ABSTRACT
The effect of banana treatment with traditional kerosene smoking and ethrel released ethylene were investigated to determine their efficacy on ripening, shelf life and physicochemical quality attributes. Fruits at full maturity stage that are light green and three quarter full were used. The study was consisted of three factors namely ripening techniques (conventional kerosene smoking and Ethrel), exposure times (that is, 18, 24 and 30 h), and cultivars (Williams I, Poyo and Giant Cavendish). Fruits were conventionally treated with kerosene smoke released from kerosene burners and ethylene released from 10 ml of ethrel solution (2-chloro ethyl phosphonic acid). They were equally treated under airtight conditions over three sets of exposure times inside locally standard 3 m × 2 m × 3 m sized six separate commercial banana ripening chambers. Fruits were then sequentially withdrawn from the chambers on the basis of their respective exposure times and studied under ambient conditions (23±1°C and 73±1% RH). All parameters tested were invariably and progressively affected by treatment combinations over the experimental period. Significant differences (p ≤ 0.05) in mean values were also recorded in all parameters at different stages of the ripening period. A three way significant (p ≤ 0.05) interaction effect of the three factors was revealed on the 7th day of the ripening period on the major quality parameters, starch, TSS, and TSS/TA. Sensory quality evaluation results conducted on the 7th day of the ripening period also showed a similarly highly significant interaction effect among the treatment factors on all quality attributes tested. Ethrel treated fruits demonstrated higher sensory quality mean score values in color (3.85), flavor (3.89), taste (3.80), aroma (3.66) and total acceptability (3.67), other than mouth- feel (3.37) and degree of ripening (3.49). Fruits treated with all treatment combinations of the kerosene smoking system equally completed their maximum ripening stage on the 7th day of the ripening period. However, at this stage, fruits were found developing some off ripening effect black scars on the peel in addition to the relatively low quality attributes recorded upon them through the sensory evaluation panel. Fruits treated with ethrel completed their ripening stage on the 7th day only at the exposure time of 30 h. Those exposed to 18 and 24 h exposure times took more time and extended their ripening stage to up to the 11th day. Thus, in terms of ripening efficiency, the kerosene smoking system can be used at the lowest exposure time of 18 h under the conditions tested. The ethrel-based ripening system can similarly be used for equal ripening efficiency and better sensory quality attributes but only at the highest exposure time of 30 h.
Key words: Banana, ripening, shelf life, physicochemical quality, kerosene smoking, ethrel.
Dessert banana and plantain (Musa sp.) are the fourth most important staple food crops in the world after rice, wheat and maize (Salvador et al., 2007a). They are also important sources of income for many smallholder Sub-Saharan Africa farmers (FAOSTAT, 2012).
In Ethiopia, dessert banana is the most important fruit crop, which grows in several parts of the country where the growing conditions are conducive. It is predominantly grown by smallholder farmers and is of great socioeconomic importance, especially in the south and southwestern parts of the country. In 2011, the area cultivated with banana in Ethiopia was 24,212 ha with a total production of 235,824 tons (FAOSTAT, 2012). However, although the demand for banana is steadily increasing in Ethiopia, it is so far grown only on less than one percent of the gross cultivated area, contributing only less than 0.8% of the gross value of agricultural outputs and quite negligible in export earnings (CSA, 2009). Besides, although some studies have been sporadically reported, no significant information is so far available regarding the marketing and post-harvest handling systems of banana including ripening.
The dominant commercial cultivars currently cultivated in Ethiopia are Dwarf Cavendish, Giant Cavendish, and Poyo. Cultivars like Williams I, Williams II and Grand Naine are also recently becoming into picture particularly in the Arba-Minch area where around 90% of the banana marketed in Ethiopia comes from (CFC, 2004). Others like Robusta and Butuzua are also among the recently released Cavendish banana cultivars in Ethiopia but their production is limited to certain commercial farms. The rest are less popular land races grown to a very limited extent in certain localities across the country.
Natural ripening of mature banana may result in softening with non-uniform, dull, pale yellow and unattractive color (Eduardo, 2012). In order for the fruit to attain a bright yellow peel color, a firm pulp texture, and good flavor, bananas are commercially ripened by relea-sing ethylene into a sealed chamber under controlled temperature and relative humidity. In doing so automated ethylene gas generators are used with calculated ethylene concentrations and exposure time. One main reason for such controlled ripening is to provide retailers and wholesalers with fruits at a stage of ripeness desired by consumers (Eduardo, 2012).
Conversely, in Ethiopia, different traditional ripening techniques are employed; which their effectiveness and effect on shelf-life and the subsequent physicochemical quality attributes are not yet studied and quantified. The most common traditional commercial banana ripening system in Ethiopia where different layers of bunches, in some cases hands, are treated involves the use of what is locally alternatively called “Chella” or “Muket” houses. Such houses, often owned by banana wholesalers, are basically airtight rooms inside which people apply kerosene smoking using kerosene burners. This traditional practice is generally known to accelerate the banana ripening process due to the presence of both acetylene (C2H2) and ethylene (C2H4) in the smoke (Sarananda, 1990). However, as stated by the same author and other previous reports, the system is often liable for lower consumer attraction, and so is being disregarded in many countries, owing to the resultant burnt scars, bruises, microbial infections, poor appea-rances on the peel, and contamination of the natural aroma of the fruits with the smoke. As an alternative and relatively improved ripening technique, ethylene generated from 10 ml of ethrel solution (ethephon or 2-chloro ethyl phosphonic acid), is also practiced in some developing countries for ripening of banana fruits often at semi-commercial levels for exposure time of 12 to 24 h (ICAR, 2009).
The aim of this study was thus to compare the efficacy of the traditional commercial kerosene smoking system in Ethiopia with the ethrel released ethylene system used elsewhere in the world in terms of ripening, shelf life and physicochemical quality attributes.
