ABSTRACT
The crambe is an oilseed plant of the Brassicaceae family, which has the potential to grow in the winter, in succession to soybean crop, and whose seeds have been used for extraction of oil for biofuel production. However, in areas with nematode infestation, special care is needed in the selection of the species to be used in crop succession. Thus, the present study aimed to assess the susceptibility of crambe to plant-parasitic nematode, Meloidogyne javanica and its interference in vegetative growth parameters: crop yield and seed oil content. Therefore, crambe (Crambe abyssinica Hochst) seedlings were inoculated with a suspension containing 0, 1300, 2600 and 5200 eggs and second-stage juveniles (J2) of M. javanica, in experiment 1 (January to March, 2012), and 0, 1000, 2000 and 4000 eggs and J2, in experiment 2 (May to July, 2012). The tomato plant was inoculated to assess the viability of the inoculum. The assessments were done at two different times: 60 days after inoculation, assessment of the nematode reproduction factor, plant height and fresh and dry mass of the aerial part, was done with four repetitions. At the end of the crop cycle, 90 days after inoculation, seed yield and oil content were assessed in the remaining four replications. When grown in a period of higher temperatures, the crambe showed susceptibility to root-knot nematodes, with a negative impact on plant yield. However, this did not occur when the plant was grown in a more favorable season. In both experiments, seed oil content was not affected by the presence of the nematode.
Key words: Crambe abyssinica, oil, susceptibility, nematodes.
The crambe is a cruciferous winter oilseed, belonging to the Brassicaceae family (Machado et al., 2008), originating in the Mediterranean and with short cycle, which ranges from 90 to 100 days (Oplinger et al., 1991). The plant is 70-90 cm tall, blooms 35 days after seeding, and the seeds are rounded and covered with a gray integument. Its main characteristic is the high concentration of oil, with levels ranging from 35 to 60% and used in biodiesel production (Oliveira et al., 2011).
Being a very robust plant, the crambe can grow in very adverse climate conditions, tolerating the frosts typical of the south of the country and temperatures as low as minus 6°C (Pilau et al., 2011), and show good yield in the dry season (Oplinger et al., 1991). Because it is resistant to drought and low temperature periods, crambe has been an alternative to sowing the winter crop in no-tillage system, with subsequent planting of soybeans (Pitol et al., 2010; Oliveira et al., 2011). However, there is little information on the management and potential of this plant, including its use as an alternative control importance of nematodes to soybeans, as Meloidogyne javanica (Treub) Chitwood and Pratylenchus brachyurus (Godfrey) Filipjev and Schuurmans Steckhoven.
The soybean, Glycine max (L.) Merrill is currently the most important crop in the world. According to data of USDA (2015), soybean yield in the 2015/16 harvest was 106.58 million of tons, in a total of 33.79 million hectares cultivated.
Despite the high yield, damage caused by phytonematodes are very common, and the losses caused by them have become a matter of growing concern among producers and researchers (Asmus, 2004; Kubo et al., 2004; Machado et al., 2006). The main nematodes associated with soybean crops include Meloidogyne incognita (Kofoid & White) Chitwood and M. javanica (Treub) Chitwood, that form root galls, Pratylenchus brachyurus (Godfrey) Filipjev & Schuurmans Stekh which cause root lesions, as well as the cyst nematode, Heterodera glycines Ichinohe, and the reniform nematode, Rotylenchulu sreniformis Linford & Oliveira (Dias-Arieira and Chiamolera, 2011).
The nematode galls are important plant pathogens that affect agricultural production worldwide (Al-Raddad, 1995). This is favored by the high reproductive capacity of the nematodes, which leads to the rapid growth of populations in the field (Ferraz, 1985).
Due to the limited number of varieties resistant to nematodes, adapted to the different farming regions and the lack of nematicides registered for the crop, alternative methods to control the pest are constantly sought. Therefore, the use of non-host plants in rotation or succession of cultures with soybean is one of the main management strategies. With this practice, the populations of nematodes can be kept below the economic damage threshold, without any risk to the environment (Carneiro et al., 2007). The main crops to be used in succession to soybean are corn, oats, wheat, and, to a lesser extent, sunflower (EMBRAPA, 2004).
