has the following: 3 to 5% K, 10 to 15% Mg and 50 to 70% Ca. At a depth of 0.60 m, it was observed that the Ca content was very close to the range considered appropriate for that nutrient, and the Mg content was at the proper range for all measured depths (Table 1).
K showed a high saturation (above 20%), which may be because this nutrient is not connected to any organic constituent, is easily leached, and the concentration will decrease to the proper range during the rainy season because there were no new vinasse or mineral fertilizer applications.
Phosphorus (Table 1) showed adequate levels in the 0.30 to 0.40 and 0.50 to 0.60 m layers but was high in the other layers (Sousa and Lobato, 2004). Notably, the soil did not show any chemical (Table 1) or physical impediments to root growth. At 60 DAP, there were no roots at the 0.45 to 0.75 m distance from the planting line. At 60 DAP, cane plants had less roots than soca cane because the cane root system begins to develop at planting. In addition, the water deficit experienced during the months of August and September (Figure 1) may have contributed to the absence of roots in this period (Vasconcelos and Garcia, 2005). Water stress is one of the main limiting factors for sprout and root growth, and also reduces the total mass of the root system.
At 0.45 m from the planting line (Figure 3), there was effect of straw on the root system to a depth of 0.20 m. At both 180 and 270 DAP, there was a greater mean root mass in the treatments with 50, 75 and 100% straw, which was higher than for the other treatments, and these treatments did not significantly differ from each other. The 25% straw treatment was insufficient to make changes at any depth, and did not differ from the treatments with 0% straw and burned cane. At 0.75 m from the planting line (Figure 4), the same was observed at 0.45 m, indicating that the straw influenced the root system to the middle of the spacing.
Comparing the dry mass of roots (mg cm-3 soil) in both periods, we observed an increase in the amount of roots at 270 DAP at 0.20 to 0.40 m and at 0.40 to 0.60 m depth, both at 0.45 and 0.75 m from the planting line (Figures 3 and 4, respectively) for all treatments. This indicates a greater depth of the root system. These results confirm those of Vasconcelos and Garcia (2005), who found that under water deficit conditions, there is an increase in roots in the deeper soil layers because humidity is maintained for a longer time in these layers and this provides less resistance to new root penetration.
The significant effect of straw in the first year of cultivation may be due to the prolonged drought that occurred, with rainfall below the historical average, resulting in high water stress (up to 200 mm (Figure 1)). This drought caused low sugarcane yields throughout south-central Brazil, representing a decrease of 11.20% (CONAB, 2015). The water deficit was especially apparent in the early stages of crop development (up to 180 DAP) (Figure 1). Water deficit has a direct influence on the development of the root system. However, the results of this study show that the 50% straw treatment mitigated this effect, allowing a greater mass of roots.
The death or renewal of the roots is directly related to water availability and soil temperature. Water deficit results in a high expenditure of energy for the formation of new roots, which can vary according to the time of exposure to the deficiency (Vasconcelos and Garcia, 2005), and this also impacts productivity (Tavares et al., 2010 and Costa et al., 2014).
Thus, retaining sugarcane straw residue results in the highest rate of water infiltration and retention in the soil and lowers temperatures on the soil surface (Souza et al., 2005b; Awe et al., 2015), which are important benefits for the crop, especially in periods with water stress or irregular rainfall. Others studies have reported an approximately 70% reduction in soil water loss when planting under straw (Braunbeck and Magalhães, 2010).
In addition to the benefits gained by increased humidity, several authors (Oliveira, 1999; Resende et al., 2006) have found that as the straw decomposes, it releases nutrients into the soil, contributing to enhanced soil fertility and better rooting.
Soares et al. (2004) reported that periods of water stress can occur throughout the crop cycle, but damage to the plant and final stalk yield varies, greatly depending on the interaction between the time of the year in which the stress occurs and the plant’s phenological phase. They also suggest that water stress has a greater influence on sugarcane yield when it occurs in the early stages of culture and may hinder or delay the development of the root system and aerial parts, which agrees with the results obtained in the present study. According to Soares et al. (2004), when water stress occurs in other phenological phases, yield is rarely affected.
Alvarez et al. (2000) evaluated the effects of the management of raw cane and burned cane on rooting in a Red Latosol dystrophic. They found no significant differences in the first and second years of cultivation, to a depth of 0.60 m, which differs from the results of the present study. This lack of effect of the straw may be due to differences in genotype, age and the production environment of the evaluations. Alvarez et al. (2000) reported that there were practically no adverse weather events in either year of their study. Under relatively stress-free climatic conditions, it is likely that the effect of straw is not immediate, and the effects differ across regions or periods that have less favorable conditions, such as was found in the present study.