Description of the study area
The experiment was carried out at Jimma University College of Agriculture and Veterinary Medicine (JUCAVM), under the laboratory of the Department of Postharvest Management (PHM). JUCAVM is found in Jimma town, which is 355 km southwest of Addis Ababa laying at about 7040’N latitude and 360 50’ E longitudes with an altitude of 1780 masl. The mean maximum and minimum temperatures were 26.8 and 11.4°C respectively, and the mean maximum and minimum relative humidity were 91.40% and 39.92% respectively.
Experimental design and treatments
The experiment was laid out in a Randomized Complete Block Design (RCBD) with a 2*3*3 factorial arrangement in three replications. The factors consisted of (1) Factor A: Ripening Techniques in two levels (i.e. kerosene smoking with kerosene burners (K) and ethrel generated ethylene (E); (2) Factor B: Exposure-Time in three levels (that is, 18 h, 24 h = local standard, and 30 h); and (3) Factor C: Cultivars in three levels (that is, Williams I = C1, Poyo = C2 and Giant Cavendish = C3ï´¿. In total, there were 18 treatment combinations and 54 experimental units.
Experimental procedures
Harvesting and transportation of banana bunches
Banana bunches of three selected Cavendish cultivars; Williams I (C1), Poyo (C2) and Giant Cavendish (C3) were obtained from Tepi Agricultural Research Center, which is located about 274 km southwest of Jimma town or 629 km southwest of Addis Ababa. Fifteen matured bunches of light-green color and about three quarter full fingers, were selected from the “Banana Variety Maintenance Trial” field. They were selected from prior selected and tagged plants that were healthy, robust and relatively uniform. Fruit bunches were carefully harvested late in the afternoon (4:00 to 5:00 p.m.) and carried on a shoulder pad of the harvesters and kept under natural tree shade for three hours in order to pre-cool the bunches, remove the field heat and slow down metabolism. Initial temperature of each bunch was recorded using a Thermo-Hygrometer (model: PWT-101, Mauritius). The bunches were then night transported to the traditional commercial banana ripening houses in Jimma town by ISUZU truck cushioned with banana leaves from all sides except from the top.
Preparation of banana hands for ripening treatment
Upon arrival at the commercial ripening houses in Jimma town, bunches were carefully unloaded and stabilized for two hours inside a ventilated shade house. Once again, the temperature of each bunch was recorded immediately after unloading using the same Thermo-Hygrometer. Bunches were then carefully de-handed in a cluster of five fingers per crown with a very sharp curved knife in order to remove most of the rachis. De-handing was performed by hanging bunches down wards from a hook and the process proceeded from the apex to the basal end in order to avoid damages to individual fingers.
In order to maintain the uniformity of the experimental materials, only uniform (in terms of overall appearance, size and stage of maturity), clean and undamaged hands were de-handed from the central part of each bunch by avoiding those in the basal and apical rows. Care was also taken to prevent the hands from bumping against each other during de-handing and washing. Immediately after de-handing, but before treatment, hands were carefully placed into the washing tank or tub and washed using 2% normal “Barakina” (Sodium hypochloride) for 3 min in clean tap water. This was done for surface disinfection and removal of the latex and any other dirt. The hands were inspected one by one for any remaining dirt, which was gently removed using wet sponge. In order to completely remove the “Barakina” from the hands, hands were sequentially transferred into the second and third tanks and rinsed with clean tap water. In the end, the hands were drained off (air dried) on a wire-meshed platform with newspaper bedding. The crowns of the hands were also brushed with Aluminum Sulphate to avoid latent infection. This process of washing and draining off the hands was carried out under shade house and took around two hours.
Preparation and treatment of banana cultivars using kerosene smoking
Three traditional commercial banana ripening chambers, locally called “Chella” or “Muket” houses, with an area of 3 m * 2 m * 3 m = 18 m3 were used from the commercial banana ripeners in Jimma town. The washed and air-dried banana hands of the three banana cultivars were put separately inside perforated plastic boxes and placed on top of weaved wooden shelves. The hands were then conventionally treated with smoke generated from two kerosene burners per room, each filled with 2 L of kerosene, for the respective durations stated above (that is, 18, 24 and 30 h). The ripening treatment process was started simultaneously at 12:00 a.m. in all ripening rooms. As conventionally practiced by the “Chella” house operators in Jimma town and elsewhere in Ethiopia, the ripening rooms were closed and air tightly sealed up until the end of their respective exposure times. The pulp temperature and relative humidity of the respective ripening rooms were recorded at the beginning and end of the ripening process.
Preparation and treatment of banana cultivars with ethrel
Ethylene, in the presence of alkaline medium such as sodium hydroxide (NaOH), evolves from Ethrel to treat climacteric fruits such as banana and promote ripening (Mohamed and Abu-Goukh, 2003; ICAR, 2009). Using the same procedure, a 10 ml of Ethrel solution and 2 g of NaOH pellets were mixed in 5 L of water in a wide mouthed plastic buckets inside the respective ripening chambers of the three exposure times (that is, 18, 24 and 30 h). This was presumed to release 100 to 150 μL L-1ethylene, which is the common concentration level used for banana ripening at destination markets (Mohamed and Abu-Goukh, 2003; ICAR, 2009). This, in place of the smoke released through kerosene burners, was used to treat fruits of the three banana cultivars through the liberated ethylene inside the respective air tight ripening chambers for the specified exposure times. A small portable electric operated fan (Model: REE-NOVA FH-04: 220-240V/50~60Hz, China) was placed near the buckets in each of the respective ethrel treatment chambers to facilitate the release and uniform circulation of the ethylene gas from the solution. The chambers were then sealed air-tight immediately after recording the prevailing temperature and relative humidity.