Despite the aggressiveness of phytonematodes, which attack crops of commercial interest, there is limited information on the reproduction of M. javanica and its interference in vegetative and productive parameters of the crambe. In view of the above considerations, the present study aimed at assessing the susceptibility of the crop to M. javanica, the interference of this nematode on the vegetative parameters of the crop and on the seeds and oil content.
The experiments were conducted in greenhouse, at Universidade Estadual de Maringá, Campus Regional de Umuarama, Umuarama, Paraná, Brazil, in completely randomized designs, with four treatments (levels of initial inoculum) and eight replications. Period of the experiments: January to March 2012 (experiment 1) and repeated from May to July 2012 (Experiment 2).
The average temperatures recorded during the experimental periods were minimum of 21.2 and maximum of 31.9°C in experiment 1, and minimum of 12.9 and maximum of 21.7°C in experiment 2, according to data obtained from SIMEPAR (2013).
Initially, crambe seeds of the variety MS Brilhante were sown in trays containing the substrate Plantmax®. Seedlings were transplanted 15 days after germination to vases with capacity of 2 L, with two seedlings per vases. The substrate used was soil classified as Oxisol Dystrophic with a sandy texture (USDA, 1998), which was previously autoclaved for two hours at constant temperature of 120°C.
Two days after transplantation, the crambe was inoculated with suspension of M. javanica. In Experiment 1, the initial populations (Pi) were 0, 1300, 2600 and 5200 eggs and second-stage juveniles (J2), and in experiment 2, they were 0, 1000, 2000 and 4000 eggs and J2. The tomato plant of Santa Clara variety was inoculated with 5200 and 4000 eggs, in the respective experiments, to demonstrate the viability of the inoculum.
The inoculum was obtained from a pure population of nematodes, kept in the tomato plant, and extraction began in the roots, which were ground with a sodium hypochlorite solution in a blender, according to the method proposed by Hussey and Barker (1973) and adapted by Boneti and Ferraz (1981). The suspensions were calibrated using Peter’s chamber, under optical microscope. Inoculation was performed by adding 4 mL of the respective suspensions to four equidistant holes, opened in the soil, around the plant.
After 60 days of cultivation, four plants of each treatment were randomly evaluated on vegetative parameters: plant height, dry mass of the aerial part, the latter obtained by drying in forced circulation at 65°C for three days. Regarding the nematological parameters, the number of galls and eggs per root was determined, which was considered the final population (Pf). The eggs were extracted according to the cited methodology and assessed in optical microscope in Peter’s chamber.
The nematode reproduction factor (RF) in the crambe was calculated using the formula RF = Pf/Pi, as proposed by Oostenbrink (1966), in which plants are considered susceptible when RF≥1, resistant when RF<1 and immune when RF=0.
At the end of the crop cycle, the remaining plants were assessed for the number of seeds per plant, seed weight and oil content. The extraction of oil from the seeds was performed in laboratory using methodology proposed by Silva et al. (2015).
The data were subjected to analysis of variance and the means to regression analysis, with a significance level of 5%, for the data concerning the inoculum levels used in the crambe. For the comparison of data with the susceptibility pattern (tomato plant), the means were compared by Tukey test at 5% probability, using the SISVAR 5.3 software (Ferreira, 2010).
In Experiment 1, the crambe was susceptible to M. javanica, with reproduction factors equal to 3.3, 2.6 and 1.5, when inoculated with 1300, 2600 and 5000 eggs, respectively. In the second experiment, RF values were equal or close to 1, with means of 1.0, 0.7 and 0.8 for plants inoculated with 1000, 2000 and 4000 eggs, respectively. In both experiments, the reproduction values of the nematode in the crambe were significantly lower than those obtained in the tomato plant, used as control treatment, whose RF values were 14.9 and 53.8 in Experiments 1 and 2, respectively.