Vasconcelos and Garcia (2005), working with six varieties of cane over two years, evaluated the root dry matter in raw cane and burned cane at 0-0.20, 0.20-0.40, 0.40 to 0.60 and 0.60 to 0.80 m soil layers. Greater root development of the harvested green cane was found in only the 0-0.20 m layer, and this is in agreement with the results of our study. Vasconcelos and Garcia (2005) attributed this difference between treatments to greater soil moisture under the straw in the dry season, a higher calcium content from the decomposition of the straw on the soil surface, and a higher content of organic matter from microbial activity on the straw.
The distribution of the roots (Figures 3 and 4) on the assessed profile (up to 0.60 m depth) was similar to that of other tropical grasses, with an exponential decline in the biomass function with depth and a variability in their distribution (Smith et al., 2005).
The actual depth was 0.20 m for the distance of 0.45 m (Figure 3) from the planting line at 180 DAP. After the new water deficit period (April and May), the depth was 0.28 m at 270 DAP. For the distance of 0.75 m (Figure 4), the effective depth was 0.34 at 180 DAP and 0.40 m at 270 DAP, confirming the results obtained by Vasconcelos and Garcia (2005). This is a characteristic of the root system of the cane plant, which explores beyond superficial soil layers, in contrast with that of ratoon (Vasconcelos and Garcia, 2005). The greatest water infiltration, low humidity and temperature in the soil were provided by the straw in the surface layers in 0 to 0.20 m. This finding is important because it allows for the development and maintenance of roots during periods of lower water availability and high temperatures, precisely at the depth where the highest concentration of roots occurs.
Medina et al. (2002) found similar results when evaluating roots of the RB 785148 variety in a Rhodic, to a depth of 0.50 m. They found that there was a higher concentration of roots in the 0 to 0.25 m layer. Costa et al. (2007) evaluated the vertical distributions of RB83-5486 and RB83-5089 cultivar roots in an Oxisol and found that there was a greater root length in the first 0.18 m of soil. The root lengths declined from a 0- to 0.18- m depth to a 0.18 to 0.36-m depth.
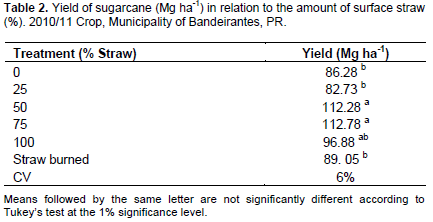
Straw coverage had a significant effect on final yield (Mg ha-1). The 50 and 75% treatments did not differ (112 Mg ha-1) and showed an average yield that was 23% higher than the 0, 25% straw and burned cane (86, 82 and 89 Mg ha-1, respectively) treatments, representing an increase of 26 Mg ha-1 (Table 2).
It was observed that the treatments influenced the root system to 0.20 m deep, resulting in a greater mass dry root (50, 75 and 100% straw treatments) and resulting in higher yield of sugarcane.
A natural characteristic of sugarcane is that it has the highest concentration of roots in the 0- to 0.20 m layer. This layer, being superficial, is vulnerable to climatic conditions, directly influencing the root system and causing a sharp drop in production under unfavorable environmental situations. Thus, the use of a management practice that provides a greater stability in this layer will result in a more developed root system, which consequently helps mitigate yield declines in sugarcane exposed to adverse weather conditions.
Resende et al. (2006) evaluated the effect of harvesting with and without straw burning after 16 years of cultivation. They found a 15% increase in yield of sugarcane under straw when compared with burned cane in the evaluated ratoons from 1988 to 1992. From 1992 to 1999 (after the renewal of the sugarcane culture), these values reached 59 and 12 to 28% in harvest ratoons. Resende et al. (2006) also stressed that the greatest differences occurred mainly in years with a lower rainfall, indicating that the presence of straw in the field was beneficial in conserving soil moisture. These results agree with those obtained by Oliveira et al. (1999) and those of this study.
Despite the benefits obtained with straw retention in this study and those described by these authors, there are others who reported disadvantages in using 100% straw for raw cane (Campos et al., 2008; Campos, 2010). Notably, the negative results described have typically been found in 100% straw retention; therefore, it is not possible to determine whether the same effect would occur with smaller amounts of this material. We observed that different amounts of residue retained in the field have different effects. This is particularly important if we consider that recently, the use of this waste as a feedstock for second generation ethanol and bioelectricity production has been one of the main alternatives to supply the world's growing energy demand, and we must determine how much straw retention is required to guarantee the sustainability of the production system.
Overall, 50% (10 Mg ha-1) straw retention provided enough residue to promote improvements in the root system and yield of sugarcane. Above 50%, there were no statistically significant responses. The withdraw of the 50% surplus straw from the field for use in industrial processes did not cause damage to the crop yield (Figures 3, 4 and Table 2).