Termination of the ripening process and subsequent handling of treated fruits for analysis
At the end of the respective treatment periods, the temperature and relative humidity of the rooms were recorded and the treated fruits were withdrawn from the ripening rooms in order to terminate the process and keep them under ambient conditions inside the laboratory of the Department of Postharvest Management at JUCAVM. Then, fruits were assigned into meshed plastic trays (35 fingers per tray), which were randomly laid out as per the design of the experiment across the laboratory benches. Finally, samples of fruits were periodically taken for physicochemical quality analysis up until the end of their marketable life. In addition to the physicochemical measurements taken immediately after harvest (that is, before the ripening treatment), which is considered as a Day Zero measurement, random samples of 3 to 5 fruits were used for subsequent analysis at three days interval. The temperature and relative humidity of the display room were recorded three times a day (that is, morning, mid-day and late afternoon) throughout the experimental period. Ripening fruits were also inspected and records were taken everyday for problems like disease incidence and physiological deterioration.
Data collected
Data collection was started right after the arrangement of the hands in the laboratory to the respective treatments and before the application of the ripening treatments. In addition to observations taken on Day one after treatment, data collection was then continued at three days interval up until the fruits became unmarketable. Data were collected upon the following physical and chemical properties of the experimental fruits.
Physical parameters
Physiological weight loss of fruits (PWL %)
Physiological weight loss of fruits was recorded through periodical weighing of five sample fingers from each experimental unit throughout the experimental period using a precision scale (model: LS200 Sartorius GMBH Gottingen, Germany). Physiological Weight Loss of fruits in the respective treatments was calculated and expressed as a percentage of the initial weight using the following equation.
Fruit firmness (N)
Fruit firmness was measured using a Texture Analyzer (Model: TA-XT Plus, UK) and calculated by employing the method used by Fan et al. (1999). Two whole unpeeled fingers were used periodically from each treatment to measure the penetration force required to penetrate the fingers by the stainless plunger and the force was automatically recorded and expressed in Newton (N). The mean value of measurements taken from two fingers of the respective treatments was recorded as the mean firmness.
Fruit peel color
Peel color was measured using the Tri-Stimulus Colorimeter (AccuProbe HH06, USA), which was regularly calibrated before measurement with a Minolta standard white tile to L = 83.14, a = -3.67 and b = 10.79. Non-destructive measurements were taken periodically starting from Day 0 (Day Zero) at three days interval to the end of the experimental period and values were recorded as a, b and L. Multiple measurements were taken from two sample fruits on two opposite sides and mid- point to the two ends of each fruit. Color values for each sample were calculated as the means of the four measurements taken. Total colour change of samples was determined using the CIE (Commission Internationale de L’Eclairage) 1976 L*a*b color scale system, where L*scale measures lightness (‘light vs. dark’) and varies from 100 for perfect white to zero for perfect black (that is, 0 to 50 indicates dark while 51 to 100 indicates light); a* measures redness (‘red vs. green’) (positive when red and green when negative); and b* measures yellowness (‘yellow vs. blue’) yellow when positive and blue when negative). The total color change (ΔE) was calculated by employing the following formula:
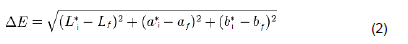
Where I = initial, and f = final.
Chemical parameters
Pulp starch content
Starch staining was carried out by a 3 to 5 min dipping of a cross-sectional cut of the unpeeled sample fruits in a starch-iodine staining solution with10 g potassium iodide (KI) and 2.5 g iodine (I2) in distilled water. It was carried out as per the rapid starch staining method and chart developed by Sylvia et al. (1993). The stained part of the sample fruit slices were then turned up and put in an upright position for about 2 min in order to allow the excess solution to be drained off. A transparent plastic sheet was then traced on the patterns of the stained areas and estimated in relation to the whole surface area of the cut dipped portion of the fruits. The sample fruits were then rated based on the chart developed previously into 10 distinguishable stages with rating numbers ranging from 1 to 10 based on the percentage of unstained area, that is, 1 means <5% unstained area, 2 (5%), 3 (10%), 4 (15%), 5 (25%), 6 (35%), 7 (45%), 8 (55%), 9 (65%) and 10 (>65%). The degree of ripening as well as the corresponding starch contents of the sample fruits was determined accordingly. A trend of increase in the rating number, from 1 to 10, was expressed as a characteristic pattern of starch loss during ripening.
Total soluble solids-TSS (°Brix)
Sample fruits were peeled and blended using a juice blender and TSS of the pulp juice was measured by the refractive index, expressed as °Brix, by a portable hand Refractometer (Model: SN-003007). The macerated samples were homogenized by adding about 40 ml of distilled water and filtered with cheese cloth. One to two drops of the filtrate was then placed on to the glass prism of the refractometer for reading within the scale. The glass prism was rinsed with distilled water and cleaned with cheese cloth in between the measurement of each of the samples drawn from the experimental units.
Titratable acidity (TA)
Titratable acidity was measured for samples of each experimental unit by titrating the pulp filtrates with 0.1N NaOH solution with 2 to 5 drops of phenolphthalein up until the indicator light pink color appeared. The volume of NaOH used up until the indicator reach the end point color was then recorded and TA was expressed as percentage of malic acid in the pulp weight of the titrate as calculated using the following formula:
Where, N = Normality of NaOH, V1 = volume of NaOH used, E = equivalent weight of acid, and V2 = volume of sample taken for estimation.
TSS to TA ratio
The ratio between total soluble solids and titratable acidity was determined by dividing the values for TSS to the values of TA of the same sample fruit.
Pulp pH
The pH of the sample fruit juice was measured using a bench top digital pH meter (model: CP-505, Poland). The pH meter was periodically calibrated with buffer at pH 4.0 and 7.0 before taking the measurements. pH was expressed as the equilibrium measure of hydrogen ion concentration in the sample fruit juice.
Sensory quality
The organoleptic or sensory quality of sample fruits from each treatment was determined using 5-point hedonic scale (where 1 = dislike, 2 = slightly, 3 = neither dislike nor like, 4 = slightly like, and 5 = like). A panel of 18 pre-oriented under and post graduate students from the departments of Horticulture and Post-Harvest Management were considered for the evaluation. The evaluation was carried out on the 7th day of the ripening period when most of the sample fruits attained the 6th color chart scale according to methods reported by Larmond (1987).The evaluation was made for such quality attributes like pulp color, flavor, taste, aroma, mouth-feel, level of ripening and overall acceptability. The sample fruits were peeled, hand sliced into smaller equal pieces and served in a random order across the laboratory benches of the Department of Post-Harvest Management in 3-digit coded flat glass trays. Distilled water was provided for the evaluators to cleanse their palates between each test sample. The panel test was carried out from 10:00 to 11:30 a.m. in the morning. The evaluation results were statistically analyzed to determine whether or not there were statistically significant differences in consumer preferences.
