Maize (Zea mays L.) has a range of uses, from animal to human feed and can be consumed in nature, or as feedstock material in the preparation of starches, flours, canjicas, breads, beverages and porridges. This study aimed to determine the linear associations, estimates of genetic parameters and heterosis of bioactive and micronutrients compounds in maize. The experimental design was randomized blocks, the treatments consisted of six open pollinated varieties (OPVs) of maize: Dente de Ouro Roxo (P1), BRS Missões (P2), Caiano Rajado (P3), AL 25 (P4), Bico de Ouro (P5), Argentino Branco (P6) and five hybrids maize derived from crosses, P2 x P1, P3 x P1, P4 x P1, P5 x P1 e P6 x P1. Linear high and positive associations are expressed between the seed width and hundred kernel weight, between the antioxidants DPPH and ABTS radical, and through the manganese and zinc. The genetic parameters determine the progenies and the parent-average relationship are superior to the length, width and acidity of seeds, total phenols and copper. The heritability in a wide sense expressed is greater for the traits: acidity seeds, antioxidant DPPH radical and copper. The heritability in a restricted sense is superior to the traits length, width and acidity of seeds, carotenoids and sodium content. The traits thickness and color of seeds, total phenols, antioxidant ABTS radical and zinc are increased through the effects of intervarietal heterosis in maize. Estimates of genetic parameters obtained can be used in genetic maize breeding programs, in order to obtain biofortified genotypes using bioactive compounds and micronutrients.
Maize (Zea mays L.) is in the family of Poaceae, with agricultural, social and economic importance. In Brazil, its cultivation covers the various producing regions, where emphasis is laid on increasing productivity per unit area. This cereal has a range of uses, which range from animal and human feed, and can be consumed in nature, or as feedstock material in the preparation of starches, flours, canjicas, breads, beverages and porridges (Estrada et al., 2014). Thus, in many parts of Africa, Mexico and Brazil, maize is a source of energy and mineral, affordable and low cost to the population (Paes, 2006). Currently, the programs of genetic improvement of maize recommend obtaining superior genotypes, efficiently improving management techniques available (Ribeiro et al., 2016).
In this context, to minimize the effects of the food and nutritional deficiency, and prevent cardiovascular and chronic diseases non transmissible as cancer in low-income populations (Costa et al., 2013), an efficient alternative and economically variable is direct breeding programs to obtain maize genotypes enriched by bioactive and micronutrients compounds (Zilic et al., 2016). Bioactive compounds (carotenoids and flavonoids) are derived from secondary plant metabolism (Silva et al., 2010), confer to the food, the antioxidant capacity that minimizes the negative effects of reactive oxygen species and nitrogen (Wen et al., 2012; Costa et al., 2013), reducing the harmful free radicals to the human organism (Sikora et al., 2008). In order to obtain superior genotypes for bioactive and micronutrients compounds, it is necessary to search for greater genetic variability for traits of interest (Cardoso et al., 2009), thus, the use of open pollinated varieties (OPVs) presented variable to clarify the needs of improving the culture (Setimela et al., 2007). However, it is possible that the obtaining of intervarietal hybrid can contribute substantially to the increase of these compounds. With the lack of information regarding the genetic parameters of these traits, this study aimed to determine the linear associations, estimates of genetic parameters and heterosis of bioactive and micronutrients compounds in maize.
The experiment was conducted in 2015 at Genomics Center and Plant Breeding of the Federal University of Pelotas. The seeds were obtained in the 2014/2015 crop year in the Agricultural Center of Palma in Capão do Leão-Brazil, in latitude 31 47 '58' 'S and longitude 52° 31' 02 '' O, with an altitude of 13.2 m. The soil is characterized as Argisol red yellow dystrophic. The experimental design was randomized blocks with 11 treatments arranged in three replications. The treatments consisted of six open pollinated varieties (OPVs) of maize: Dente de Ouro Roxo (P1), BRS Missões (P2), Caiano Rajado (P3), AL 25 (P4), Bico de Ouro (P5), Argentino Branco (P6), and five hybrids maize derived from crosses, P2 x P1, P3 x P1, P4 x P1, P5 x P1 e P6 x P1.