The difference in the reproductive activity of M. javanica in the two years can be explained by the experimental period. In Experiment 1, plant growth occurred from January to March, being affected by the high temperatures, resulting in physiological stress that made the plants susceptible to the establishment of the pathogen in the roots, while Experiment 2 was conducted in May and June, period of milder temperatures, which is more appropriate for the crambe crop (Knights, 2002; Pitol et al., 2010; Silva et al., 2013).
The climate factors have also directly impacted the reproductive activity of root-knot nematodes, because the genus Meloidogyne spp. usually has high activity in the soil and roots under high temperatures, and its ideal temperature for reproduction is 25°C. However, species such as M. incognita and M. javanica cannot survive at temperatures lower than 10°C (Ritzinger et al., 2010; Campos et al., 2011). Campos et al. (2011) reported that the reproduction of M. javanica in soybean plants increased at a temperature of 28°C, both in resistant and susceptible varieties.
The number of galls and eggs was found to be directly proportional to the population levels of M. javanica (Figures 1 and 2). In experiment 1, the relationship between the number of galls and eggs in crambe roots and the population of phytonematodes was best fitted by the quadratic equation (Figures 1A and 2A). Both parameters showed decrease when the population of nematodes was higher than 4000 eggs (Figure 1A). This was possibly due to food restriction, as it has been observed in other pathosystems involving Meloidogyne spp., in which increase in nematode population reduced the number of galls, giving rise to the hypothesis that the parasite had difficulty establishing feeding sites, due to the competition generated by the penetration of a large number of J2 in the roots (Schochow et al., 2004; Fabry et al., 2009).
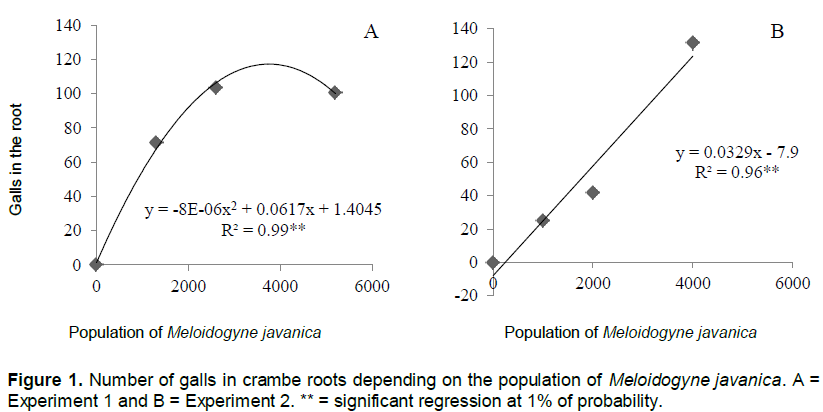
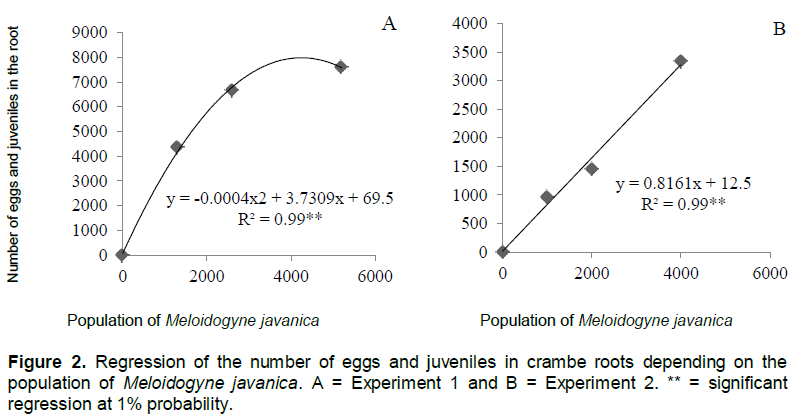
In Experiment 2 (Figures 3B and 4B), the number of galls and eggs showed linear increase in relation to the initial population of M. javanica. The data obtained corroborate the findings of Souza et al. (2011), that classified the crambe as susceptible to this nematode, and its reproduction was directly proportional to the increase in inoculums levels of the parasite.