Statistical analysis
The experimental data were analyzed through the analysis of variance (ANOVA) by employing SAS software version 9.2. The least significant differences (LSD %) test was used to determine the level of significance at 5% (P < 0.05) and for mean separation and comparison of their differences among the treatment means. Data on main factor effects and their corresponding interaction effects are presented as results of the experiment only when they showed statistically significant differences.
Physical characteristics
Physiological weight loss (PWL %)
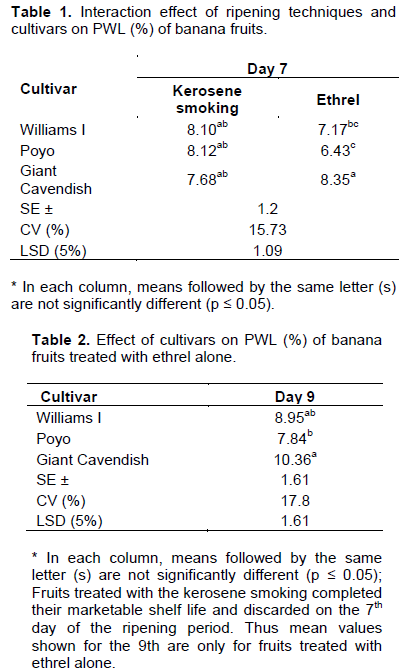
Physiological weight loss increased consistently as the ripening period prolonged in all treatments. The increase in weight loss with the application of the kerosene smoking and ethrel-based ripening systems was probably due to the upsurge in respiration rate of the sample fruits over the ripening period. Dhall and Singh (2013) also reported a similar increase in PWL (2.23 to 8.60%) during tomato ripening with ethrel and ethylene which was attributed to the increased rate of respiration as well as transpiration from the peel surface with the advance of ripening. The two-way interaction effect of ripening techniques and cultivars showed a significance difference (p<0.05) among cultivars treated with the kerosene smoking and ethrel-based ripening system on the 7th day of the ripening period (Table 1). However, while all cultivars recorded the highest weight loss (7.68 to 8.35%) on the 7th ripening day with the kerosene smoking-based system, only Giant Cavendish recorded a similar loss under both ripening systems on the same day. All cultivars showed similar levels of weight loss with the ethrel-based ripening system after the 9th day of ripening (Table 2). Thus, the kerosene smoking-based system significantly increased the weight loss invariably much earlier than the ethrel-based system signified that it was more efficient in the acceleration of the ripening process than the later.
Firmness (N)
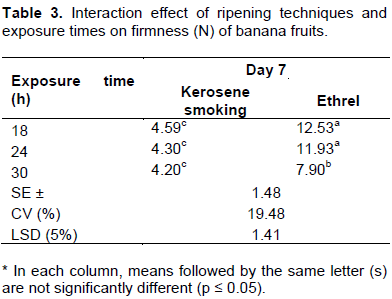
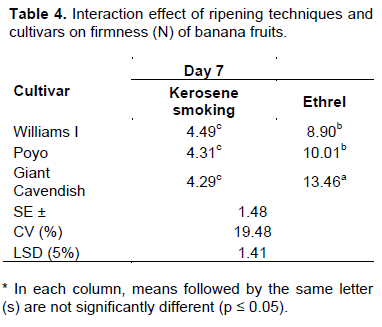
The data on banana fruit firmness revealed an increasing trend during the ripening period for all treatments. Significant differences (p≤0.05) among mean values of firmness were recorded at different stages of the ripening period. The firmness of fruits averagely decreased significantly from 21.87 N on Day 0 to 4.36 N on the 7th day of the ripening period. While fruits treated with the kerosene smoking system attained the above stated minimum firmness level on the 7th day of ripening with all exposure times (Tables 3 and 4), those treated with ethrel attained a similar level of firmness only at the 11th day of the ripening period with the exposure time of 30 h (Table 5). With the exposure times of 18 and 24 h, varieties treated with ethrel showed differences with only Williams I and Poyo attaining the stated level of firmness at the 11th day of the ripening period. Venkata et al. (2012) also reported a similar finding on Grande Naine banana fruits, treated with smoking and ethrel, declined in firmness to 4.83 on the 8th day of the ripening period. The decline in firmness was so drastic between the initial two days and thereafter decreased gradually throughout the ripening period. This consistent softening or decline in firmness corresponds to an inter conversion of insoluble pectic substances into soluble pectin (that is, by cellular disintegration leading to membrane permeability) over the ripening period (Venkata et al., 2012). Tapre and Jain (2012) also reported that the softening of banana fruits during ripening treatment is associated with the conversion of starch to sugar, the breakdown of pectin substances and the movement of water from the rind of the banana to pulp during ripening. As stated by Sandipkumar and Shanmugasundaram (2015), changes in biochemical and other properties such as peel thickness, TSS and moisture or pulp dry matter content are the main cause of alteration in mechanical properties such as firmness in banana. The result in the present study is further consolidated by the fact that the correlations between firmness and TSS as well as firmness and pulp dry matter content were found to be strongly negative (R = -0. 86) and moderately positive (R = 0.58) respectively (Annex 1).