The seeds were stored in cold chamber at ±4°C for 180 days, with homogeneity 13% humidity. Subsequently, the samples were subjected to the cleaning to the exclusion of strange particles, 500 g of seed per genotype was obtained, which were homogenized again and the measurements of the pre-grinding of the seeds traits was done. After the seeds were crushed in Marconi® MA 020 mill fitted with a 0.053 mm sieve, the ground sample of each genotype was divided into three sub-samples of 150 g, being directed to the Secondary Metabolism Laboratory UFPel, Brazil.
The measured traits in pre-grinding were: seed length (SL), seed width (SW), seed thickness (ST) both expressed in mm and measured with the assistance of a digital paquimeter in 90 seeds per genotype; hundred kernel weight (HW), in grams. The measured traits in post-grinding were: seed color (SC) in Hue angle; seed acid (AC) in percent of citric acid; pHin hydrogenionic potential; soluble solids (SS) in °Brix (AOAC, 2005), total phenols (TP) in μg g-1 (Singleton and Rossi,1965); total flavonoids (TF) in mg g-1 (Zhishean et al., 1999); total carotenoids (TC) in mg g-1 (AOAC, 2005); antioxidant potential by DPPH (DP), as a inhibition percentage (Brand-Williams et al., 1995); antioxidant potential by ABTS radical (AB), in inhibition percentage (Rufino et al., 2007). The micronutrients: copper (Cu), zinc (Zn), sodium (Na), manganese (Mn) and iron (Fe) were expressed in mg kg-1 (Tedesco et al., 1995).
The data were submitted to analysis of variance at 5% probability in order to verify their presuppositions, the normality test of Wilk (1965), and homogeneity of variances by Bartlett (Steel et al., 1996) was used. Subsequently, a descriptive analysis by the distributions of phenotypic frequencies, and in conjunction the parent average (PA) for the distinction of upper and lower classes were used. Pearson correlation was performed in order to show the trend of associations among traits where their coefficients were based on the classification of Carvalho et al. (2004). The data were standardized by the rating of Z scores, as:

Where xi: is the observed value, μ: corresponds to the average, and θ: the standard deviation of the trait (Cruz et al., 2014). The genetic parameters were obtained by the father-average regression method and progenies (covariance of additive variance), and the angle β coefficient corresponds to the total genetic variance (Falconer, 1987). The father-average correlation with the progenies was estimated (r), the phenotypic variance (Vp), additive variance (Va), heritability in the broad sense (H²), heritability in the narrow sense (h²) and heterosis percentage were estimated with the methodology proposed by Ramalho et al. (2012). The analyses were performed using Excel and Genes software (Cruz, 2013).
Analysis of variance for traits
Analysis of variance revealed significant difference at P>0.05 probability for the 18 traits measured in open pollinated varieties (OPVs) and maize hybrids. The coefficients of variation (CV%) obtained in the experiment presented range of 0.74 to 19.76% and showed accuracy in conducting the experiment, transmitting reliability of the data. Thus, nine traits obtained CV less than 10% with high precision, others are evident in the range of 10 to 20% being ranked with good experimental precision (Gomes, 2000).
Descriptive analysis by the distributions of phenotypic frequencies
The hybrids maize were submitted to descriptive analysis by frequency distributions, in order to reveal the number and amplitude of the phenotypic classes formed, besides showing that these comprise the largest number of genotypes. The seed length (SL) formed five classes with amplitude of 9.9 to 12.9 mm (Figure 1). Classes of 10.5 and 12.3 mm comprise 66.7% of hybrids maize, however, the overall parents average (PA: 12.10 mm) discriminated classes 12.3 and 12.9 mm with 46.7% upper progenies due to intervarietal crossing. The seed width (SW) formed four classes with amplitude of 9.2 to 10.8 mm (Figure 1B). The classes of 9.2 and 9.6 mm comprise 73.3% of hybrids maize, and the overall average of the parents (PA: 9.78 mm) points out that the class of 10.0 and 10.8 mm have 26.6% of the upper progenies.