Asmus and Andrade (2001) also reported crambe susceptibility to M. javanica. However, the authors observed that the reproduction of the phytonematode was less intense than in other hosts, such as canola and quinoa. Despite the multiplication of M. javanica in the crambe, RF indexes are still lower than those of other cultures commonly used in crop succession, such as sunflower, RF = 29.15 (Rosa et al., 2013) and black oat, RF=6.85 (Asmus et al., 2005).
The crambe is a commercially new culture, and, thus, further studies are needed on this species. Nevertheless, some authors have investigated the susceptibility of other brassicas to phytonematodes, and their results corroborate our experiment showing the susceptibility of Brassica napus Linnaeus (rapeseed) and Brassica campestris L. (turnip rape) to Meloidogyne chitwoodi Golden, O’Bannon, Santo and Finley 1 and 2, and M. hapla Chitwood (Mojtahedi et al., 1991), canola to M. hapla and M. incognita (Bernard and Montgomery-Dee, 1993), and Sinapis alba L. (mustard) to M. enterolobii Yang and Eisenback (M. mayaguensis Rammah and Hirschmann) (Brito et al., 2007), Raphanus sativus L. var. oleiferus Metzg. (forage turnip) to M. javanica (Rosa et al., 2013) and Brassica oleracea to M. javanicae and M. incognita (Dias-Arieira et al., 2012). In turn, studies conducted by Charchar et al. (2007) indicated that black mustard (Brassica nigra (L.) Koch.) was considered a bad host for a mixed population of M. incognita and M. javanica.
Analysis of vegetative parameters of the crambe (Table 1) showed that the presence of the nematodes did not affect plant growth. In comparison with data related to plant height found in the literature and cited by Oliveira et al. (2011), experiment 2 showed that, the development of the aerial part was nearly ideal for the culture, confirming that the period of the experiment was more favorable.
Despite the susceptibility of the crop, RF values close to 1 indicate that the crambe could be an option for use in crop succession systems, particularly because it ensures economic benefits to the producer. Also, since it is a species of Brassicaceae, release of glucosinolates may occur in the process of waste decomposition, as already observed for several species of the same family (Mojtahedi et al., 1991; Potter et al., 1999; Mazzola et al., 2001; McCully et al., 2008; Rizzardi et al., 2008; Reardon et al., 2013).
However, Mojtahedi et al. (1991) observed that the suppressive effect of rapeseed on the nematodes was only possible when crop residues were incorporated to the soil.
In experiment 1, the increase in the population of M. javanica promoted the proportional decrease in seed production (Figure 3A). The same was observed in experiment 2 (Figure 3B). However, this decrease occurred in populations of up to 2000 eggs. The increase in population levels led to increase in the production of crambe seeds. Similar results were observed for the parameter mass of seeds per vases (Figure 4).
Although increase in inoculum levels of the nematode affected the number of seeds and mass of seeds, a difference was observed in the fitting of the regression equation in experiment 1, the reductions were linear and in Experiment 2, there was reduction up to the level of 2000 eggs per plant.
Some factors can explain these results, and the temperature during the experimental period is possibly the most important. According to Falasca et al. (2010), the ideal temperatures for the vegetative period of the crambe are between 15 and 25°C. But in experiment 1, the experiment was conducted from January to March, during which temperatures were above the ideal for the crop, ranging from 21.2 to 31.9°C. Thus, the decrease in seed production cannot be associated only with increase in nematode population, but also to adverse climate conditions, as observed in previous studies that reported that the crambe had lower growth rates under higher temperatures, resulting in lower grain production (Silva et al., 2013).