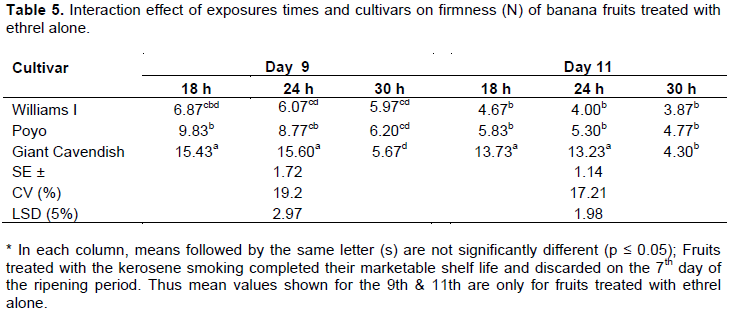
Peel color
Significant differences (p ≤ 0.05) in mean values of total peel color change were found at different stages of the ripening period. The obvious manifestations of banana color change during ripening such as the disappearance of the green peel color and the corresponding yellowing were clearly observed in the present experiment irres-pective of variations in treatments. The results were in agreement with the reports of Tourky et al. (2014) and Salvador et al. (2007b) in that the progressive loss of the green color in the peel was due to the continuing degradation of the chlorophyll structure during ripening.
The highest total peel color change was recorded on the 7th day of the ripening period for fruits treated with kerosene smoking across all exposure times (Table 6 and 7). On the other hand, fruits treated with ethrel ex-tended the trend till the 9th day and then declined on the 11th day (Table 8). This could be attributed to the much accelerated ripening effect of the kerosene smoking ripening system, which triggered all cultivars to complete their ripening period much earlier than the ethrel-based system. Similar results were also found by Ding et al. (2006) who reported that the color change of the peel implied the ripeness of Berangan banana and William Cavendish in that as the fruits ripen, they progressively developed a bright yellow color as chlorophyll gets degraded and carotenoids become visible. Thereafter the peel color declined as brown spots (senescent) appeared on the skin and the fruits became overripe.
On the other hand, it was observed that fruits treated with the ethrel-based ripening system developed uniform yellow peel color; while those treated with kerosene smoking exhibited deep yellow color with black spots on the peel surface leading to over-softening of the fruits at the end of the ripening period. A similar result was earlier reported by Sarananda (1990) that although the traditional banana ripening practice by smoking and subsequently storing at room temperature accelerates the ripening process, the resultant burnt scars, bruises, microbial infections and poor appearance of smoked ripe fruits lower consumer attraction.
Chemical characteristics
Starch
The starch stained a blue-black color, while areas that have lost starch remained white. Significant differences (p ≤ 0.05) in starch un-staining were found at different stages of the ripening period. The proportion of unstained area increased faster from 1(<5% unstained) at the beginning to 10 (>65% unstained) on Day 7 of the ripening period with the kerosene smoking system for all three exposure times and cultivars (Table 9). Under the ethrel-based ripening system, cultivars showed a similar extent of starch un-staining on Day 7 of the ripening period only at the exposure time of 30 h (Table 9). With the 18 and 24 h exposure times, cultivars exhibited similar level of starch un-staining only on the 11th day of the ripening period (Table 10). This significant disappearance of starch on Day 7 of the ripening period of the cultivars showed a highly significant correlation with peel color (R = 0.82), TSS (R = 0.96), and pH (R = -0.80) (Annex Table 1). The results are in agreement with previous findings by Tourky et al. (2014) in that starch hydrolysis is the most important post-harvest biochemical change which occurs during the post-harvest ripening of banana and causes the accumulation of sugar that are responsible for the increase in pulp TSS content and sweetening of the fruit.
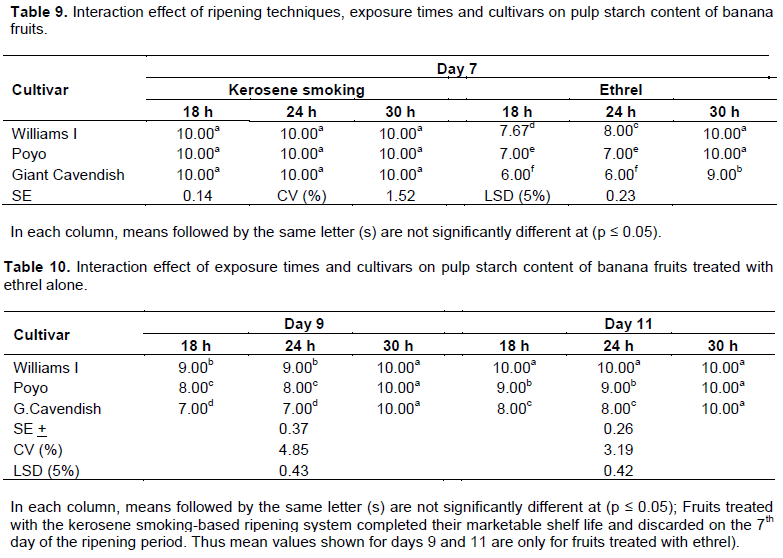
TSS (0Brix)
A significant difference (p ≤ 0.05) was generally observed in respect of the total soluble solids (TSS) of banana fruits among the treatments at different stages of the ripening period. TSS content increased consistently throughout the ripening period irrespective of treatments. Results also indicate a progressive increase in TSS and decrease in starch content with the maximum TSS (25.21°Brix) attained at the end or 7th day of the ripening period under the kerosene smoking system followed by the 9th and 11th day under the ethrel-based system (Tables 11 and 12). These results are almost in consistency with results (23.07 °Brix) obtained by Tapre and Jain (2012) at stage 7 of the ripening period of the banana fruits. Sandipkumar and Shanmugasundaram (2015) also reported an increase in TSS from 3 to 22.24°Brix during nine days of banana storage. In addi-tion, Kulkarni et al. (2004) reported an increase in TSS and sugars in mango fruits treated with ethrel with progress in storage time. The increase in TSS during ripening may result from an increase in concentration of organic solutes as a consequence of water loss and hydrolysis of starch into soluble sugars such as sucrose, glucose and fructose (Tapre and Jain, 2012). Tourky et al. (2014) reported that banana and some other fruits contain many water soluble compounds (sugars, acids, vitamin C, amino acids and some pectin) that form the TSS. Total soluble solids, having sugar as their main component are known to be important postharvest quality attributes of the banana fruits that serve as the most useful index of ripeness.