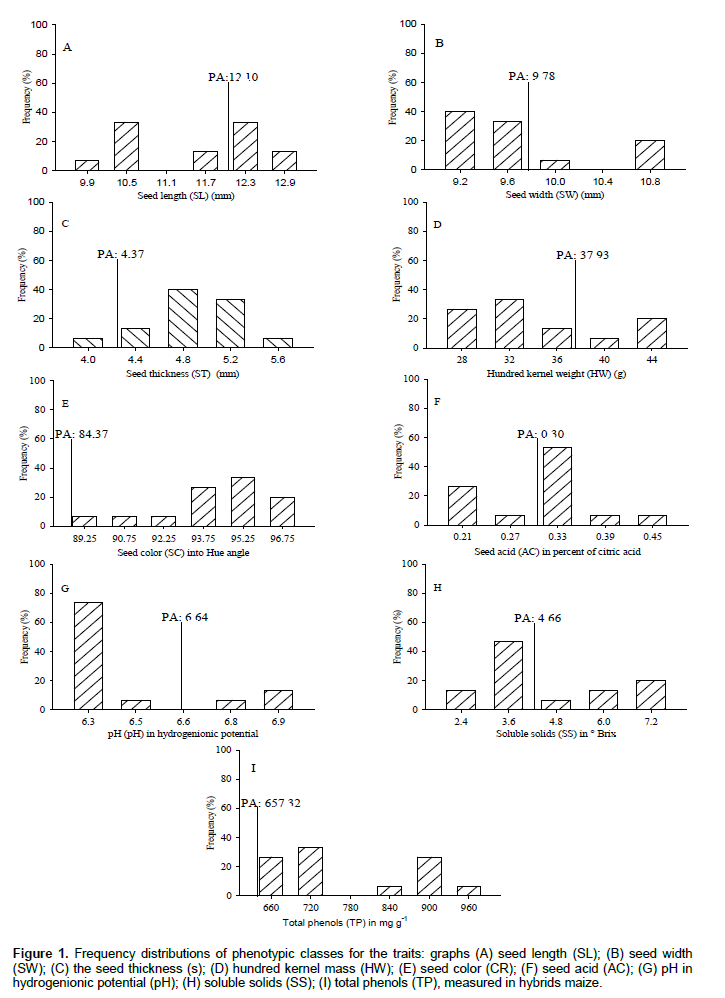
The seed thickness (ST) formed five classes with amplitude of 4.0 to 5.6 mm (Figure 1C). The class of 4.8 mm corresponds to 40% of hybrids maize, and the overall parents average (PA: 4.37mm), revealed that the classes of 4.4; 4.8; 5.2 and 5.6 mm included 93.3% of the superior progenies. Research has shown that the dimensions of maize seeds only changed the initial establishment of plants, morphological and productivity are not influenced (Vazquez et al., 2012). In another report, effects of seed size on maize physiological characteristics and seedling growth were not observed (Sangoi et al., 2004). The hundred kernel weight (HW) formed five classes with amplitude of 28 to 44 g (Figure 1D). The class with 32 g included 33.3% of hybrids maize, and the overall parents average (PA: 37.9 g) distinguished the classes of 40 and 44 g with 26.7% of the superior progenies. Crossings intervarietal in partial diallel with 18 OPVs obtained 96 hybrids of maize where 46.1% increased hundred kernel weight from the effects of heterosis (Oliveira et al., 2007). Research determined that regardless of the type of intersection that get simple hybrid of maize, double or triple, direct and positive associations are consolidated among the hundred kernel weight, kernel weight of ear and productivity (Lopes et al., 2007; Nardino et al., 2016).
The seed color (SC) formed six classes with amplitude of 89.2 to 96.7 of the Hue angle (Figure 1E). The class of 95.2 corresponded to 33.3% of hybrids maize which can be classified as yellow (Hue angle ± 90), the overall parent average (PA: 84.37 Hue) is intermediate to red colorations (Hue angle<90) and yellow (Hue angle ± 90), where all intervarietal crossings provided increase in the color of the seeds with superior progenies angle Hue to parents. The color of maize seeds is due to the proportion of existing carotenoid (Kimura et al., 2007). The most frequent carotenoids in maize are lutein, zeaxanthin and β-carotene (Silva et al., 2010). The seed acid (AC) formed five phenotypic classes with amplitude of 0.21 to 0.45% (Figure 1F). The class with 0.33% showed 53.3% of hybrids maize, and the overall parents average (PA: 0.30%) distinguished classes of 0.33; 0.39 and 0.45% acidity with 66.7% of the progeny superior to parents. The seeds pH (pH) formed with four classes amplitude from 6.3 to 6.9 (Figure 1G). The class of 6.3 is higher with 73.3% of hybrids maize, and the overall parents average (PA: 6.64) revealed that the classes of 6.8 and 6.9 obtained 20% of the superior progenies. The acidity is inversely proportional to pH, with the higher acidity of the fruit, conducive for higher respiratory rate of plant stages, in physiological maturity, the acidity tends to decrease due to the decrease in metabolic activity (Junior et al., 2008). In maize, the major metabolic rates are obtained during the reproductive period after fertilization of the ovules, which coincides with the definition of the magnitude of seeds per ear (Argenta et al., 2003).