On the other hand, in experiment 2 carried out in the months of May and July, the climate conditions were more favorable to plant development, with temperatures between 12.9 and 21.7°C, which are indicated for crambe crop (Knights, 2002; Pitol et al., 2010; Silva et al., 2013). Therefore, the decrease in seed production, and, consequently, in seed mass, can be directly associated with the increase in the population of M. javanica up to the level of 2.000 eggs per plant, in which the plant production recovered. The increase in seed production may have occurred because of the competition between nematodes for feeding sites, as already discussed.
Despite the decrease in seed production (Table 2), oil content was not significance, in the two experiments, showing that the population levels of M. javanica did not influence oil content in crambe seeds. The average oil production of the analyzed plants was 30.5 and 30.3% in experiments 1 and 2, respectively. According to Laghetti et al. (1995), the crambe has an oil content of approximately 38% in the mass, higher than the one found in the present study, which was about 30%.
Being a rustic species, the crambe did not show changes in oil content in the seeds, even under unfavorable climate and soil conditions, as those found in experiment 1. Silva et al. (2013) also observed the oil content in crambe seeds was not affected when the plants were grown in different climate conditions. Thus, it can be inferred that even in conditions of higher population levels of M. javanica in the soil, oil content in the seeds will not be affected.
The reproduction of M. javanica was lower at the season most favorable for crambe vegetative development, indicating that winter cultivation can be recommended, since the pathogen will have decreased reproductive activity. Also, the crop will not be affected, which demonstrates that crambe is a cost-effective crop. However, further research in this field is needed to confirm this hypothesis.
The crambe was susceptible to root-knot nematodes. However, parasitism did not affect the vegetative growth of the crop. Regarding the production of crambe seeds, it was affected by the nematode population, in association with climate factors, such as high temperatures. The population of nematodes in the plants did not affect the seed oil content.
The authors have not declared any conflict of interests.
REFERENCES
|
Al-Raddad AM (1995). Interaction of Glomus mosseae and Paecilomyces lilacinus on Meloidogyne javanica of tomato. Mycorrhiza 5:233-236.
Crossref
|
|
|
|
Asmus GL (2004). Ocorrência de nematoides fitoparasitos em algodoeiro no Estado de Mato Grosso do Sul. Nematol. Bras. 28:77-86.
|
|
|
|
|
Asmus GL, Andrade PJM (2001). Reprodução do nematoide das galhas (Meloidogyne javanica) em algumas plantas alternativas para uso em sucessão à cultura da soja. Comunicado Técnico, 37. Embrapa Agropecuária Oeste, Dourados.
|
|
|
|
|
Asmus GL, Inomoto MM, Sazaki CSS, Ferraz MA (2005). Reação de algumas culturas de cobertura utilizadas no sistema plantio-direto a Meloidogyne incognita. Nematol. Bras. 29:47-52.
|
|
|
|
|
Bernard EC, Montgomery-Dee ME (1993). Reproduction of plant-parasitic nematodes on winter rapeseed (Brassica napuss sp. oleifera). J. Nematol. 25:863-868.
|
|
|
|
|
Boneti JIS, Ferraz S (1981). Modificação do método de Hussey & Barker para extração de ovos de Meloidogyne exigua em raízes de cafeeiro. Fitopatologia Bras. 6:553.
|
|
|
|
|
Brito JA, Stanley JD, Mendes ML, Cetintas R, Dickson DW (2007). Host status of selected cultivated plants to Meloidogyne mayaguensis in Florida. Nematropica 37:65-71.
|
|
|
|
|
Campos HD, Silva JRC, Campos VP, Silva LHCP, Costa LSAS, Silva WJR (2011). Efeito da temperatura do solo na infectividade e reprodução de Meloidogyne javanica e Heterodera glycines em cultivares de soja. Ciênc. Agrotecnologia 35:900-907.