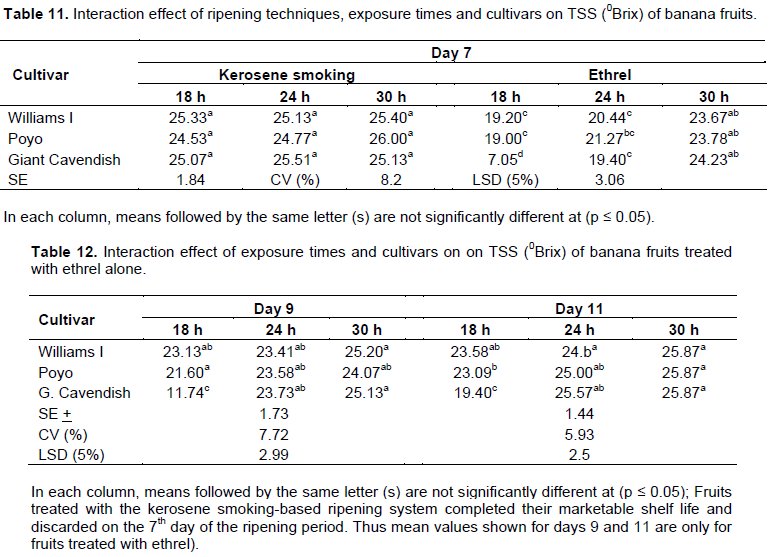
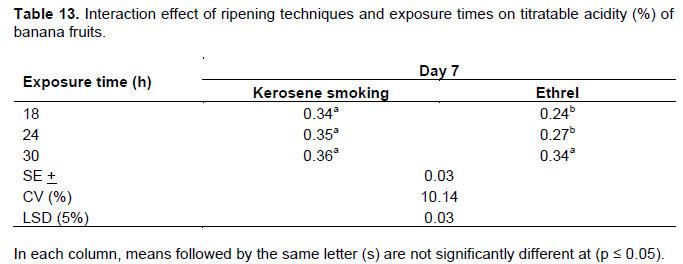
Titratable acidity (TA %)
Although the percentage values were recorded in the narrow range, a progressive increase in TA was found with advances in the ripening period in all the treatments. The highest percentage of TA (0.34-0.36%) was recorded on the 7th day of the ripening period similarly under the kerosene smoking system equally with all the cultivars and exposure times. Although a slight decline in TA (0.28%) was observed with the kerosene smoking treatment after the 7th day of the ripening period, the fruits were by then became unmarketable and discarded (Table 13). Fruits treated with the ethrel-based system attained similar level of TA percentage at the 7th day of the ripening period only at the exposure time of 30 h (Table 13). There was also a two way interaction effect of exposure times and cultivars on the 9th day of the ripening period where fruits attained similar level of titratable acidity (0.34 to 0.35%) at 30 h of exposure time (Table 14). Those treated with the exposure times of 18 and 24 h extended the stage to the 11th day of ripening (Table 15). Although results were not statistically significant, similar results were reported by Tapre and Jain (2012) with values ranging narrowly from 0.3 to 0.45% among treatments at the end of the banana ripening period. The result is as well concurring with the studies made by Zeweter (2008), and Siriboon et al. (2004) who reported that TA increased to its peak as ripening progressed, which coincided with the accumulation of ethylene and ripening, and then started declining afterwards. Thus, as stated by Dadzie and Orchard (1997), the increase in TA over the ripening period could also be regarded as a useful index of banana fruit.
.png)
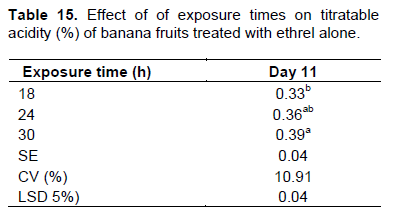
Fruits treated with the kerosene smoking-based ripening system completed their marketable shelf life and discarded on the 7th day of the ripening period. Thus mean values shown on the 11th are only for fruits treated with ethrel.
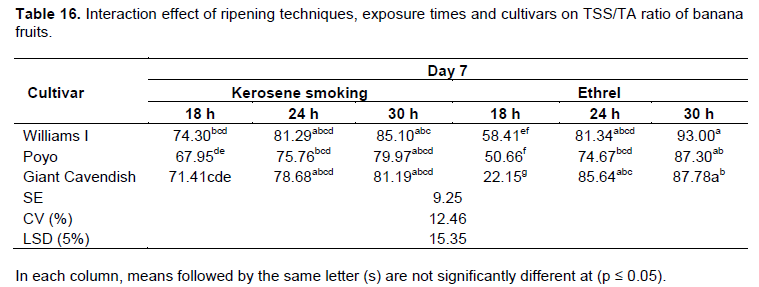
TSS/TA ratio
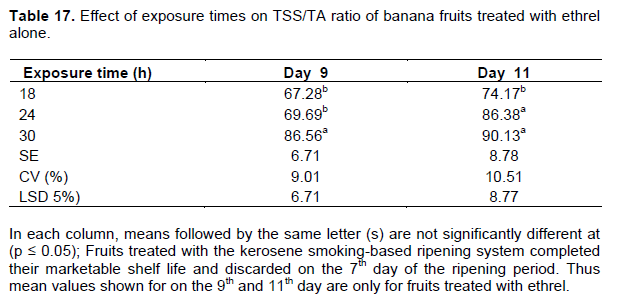
The TSS/TA ratio, which is also taken as a ripening index (RI), generally increased as the ripening period progressed in all treatments. The maximum TSS/TA ratio (67 to 81) was recorded on the 7th day of the ripening period across all the treatments under the traditional kerosene smoking ripening system (Table 16). Cultivars treated with the ethrel-based system also showed a similar ratio in TSS/TA on the 7th day under the exposure times of 24 and 30 h (Table 16). Cultivars treated for 18 h with the ethrel-based system attained a similar TSS/TA ratio as of the 9th day of the ripening period (Table 17). This increase in TSS/TA ratio was attributed to the increase in the amount of sugars and the parallel decline in organic acids (Dadzie and Orchard, 1997). The proportional organic acids increase, which are important in giving a desired sugar-to-acid balance and pleasing fruit taste, usually decline during fruit ripening as they are respired and converted into sugar (Tourky et al., 2014).