The soluble solids (SS) formed five classes with amplitude of 2.4 to 7.2 °Brix (Figure 1H). The class of 3.6 °Brix is higher with 46.7% of hybrids maize, and the overall parents average (PA: 4.66 °Brix) distinguish classes of 4.8, 6.0 and 7.2 °Brix with 40% of the superior progenies. The soluble solids in maize are directly influenced by the characteristics of genotype, photosynthetic efficiency of the plant and the water supply, where the largest proportions are expressed as milky seed in R3 stadium. In this way, the increase in soluble solids provides larger dimensions and kernel weight (Magalhães and Durães, 2006). The total phenols (TP) formed five classes with amplitude of 660 to 960 μg g-1 (Figure 1I). The 720 μg g-1 class corresponds to 33.3% of hybrids maize, and the overall parents average (PA: 657.32 μg g-1) indicated that all obtained progenies were superior to their parents due to heterosis of intervarietal crossings. Phenols are compounds of secondary metabolism and contribute positively to the antioxidant potential (Silva et al., 2010).
The total flavonoids (TF) formed five classes with amplitude of 400 to 800 μg g-1 (Figure 2A). The class of 400 μg g-1 is higher with 33.3% of hybrids maize, and the overall parents average (PA: 495.45 μg g-1) discriminated classes 500, 600, 700 and 800 μg g-1 with 66.7% of the superior progenies. The flavonoids contributed to the development of the plant and assist in protecting the tissues to light stress, the incidence of insect pests minimize the free radicals by acting as antioxidants, regulating the hormonal and enzymatic balance, thus, searching maize define the exogenous application of flavonoid that influenced increased productivity (Oliveira and Fernandes, 2008). The total carotenoids (TC) formed four classes with amplitude of 15 to 165 μg g-1 (Figure 2B). The class with 105 μg g-1 is higher with 53.3% of hybrids maize, and the overall parents average (PA: 83,64μg g-1) provided distinguished the classes of 105, 135 and 165 μg g-1 with 80% of the superior progenies. Studies carried out on 10 OPVs of maize revealed that total carotenoids are formed by larger fractions of xanthophylls, zeaxanthin, β-cryptoxanthin and β-carotene (Rios et al., 2012). The maize breeding is aimed increasing carotenoids in the seeds to obtain genotypes with higher content of provitamin A (Cardoso et al., 2009).
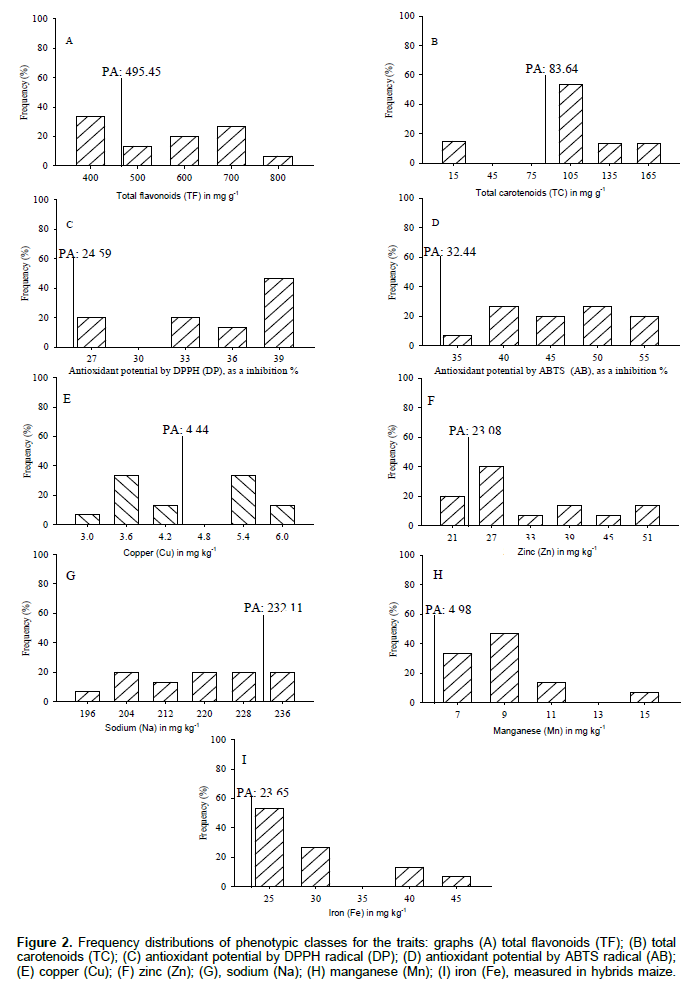
The antioxidant potential was obtained against DPPH (DP) formed in four classes of amplitude 27-39% inhibition of the radical (Figure 2C). The class with 39% inhibition corresponds to 46.7% of hybrids maize, and the overall parents average (PA: 24.59%) shows that all progenies are superior to parents. The antioxidant potential obtained against ABTS (AB) was formed with five classes with amplitude of 35-55% inhibition of the radical (Figure 2D). Classes of 40 and 50% were higher with 53.3% of hybrids maize, and the overall parents average (PA: 32.44%) shows that all progenies are superior to parents. The antioxidant activity minimizes free radicals and oxidative stress in humans, leading to compounds with antioxidant activity such as the carotenoids, phenols and flavonoids (Silva et al., 2010).