Crossref
|
|
|
|
|
Carneiro RG, Moritz MP, Mônaco APA, Nakamura KC, Scherer A (2007). Reação de milho, sorgo e milheto a Meloidogyne incognita, M. javanica e M. paranaensis. Nematol. Bras. 31:9-13.
|
|
|
|
|
Charchar JM, Gonzaga V, Vieira JV, Oliveira VR, Moita AW, Aragão FAS (2007). Efeito da rotação de culturas no controle de Meloidogyne spp. em cenoura na região norte do estado de Minas Gerais. Nematol. Bras. 31:173-179.
|
|
|
|
|
Dias-Arieira CR, Chiamolera FM (2011). Cresce a incidência de nematoides em milho e soja. Rev. Campo. Negócios 7:18-20.
|
|
|
|
|
Dias-Arieira CR, Cunha TPL, Chiamolera FM, Puerari HH, Biela F, Santana SM (2012). Reaction of vegetables and aromatic plants to Meloidogyne javanica and M. incognita. Hortic. Bras. 30:322-326.
Crossref
|
|
|
|
|
EMBRAPA. Tecnologia de produção de soja, região central do Brasil 2004: sistemas de produção. Embrapa Soja (2004). Available at: http://www.cnpso.embrapa.br/producaosoja/rotacao.htm
|
|
|
|
|
Fabry CFS, Freitas LG, Lopes EA, Ferraz S (2009). Eficiência de Rhizobiumetli como agente de biocontrole de Meloidogyne javanica e M. incognita. Nematol. Bras. 33:99-102.
|
|
|
|
|
Falasca SL, Flores N, Lamas MC, Carballo SM, Anschau A (2010). Crambe abyssinica: An almost unknown crop with a promissory future to produce biodiesel in Argentina. Int. J. Hydrogen Energy 35:5808-5812.
Crossref
|
|
|
|
|
Ferraz S (1985). Summary report on the current status, progress and needs for Meloidogyne research in Brazil (region III). In: Sasser JN, Carter CC (ed). An Advanced Treatise on Meloidogyne 1.Biology and Control. North Carolina State University, Raleigh. pp. 351-352.
|
|
|
|
|
Ferreira DF (2010). SISVAR - Sistema de análise de variância. Versão 5.3. Lavras-MG: UFLA.
|
|
|
|
|
Knights SE (2002). Crambe, a North Dakota case study. North Dakota: RIRDC. P 25.
|
|
|
|
|
Kubo RK, Oliveira CMG, Monteiro AR, Ferraz LCCB, Inomoto MM (2004). Ocorrência de nematoides do gênero Pratylenchus em cafezais do estado de São Paulo. Nematol. Bras. 28:159-165.
|
|
|
|
|
Laghetti G, Piergiovanni AR, Perrino A (1995). Yield and oil quality in selected lines of Crambe abyssinica ex R.E. Fries and C. hispanica L. grow in Italy. Ind. Crops Prod. 4:203-212.
Crossref
|
|
|
|
|
Machado ACZ, Beluti DB, Silva RA, Serrano MAS, Inomoto MM (2006). Avaliação de danos causados por Pratylenchus brachyurus em algodoeiro. Fitopatologia Bras. 31:11-16.
Crossref
|
|
|
|
|
Machado MF, Brasil NA, Oliveira LS, Nunes DL (2008). Estudo do crambe (Crambe abyssinica) como fonte de óleo para produção de biodiesel. Itaúna/MG – UFMG.
|
|
|
|
|
Mazzola M, Granatstein DM, Elfving DC, Mullinix K (2001). Suppression of specific apple root pathogens by Brassica napus seed meal amendment regardless of glucosinolate content. Phytopathology 91:673-679.
Crossref
|
|
|
|
|
McCully ME, Miller C, Sprague SJ, Huang, CX, Kirkegaard JA (2008). Distribution of glucosinolates and sulphur-rich cells in roots of field-grown canola (Brassica napus). New Phytol. 180:193-205.