1. The 50, 75 and 100% straw treatments promoted a greater root mass to a depth of 0.20 m.
2. The effective depth for the 0.45 m distance from the planting line was 0.28 m and was 0.40 m for 0.75 m at 270 DAP.
3. The harvesting with straw burning, the total removal of straw from the field or the 25% straw maintenance reduced crop yield of sugarcane in periods with a water deficit.
4. Retaining of the 50% straw on the field is sufficient to increase the mass of roots and yield of sugarcane, even in periods of water stress.
The authors have not declared any conflict of interests.
REFERENCES
|
Alvarez IA, Castro PRC, Nogueira MCS (2000). Crescimento de raízes de cana crua e queimada em dois ciclos. Sci. Agric. 57(4).
Crossref
|
|
|
|
Azevedo MCB, Chopart JL, Medina CC (2011). Sugarcane root length density and distribution from root intersection counting on a trench-profile. Sci. Agric. 68(1).
Crossref
|
|
|
|
|
Azad MAK, Islam MS, Amin L (2014). Straw availability, quality, recovery, and energy use of sugarcane. Biomass Bioenerg. 53:11-19.
Crossref
|
|
|
|
|
Awe GO, Reichert JM, Timm LC, Wendroth O (2014). Temporal processes of soil water status in a sugarcane field under residue management. Plant Soil. 387(1):395-411.
|
|
|
|
|
Awe GO, Reichert JM, Wendroth OO (2015). Temporal variability and covariance structures of soil temperature in a sugarcane field under different management practices in southern Brazil. Soil Till. Res. 150:93-106.
Crossref
|
|
|
|
|
Braunbeck AO, Magalhães PSG (2010). Avaliação tecnológica da mecanização da cana-de-açúcar. In: Cortez LAB Bioetanol de cana-de-açúcar. 1 ed. São Paulo: Blucher. pp. 451-475.
|
|
|
|
|
Ball-Coelho B, Tiessen H, Stewart JWB, Salcedo IH, Sampaio EVSB (1993). Residue management effects on sugarcane yield and soil properties in Northeastern Brazil. Agron. J. 85:1004-1008.
Crossref
|
|
|
|
|
Campos LHF, Carvalho SJP, Christoffoleti PJ, Fortes C, Silva JS (2008). Crescimento e produtividade da cana-de-açúcar (Var.SP83-2847) submetida a três manejos da palhada. STAB 26(6):33-36.
|
|
|
|
|
Campos LHF (2010). Sistemas de manejo da palhada influenciam acúmulo de biomassa e produtividade da cana-de-açúcar (var. RB855453). Acta. Sci. Agron.
Crossref
|
|
|
|
|
Cavenaghi AL, Rossi CVS, Negrisoli E, Costa EAD, Velini ED, Toledo REB (2007). Dinâmica do herbicida amicarbazone (Dinamic) aplicado sobre palha de cana-de-açúcar (Saccharum officinarum). Planta Daninha.
Crossref
|
|
|
|
|
Christoffoleti PJ, Carvalho SJP, López-Ovejero RF, Nicolai M, Hidalgo E, Silva JE (2007). Conservation of natural resources in Brazilian agriculture: Implications on weed biology and management. Crop Prot. 26(3):383-389.
Crossref
|
|
|
|
|
Costa MCG, Mazza JÁ, Vitti GC, Jorge LAC (2007). Distribuição radicular. Estado nutricional e produção de colmos e de açúcar em soqueiras de dois cultivares de cana-de-açúcar em solos distintos (1). Rev. Bras. Cienc. Solo. R. Bras. Ci. Solo 31:1503-1514.
Crossref
|
|
|
|
|
Costa CTS, Ferreira VM, Endres L, Ferreira DTRG. Gonçalves ER (2011). Crescimento e produtividade de quatro variedades de cana-de-açúcar no quarto ciclo de cultivo. Revista Caatinga. Mossoró. v. 24(3):56-63.
|
|
|
|
|
Costa LG, Marin FR, Nassif DSP, Pinto HMS, Lopes-Assad MLRC (2014). Simulação do efeito do manejo da palha e do nitrogênio na produtividade da cana-de-açúcar. Rev. Bras. Eng. Agric. Ambient. 18(5):469-474.
Crossref
|
|
|
|
|
CONAB- COMPANHIA NACIONAL DE ABASTECIMENTO (2015).
|
|
|
|
|
Acompanhamento da safra brasileira: cana-de-açúcar. Segundo Levantamento, agosto/2014, Brasília: CONAB, 18 pp.
|
|
|
|
|
EMPRESA (1997). Manual de métodos de análise de solo. Rio de Janeiro. Centro Nacional de Pesquisa de Solos. 212 pp.
|
|
|
|
|
Ferreira DF (2010). Programa computacional Sisvar - UFLA. versão 5.3. 2010.
|
|
|
|
|
Guimarães ER, Mutton MA, Mutton MJR, Ferro MIT, Ravaneli GC, Silva JA (2008). Free proline accumulation in sugarcane under water restriction and spittlebug infestation. Sci. Agric. 65(6).