The content of copper (Cu) formed five classes with an amplitude of 3 to 6 mg kg-1(Figure 2E). The classes of 3.6 and 5.4 mg kg-1 was higher with 66.7% of hybrids maize, and the overall parents average (PA: 4.44 mg kg-1) discriminated classes of 5.4 and 6.0 mg kg-1 with 46.7% of the superior progenies. Cu is a constituent of enzymes and proteins, which operates in photosynthetic processes, respiration, electron transport, is a component of secondary metabolites primarily of phenols, and closely related to the development of seeds (Kirkby and Romheld, 2007). The content of zinc (Zn) formed six classes with amplitude of 21 to 51 mg kg-1 (Figure 2F). The 27 mg kg-1 class was higher with 40% of hybrids maize, and the overall parents average (PA: 23.08 mg kg-1) distinguishes the class of 27, 33, 39, 45 and 51 mg kg-1 with 80% superior progenies. The Zn actively participates in the interactions between enzymes and substrates, helps tryptophan synthesis and auxin which are responsible for the growth of plant tissues, influence positively the carbohydrates, proteins and membranes (Kirkby and Romheld, 2007), elongation of the stem internodes, accumulation of biomass and seed yield in maize (Andreotti et al., 2001).
The sodium content (Na) formed six classes with amplitude of 196 to 236 mg kg-1 (Figure 2G). The hybrids maize have uniform distribution among the phenotypic classes, and the overall parents average (PA: 232.11mg kg-1) distinguishes 236 mg kg-1 class with 20% of the superior progenies. The manganese (Mn) shows four classes formed with amplitude of 7 to 15 mg kg-1 (Figure 2H). The 9 mg kg-1 class stands higher with 46.7% of hybrids maize, and the overall parents average (PA: 4.98mg kg-1) shows that all progenies were superior to parents. The Mn acts directly on photosynthetic processes with the breakdown of water and electron transport molecule, minimizes the effects of reactive oxygen species and is an enzyme cofactor that assists in secondary metabolism of phenols and flavonoids. In maize, it is crucial for male inflorescence, viability of anthers and pollen, and closely linked to the physiological potential of seeds (Kirkby and Romheld, 2007). The iron (Fe) formed four classes with amplitude of 25 to 45 mg kg-1 (Figure 2I). The class of 25 mg kg-1 is higher with 53.3% of hybrids maize, and the overall parents average (PA: 23.65 mg kg-1) shows that all progenies were superior to their parents. The Fe comprises the proteins responsible for electron transport, is an enzyme activator that regulates the biosynthesis of chlorophyll and function of chloroplasts, in contrast, groups phenols fractions for lignification of tissues, especially the root (Kirkby and Romheld, 2007). Few genes control the iron accumulation in the seeds of efficiency, but are dependent on the phloem transport and the amount of minerals contained in the plant tissues (Rios et al., 2009).