pH
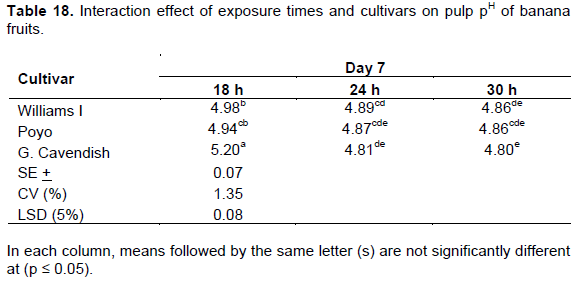
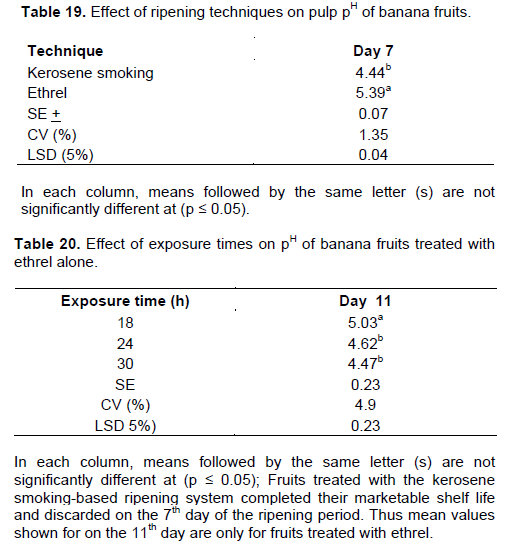
pH was expressed as the equilibrium measure of hydrogen ion concentration in the sample fruit juice. The pH value gives a measure of the alkalinity of the sample fruit juice. The pH of the pulp steadily decreased over the ripening period irrespective of treatments. A two way interaction effect on pulp pH was obtained between exposure times and cultivars on the 7thday of the ripening period (Table 18). Significantly (p ≤ 0.05) low pH was recorded through the kerosene smoking system on the same date (Table 19). On the other hand fruits treated with the ethrel-based system showed similar level of pH only on the 11th day of the ripening time at the exposure times of 24 and 30 h (Table 20). The decline in pH as ripening progresses is in agreement with the findings of Zeweter (2008) and Hernandez et al. (2006). As ripening advances, titratable acidity increases which results in a decrease of pulp pH (Dadzie and Orchard, 1997). Pulp pH and total titratable acidity are thus important post-harvest quality attributes in the assessment of fruit ripening and quality.
Sensory quality
The data in Table 21 illustrates highly significant differences (p ≤ 0.05) in mean values among the treatments across all the tested sensory quality parameters. Results also clearly indicate that ethrel treated fruits demonstrated higher mean score values for sensory quality attributes of color (3.85), flavor (3.89), taste (3.80), aroma (3.66) and total acceptability (3.67) other than mouth-feel (3.37) and degree of ripening (3.49). A similar result was reported by Kulkarni et al. (2010) that ethrel at 500 ppm induced uniform ripening without impairing taste and flavour of banana. All the same, Lakshmana et al. (2010) reported parallel results that the organoleptic quality of banana ripening treated with 2500 ppm ethrel dip (5 min) at 18 to 26°C and 60 to 70% RH was found optimal for the conversion of starch and acids and possessed better quality attributes in terms of pulp color, taste, aroma, texture, firmness and overall appearance compared to hot water dip (55°C, 5 min) and including smoking (24 h).

Slightly higher mean score value with respect to mouth-feel (3.44) was recorded for the kerosene smoking-based
ripening system, which could be related to the similar higher value accounted to it in terms of degree of ripening (3.77). The relatively low scores observed in Giant Cavendish fruits under the ethrel-based ripening system, invariable across all the sensory quality attributes, could be attributed to its inability to reach the same ripening stage on the 7th day as the other two cultivars. This situation with Giant Cavendish was likewise manifested through the mean values of most of the other physicochemical parameters tested including total peel color. In addition, the relatively low mean score values accounted to the kerosene smoking-based ripening particularly in terms of color, flavor and aroma, could be attributed to the poor performance of the system in appropriately expressing the natural traits of the fruits. Sarananda (1990) and similar other studies reported that although such traditional smoking techniques of banana using kerosene smoking generally accelerate the ripening process, primarily due to the presence of acetylene (C2H2) and ethylene (C2H4) in the smoke, they usually render the fruits into lower consumer attractions. This, among other reasons, was similarly attributed to the masked or displaced natural aroma of the fruits by the smoke, poor appearance of smoked fruits, burnt scars and bruises on the peel surface, and increasing the liability of the fruits for microbial infections.
Ripening of banana fruits using the traditional kerosene smoking ensures faster and uniform ripening in variably with all treatment combinations in just seven days of the ripening period. However, at this stage, fruits were found developing some off ripening effect black scars on the peel in addition to the relatively low quality attributes recorded upon them through the sensory evaluation panel. On the other hand, banana fruits treated with ethrel generated ethylene were able to complete their ripening stage on the 7th day of the ripening period only at the exposure time of 30 hours. Those treated for 18 and 24 h extended their ripening stage to the 9th and 11th day of the ripening period. However, ethrel generated ethylene treated fruits demonstrated higher mean score values in all sensory quality attributes tested other than mouth-feel, which could also be attributed to the slight delay in their ripening stage. Thus, in terms of ripening efficiency, the kerosene smoking system can be used at the lowest exposure time of 18 h under Jimma (Ethiopia) conditions. The ethrel-based ripening system can similarly be used for equal ripening efficiency and better sensory quality attributes but only at the highest exposure time of 30 h. In addition, further study is recommended for the ethrel-based ripening system with more concentrations in order to attain the more efficient 18 h exposure time recommended for the kerosene smoking system under similar conditions.