Crossref
|
|
|
|
|
Mojtahedi H, Santo GS, Hang AN, Wilson JH (1991). Suppression of root-knot nematode populations with selected rapeseed cultivars as green manure. J. Nematol. 23:170-174.
|
|
|
|
|
Oliveira NAMO, Guerra N, Maciel CDG, Silva TRB, Lima GGR (2011). Seletividade de herbicidas aplicados em pré-emergência na cultura do crambe. Rev. Bras. Herbicidas 10:49-56.
Crossref
|
|
|
|
|
Oostenbrink R (1966). Major characteristics of the relation between nematodes and plants. Mededeelingen der Landbouw-Hoogeschool. 66:1-46.
|
|
|
|
|
Oplinger ES, Kelling KA, Kaminsid AR, Doll JD, Oelke EA, Putnam DH, Durgan BR, Teynor TM (1991). Crambe: Alternative field crops manual. University of Wisconsin and University of Minnesota. St. Paul, MN 55108 Avaailable at:
View
|
|
|
|
|
Pilau FG, Battisti R, Somavilla L, Schwerz L (2011). Temperatura basal, duração do ciclo e constante térmica para a cultura do crambe. Bragantia 70:958-964.
Crossref
|
|
|
|
|
Pitol C, Broch DL, Roscoe R (2010). Tecnologia e Produção: Crambe. Maracaju, Editora: Fundação MS. P 60.
|
|
|
|
|
Potter MJ, Vanstone VA, Davies KA, Kirkegaard JA, Rathjen AJ (1999). Reduced susceptibility of Brassica napus to Pratylenchus neglectus in plants with elevated root levels of 2-Phenylethyl Glucosinolate. J. Nematol. 31:291-298.
|
|
|
|
|
Reardon CL, Strauss SL, Mazzola M (2013). Changes in available nitrogen and nematode abundance in response to Brassica seed meal amendment of orchard soil. Soil Biol. Biochem. 57:22-29.
Crossref
|
|
|
|
|
Ritzinger CHSP, Fancelli M, Ritzinger R (2010). Nematoides: bioindicadores de sustentabilidade e mudanças edafoclimáticas. Rev. Bras. Frutic. 32:1289-1296.
Crossref
|
|
|
|
|
Rizzardi MA, Neves R, Lamb TD, Johann LB (2008). Potencial alelopático da cultura da canola (Brassica napus L. var. oleifera) na supressão de picão-preto (Biden ssp.) e soja. Rev. Bras. Agrociênc. 14:239-248.
|
|
|
|
|
Rosa JMO, Westerich JN, Wilcken SRS (2013). Reprodução de Meloidogyne javanica em olerícolas e em plantas utilizadas na adubação verde. Trop. Plant Pathol. 38:133-141.
Crossref
|
|
|
|
|
Schochow M, Tjosvold SA, Ploeg AT (2004). Host status of Lisanthus 'mariach lime green' for three species of root-nematode. HortScience 39:120-123.
|
|
|
|
|
Silva TRB, Reis ACS, Nolla A, Dias-Arieira CR, Tavares-Silva CA, Gouveia BT, Mascarello AC, Carraro TV, Arieira JO (2013). Nitrogen top dressing application and growing season of crambe cultivated on two crop year. Int. J. Food Agric. Environ . 11:1463-1466.
|
|
|
|
|
Silva TRB, Rogério F, Santos JI, Poletine JP, Gonçalves Júnior AC (2015). Oil quantification of crambe seeds calcination method in muffle furnace. J. Agron. Sci. 4:106-111.
|
|
|
|
|
SIMEPAR. Instituto Tecnológico Simepar (2013). Dados de variáveis climatológicas da região de Cascavel entre 2010 a 2012.
|
|
|
|
|
Souza RA, Ribeiro RCF, Rocha LS, Xavier AA, Soares-Martins IP, Silva FJ (2011). Reação de crambe (Crambe abyssinica Hochst) à Meloidogyne javanica. In: Congresso Brasileiro De Fitopatologia, 44. Anais. Bento Gonçalves, RS. P 781.
|
|
|
|
|
USDA (1998). Keys to soil taxonomy. United States Department of Agriculture, New York. Available at:
View.
|
|