Pearson linear correlation between traits
The Pearson correlation was performed in order to identify the tendency of associations among 18 traits measured in open pollinated varieties (OPVs) and intervarietal hybrids maize (N=33). 153 linear associations among traits, 42 significant at the P>0.05 probability was carried out (Table 1). The linear correlation coefficients followed the classification proposed by Carvalho et al. (2004). The seed length (SL) has intermediate and positive trend with HW (r=0.42), however, intermediate and negative trends with ST (r=-0.52), pH (r=-0.53) and SS (r=-0.41). Maize seeds with larger longitudinal dimensions tend to increase its weight and reduce the thickness by changing the compliance of the endosperm. In this way, the modifications affect the accumulation of total sugars and cause a decrease in pH. Smaller ears direct assimilated available to a smaller number of seeds, the increased kernel weight is due to the transformation of soluble sugars in starch between the stages R2 and R6, which results in maximum dry matter accumulation in maize seed (Magalhães and Durães, 2006).
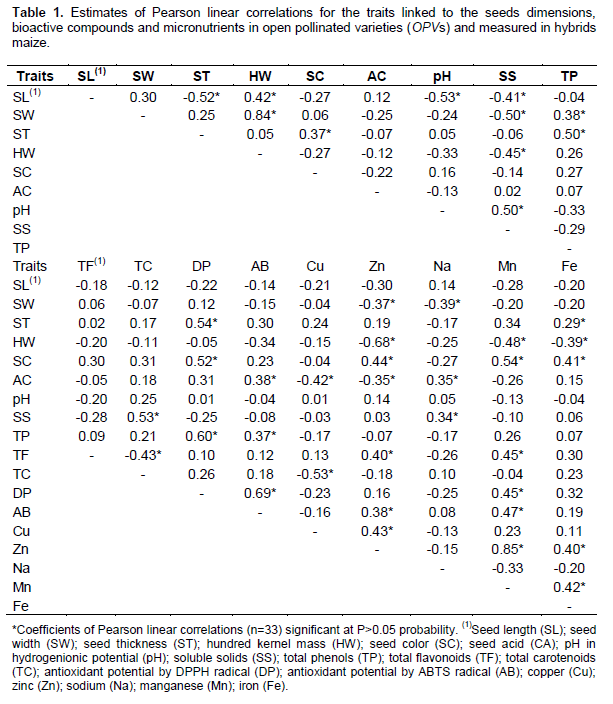
The seed width (SW) shows high positive trend with HW (r=0.84) and with the intermediate and positive TP (r=0.38), however, intermediate and negative with the SS (r=- 0.50), Zn (r=-0.37) and Na (r=-0.39). The results indicate that larger seeds have the greatest weight, and increase total phenolic concentration with decrease in the mineral fraction through the elements Zn, and Na. The seed thickness (ST) has intermediate and positive trends with SC (r=0.37), TP (r=0.50), DP (r=0.54) and Fe (r=0.29). Maize seeds when thick tend to express stronger coloration, increase the pigments contained in their integument, which is indicative of greater accumulation of phenolic compounds and carotenoids. In this way, genotypes with high phenolic fraction are superior to the antioxidant potential and iron accumulation in the seed. The antioxidant potential is closely related to the phenolic fraction of vegetable, where the largest proportion of carotenoids in maize (lutein and zeaxanthin) minimizes the negative effects of reactive oxygen species (Silva et al., 2010). Research indicates that OPVs of maize have higher concentrations of iron in the seeds as compared to hybrids and larger proportions of this mineral are contained in the embryo seeds with hard seed coat (Castro et al., 2009).
The hundred kernel weight (HW) reveals high and negative trend with Zn (r=-0.68), intermediate and negative with SS (r=-0.45), Mn (r=-0.48) and Fe (r=-0.39). Inverse associations between kernel weight and mineral buildup is justifiable because the endosperm is formed by larger proportions of starches carbohydrates, and the embryo assign the largest mineral fractions (Castro et al., 2009). Thus, the kernel weight increment does not show abrupt effect on the accumulation of minerals. In contrast, the increase in Zn and Fe ratios in maize seed can be achieved with efficient use of the best genitors, in order to explore the hybrid combination by additive and non-additive effects together with combine selection strategies that prioritize get genotypes with higher proportions of micronutrients and productivity (Menkir, 2008).