The authors have not declared any conflict of interests.
The authors are highly thankful to the Department of Post Harvest Management of Jimma University College of Agriculture and Veterinary Medicine (JUCAVM) for providing the laboratory facilities to carry out the experiment.
REFERENCES
|
Central Statistics Agency (CSA) (2009). Area and Production of Major Crops. Agricultural Sample Enumeration Survey. Addis Ababa, Ethiopia.
|
|
|
|
Common Fund for Commodities (CFC) (2004). Development of organic banana production and export in Sudan and Ethiopia to the Middle East and Europe. FC/CC/34/FISGB/10. Appraisal Report www.egfar.org/.../promoting-organic-banana-exports-ethiopia-and-sudan.
|
|
|
|
|
Dadzie BK, Orchard JE (1997). Routine post-harvest screening of banana/plantain hybrids: Criteria and methods. INIBAP Technical Guidelines 2. International Plant Genetic, Resources Institute, Rome, Italy, International Network for the Improvement of Tomato, Montpellier, France, P 47. Available at: View
|
|
|
|
|
Dhall RK, Singh P (2013). Effect of Ethephon and Ethylene Gas on Ripening and Quality of Tomato (Solanum lycopersicum L.) during Cold Storage. Dhall and Singh. J. Nutr. Food Sci. 3:6
|
|
|
|
|
Ding P, Ahmad SH, Abd Razak AR, Mohamed, MTM, Saari N (2006). Peel Color of Musa AAA 'Berangan' and 'William Cavendish' during Degreening. Agro Search 11:1-4.
|
|
|
|
|
Eduardo K (2012). Banana and Plantain. Dole Fresh Fruit International, Ltd., San Jose, Costa Rica. View
|
|
|
|
|
Fan X, Belankenship SM, Mattheis JP (1999). 1-Methylcyclopropene inhibits apple ripening. J. Am. Soc. Hortic. Sci. 124:690-695.
|
|
|
|
|
FAOSTAT (2O12). View
|
|
|
|
|
Hernandez Y, Lobo MG, Gonzalez M (2006). Determination of vitamin C in tropical fruits: A comparative evaluation of methods. Food Chem. 96:654-664. View
Crossref
|
|
|
|
|
ICAR (Indian Council of Agricultural Research) (2009). Uniform bulk ripening of mango, banana and papaya. A Science and Technology Newsletter .Volume 15 No. 4. KrishiBhawan, New Delhi 110 001, India. View
|
|
|
|
|
Kulkarni SG, Kudachikar VB, Vasantha MS, Keshava Prakash MN, Aravinda PB, Ramana KVR (2004). Studies on effect of ethrel dip treatment on the ripening behaviour of mango (Mangifera indica L.). variety 'Neelam'. J. Food Sci. Technol. 41:216-220.
|
|
|
|
|
Kulkarni SG, Kudachikar VB, Keshava Prakash MN (2010). Studies on physico-chemical changes during artificial ripening of banana (Musa sp) variety 'Robusta'. J. Food Sci. Technol. 48(6):730-734.
Crossref
|
|
|
|
|
Lakshmana L, Umesh K, Kumar D (2010). Effect of Post-Harvest Treatments on Ripening Behaviour and Quality Aspect of Banana Fruit Grown Under Hill Zone. Karnataka Journal of Agricultural Sciences, 14(3).
|
|
|
|
|
Larmond E (1987). Laboratory Methods of Sensory Evaluation of Food Research Branch Canada. Deptt. Agric. Publication. P 44.
|
|
|
|
|
Mohamed HE, Abu-Goukh AA (2003). Effect of ethrel in aqueous solution and ethylene released from ethrel on mango fruit ripening. J. Hortic. Sci. Biotechnol. 78(4):568-573.
|
|
|
|
|
Salvador A, Arnal L, Manterde A, Cuquerella J (2007b). Reduction of chilling injury sysptoms in persimmon fruit cv. 'RojoBrillante by 1-MCP, Postharvest Biol. Technol. 33:285-281. Salvador A, Sanz T, Fiszman SM (2007a). Changes in color and texture and their relationship with eating quality during storage of two different dessert bananas. Postharvest Biol. Technol. 43:319-325.
Crossref
|
|
|
|
|
Sandipkumar KP, Shanmugasundaram S (2015). Physicochemical Changes during Ripening of Monthan Banana. Int. J. Technol. Enhancements Emerg. Eng. Res. 3(2):18.
|
|
|
|
|
Sarananda KH (1990). Effect of Calcium Carbide on Ripening Of 'Embul' Banana (Musa spp.). Tropical Agriculturist. Horticulture Division, Getambe, Peradeniya, Sri Lanka. Vol 146.
|
|
|
|
|
Siriboon N, Banlusilp P (2004). A Study on the Ripening Process of 'Namwa' Banana. Faculty of Biotechnology, Assumption University Bankok, Thailand.
|
|
|
|
|
Sylvia MB, Donna DE, Ronald LP (1993). Ripening Index for Banana Fruit Based on Starch Content. Technology and Product reports. HortTechnol. 3(3):338-339.
|
|
|
|
|
Tapre AR, Jain RK (2012). Study of advanced maturity stages of banana. Int. J. Adv. Eng. Res. Stud. 1(3):272-274.
|
|
|
|
|
Tourky MN, Tarabih ME, El-Eryan EE (2014). Physiological studies on the marketability of Williams Banana Fruits. Am. J. Plant Physiol. 9:1-15.
Crossref
|
|
|
|
|
Venkata SK, Jagadeesh SL, Thammaiahand N, Chavan ML (2012). Changes in physico-chemical and sensory characteristics of banana fruit cv. Grand Naine during ripening. Karnataka J. Agric. Sci. 26(1):111-114.
|
|
|
|
|
Zeweter A (2008). Effect of 1-Methylcyclopropene, potassium permanganate and packaging materials on quality and shelf life of banana (Musa acuminate var Dwarf Cavendish). M.Sc. Thesis. Alemaya University, Ethiopia.
|
|