Crossref
|
|
|
|
|
Garcia JFG, Grisoto E, Botelho PSM, Parra JRP, Appezzato-da-Glória B (2007). Feeding site of the spittlebug Mahanarva imbriolata (STAL)(Hemíptera: Cercopidae) on sugarcane. Sci. Agric. 64(5):555-557.
Crossref
|
|
|
|
|
Lima MAP, Natalense APP (2010). Necessidade de pesquisa básica para cana e etanol. In: Cortez LAB (ed). Bioetanol de cana-de-açúcar. 1 ed. Blusher. São Paulo. pp. 150-170.
|
|
|
|
|
Medina CC, Neves CSVJ, Fonseca CBF, Torreti AF (2002). Crescimento radicular e produtividade de cana-de-açúcar em função de doses de vinhaça em fertirrigação. Semina: Ci. Agrárias. 23(2).
|
|
|
|
|
Oliveira MW, Trivelin PCO, Gava GJC, Penatti CP (1999). Degradação da palhada de cana-de-açúcar. Sci. Agric. 56(4).
Crossref
|
|
|
|
|
Panosso AR, Marques Jr J, Milorib DMBP, Ferraudo AS, Barbieri DM, Pereira GT, Scala Jr NL (2010). Soil CO2 emission and its relation to soil properties in sugarcane areas under Slash-and-burn and Green harvest. Soil Till. Res. 111(2):190-196.
Crossref
|
|
|
|
|
Resende AS, Santos A, Xavier RP, Coelho CH, Gondim A, Oliveira OC, Alves BJR, Boddey RM (2006). Efeito da queima da palhada da cana-de-açúcar e de aplicações de vinhaça e adubo nitrogenado em características tecnológicas da cultura. Rev. Bras. Cienc. Solo. 30(6).
Crossref
|
|
|
|
|
Santos HG, Jacomine PKT, Anjos LHC et al (2013). Sistema Brasileiro de Classificação de Solos.
|
|
|
|
|
Soares RAB, Oliveira PFM, Cardoso HR, Vasconcelos ACM, Landell MGA, Rosenfeld U (2004). Efeito da irrigação sobre o desenvolvimento e a produtividade de duas variedades de cana-de-açúcar colhidas em início de safra. STAB – Açúcar. Álcool and Subprodutos 22:38-41.
|
|
|
|
|
Smith DM, Inman-Bamber NG, Thorburn PJ (2005). Growth and function of the sugarcane root system. Field Crop Res. 92(2-3):169-183.
Crossref
|
|
|
|
|
Sousa DMG, Lobato E (2004). Adubação com nitrogênio. In: Sousa DMG. Lobato E (ed) Cerrado: correção do solo e adubação. 2ed. Embrapa Cerrados. Planaltina. pp. 129-144.
|
|
|
|
|
Souza ZM, Paixão ACS, Prado RM, Cesarin LG, Souza SR (2005a). Manejo de palhada de cana colhida sem queima, produtividade do canavial e qualidade do caldo. Cienc. Rural. 35(5).
Crossref
|
|
|
|
|
Souza ZM, Prado RM, Paixão ACS, Cesarin LG (2005b). Sistemas de colheita e manejo da palhada de cana-de-açúcar. Pesq. Agropec. Bras. 40(3).
|
|
|
|
|
Shukla SK, Lal M, Sing SK (2013). Improving bud sprouting, growth and yield of winter initiated sugarcane ratoonthrough tillage cum organic mediated rhizospheric modulation in Udic ustochreptunder subtropical Indian condition. Soil Till. Res. 126:50-59.
Crossref
|
|
|
|
|
Tavares OCH, Lima E, Zonta E (2010). Crescimento e produtividade da cana-planta cultivada em diferentes sistemas de preparo do solo e de colheita. Acta Sci. 32(1):61-68.
Crossref
|
|
|
|
|
Tomé Júnior JB (1997). Manual para interpretação de análise de solo. Guaíba. Rio Grande do Sul.
|
|
|
|
|
Thornthwaite CW, Mather JR (1955). The water balance. Publications in Climatology. Drexel. New Jersey.
|
|
|
|
|
Vasconcelos ACM, Garcia JC (2005). Desenvolvimento radicular da cana-de-açúcar. Cana-de-açúcar: ambientes de produção. POTAFOS. Piracicaba.
|
|
|
|
|
Viana RS, Silva PH, Mutton MA, Mutton MJR, Guimarães ER, Bento M (2008). Efeito da aplicação de maturadores químicos na cultura da cana de açúcar (Saccharum spp.) variedade SP81-3250. Acta. Sci. Agron.
Crossref
|
|