The color of the seeds (SC) expressed intermediate and positive trends with DP (r=0.52), Zn (r=0.44), Mn (r=0.54) and Fe (r=0.41). The seeds with more pigmented integument tend to have higher proportions of bioactive compounds with increased antioxidant activity and the ability to suppress reactive oxygen species (Silva et al., 2010). These effects are enhanced by Fe functionality to transport electrons, where Mn assists the secondary metabolism, Zn mainly in the synthesis of hormones auxin, all micronutrients of which are enzyme cofactors (Kirkby and Romheld, 2007). The seed acid (AC) showed positive tendencies and intermediate AB (r=0.38) and Na (r=0.35), however, intermediate and negative Cu (r=-0.42) and Zn (r=-0.35). The pH is associated with intermediate and positive SS (r=0.50). The pH is proportional to the fraction of soluble sugars and inversely to the acidity. Thus, the sodium accumulation in seeds coats resulted in increased acidity, and inhibit the ABTS radical, however, micronutrients Cu and Zn decrease. Research in coffee clarify the dynamics of associations of bioactive compounds where lower proportions of soluble sugars and proteins indicate increasing acidity, the phenolic compounds and therefore the antioxidant activity (Abrahão et al., 2010).
The soluble solids (SS) show intermediate and positive association with the TC (r=0.53), total phenols (TP) have intermediate positive trend with DP (r=0.60) and AB (r=0.37). The antioxidant potential obtained by DPPH radical expressed high positive association with ABTS radical (r=0.69). These combinations indicate linear dependence between the radicals and preliminarily, which can be explained through similar dynamics. For OPVs and hybrid of maize, antioxidant activity are attributed to total phenols, where most soluble solids fraction increases the total carotenoids (lutein, zeaxanthin, β-cryptoxanthin and β-carotene) (Kimura et al., 2007). Therefore, maize seed are more pigmented due to carotenoids which may have antioxidant activity.
Zn is associated with intermediate and positively with TF (r=0.40), AB (r=0.38) and Cu (r=0.43). While Mn presents highly positive trend with Zn (r=0.85), and intermediate and positive with TF (r=0.45), DP (r=0.45) and AB (r=0.47). In contrast, the Fe relates to intermediate and positive way with Zn (r=0.40) and Mn (r=0.42). By identifying traits which define the antioxidant potential in maize, it appears that both Fe and total flavonoids tend to increase Zn and Mn, and both inhibit ABTS radical, and contribute to antioxidant activity.
Parent-progeny regression and obtaining the estimation of genetic parameters
The relation of the progenies with the father-average (r) represents how the characteristic may be associated with offspring by crossing (Table 2). The size of the seeds show that SL (r=0.21) and SW (r=0.27) indicate greater contribution from genitors to progeny with respect to ST (r=0.13) and HW (r=0.09). Phenotypic variation of the traits SL, SL, ST and HW were determined by 11.7, 19.8, 1.5 to 6.5% of additive effects, respectively. Heritability in wide sense (H²) shows that the largest genetic variations are conditioned to traits SW, ST and HW, however, indicates that the SL is affected by 83.1% of environmental effects. The heritability in restricted sense (h²) defines actually heritable fraction of genetic variation characteristic. Thus, SL and SW show low heritability (0.11 and 0.19) originating from additive effects, the results obtained for the ST and HW results from the dominance deviations. The obtained genetic parameters in 28 intervarietal hybrids of maize derived from 8 OPVs indicate that the kernel weight is determined by heterosis and dominance effects (Bernini et al., 2012).
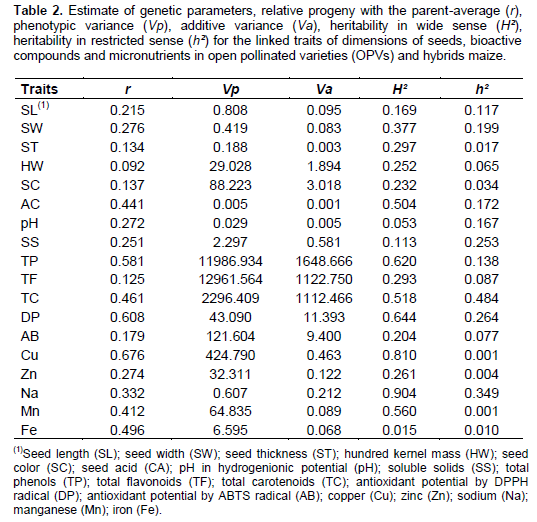
Among the constitutional parameters of seeds, AC has progenies relationship with the father-average (r=0.44) superior to SC (r=0.13), pH (r=0.27) and SS (r=0.25). Phenotypic variation of SC, AC, pH and SS are from 3.4, 20.0, 17.2 and 25.2% of additive effects, respectively. Heritability in wide sense indicates greater genetic variation for AC character (H²=0.50). On the other hand, the SS are influenced by 88.7% of environmental effects. The heritability in restricted sense shows superior additive effects for AC trait (h²=0.17), pronounceable dominance deviations are expressed for the SC (h²=0.03).
For phenolic constituents and antioxidant radicals, it is observed that the TP and the DP are higher in relation progenies father-average (r=0.58) and (r=0.60) respectively, associated with TF (r=0.12), TC (r=0.46) and AB (r=0.17). Phenotypic variation of TP, TF, CT, DP and AB are the result of 13.7, 8.6, 48.4, 26.4 and 7.7% of additive effects, respectively. Heritability in wide sense indicates greater genetic variation for TP (H²=0.62), CT (H²=0.51) and DP (H²=0.64), however, the TF and AB are influenced by 70.7 and 79.6% of environmental effects. The heritability in restricted sense indicates additive effects of TC (h²=0.48), higher dominance deviations are assigned to TF (h²=0.08) and AB (h²=0.07).
Among the micronutrients, the Cu expressed relation of progenies with father-average higher (r=0.67) than Zn (r=0.27), Na (r=0.33), Mn (r=0.41) and Fe (r=0.49). The phenotypic variation Cu, Zn, Na, Mn and Fe is derived of 0.10, 0.37, 34.92, 0.13 to 1.03% of additive effects. Heritability in wide sense shows greater genetic variation for Cu (H²=0.81) and Na (H²=0.90), however, Zn and Fe are affected by 73.9 and 98.5% of environmental effects. The heritability in restricted sense indicates superiority to additive effects of Na (h²=0.34), however, the greater dominance deviations are established to be Cu (h²=0.001), Zn (h²=0.004), Mn (h²=0.001) and Fe (h²=0.010).
Determination of the genetic effects of heterosis
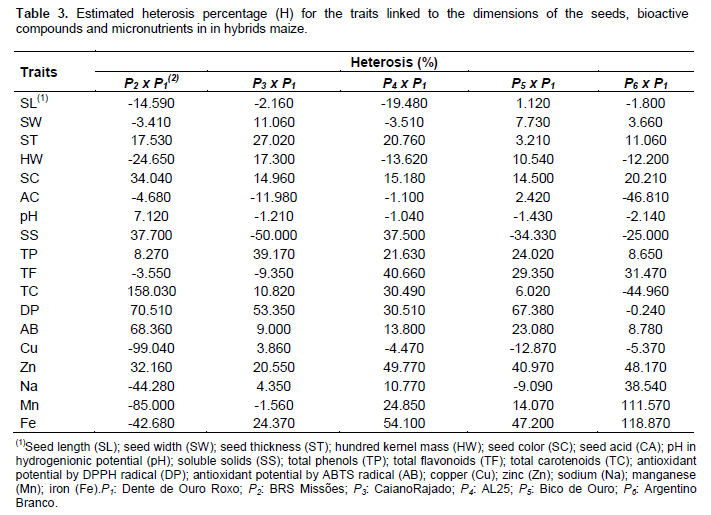
The heterosis expresses the increment of the trait through hybrid combination performed (Ramalho et al., 2012). Thus, they carried the fixing intervarietal crosses of the Dente de Ouro Roxo genotype (P1), as the male parent is held, by contrast, genotypes BRS Missões (P2), Caiano Rajado (P3), AL 25 (P4), Bico de Ouro (P5) and Argentino Branco (P6) comprised the female parents, where the traits ST, SC, TP, AB and Zn presented positive heterosis for all crossings made (Table 3), that is, the increase of the traits evaluated in relation to the parents average. The P2 x P1 crossing provided increased SC and TC with 34.04 and 158.03%, respectively. By combining the genitors P3 x P1, the traits ST, TP and DP were increased to 27.02, 39.17 and 53.35% due to the effects of heterosis. The crossing of P4 x P1 showed that traits Zn and Fe were increased by 49.77 and 54.10%, however, the crossing of P5 x P1 showed increased 67.38% DP radical. However, the hybrid combination of P6 x P1 identifies pronounceable effects of Fe and Mn with heterosis of 118.87 and 111.57% in the proportions of these elements in seeds of hybrid of maize.
The results obtained for the six open pollinated varieties (OPVs) and the five intervarietal hybrids of maize allowed evaluating the genetic parameters and gene action mechanisms that influence the phenotypic variation observed. The seed thickness and color, total phenols, antioxidant ABTS radical and zinc are increased by intervarietal heterosis, and are important for the development of maize genotypes in the region of southern Brazil.