ABSTRACT
“Chemlali” is the main olive variety cultivated in Tunisia. Seasonal factors such as rainfall and temperature vary year to year influencing the composition and quality of virgin olive oil. This work aims to investigate the effect of the seasonal climatic variations on the quantity and the quality characteristics (free acidity, specific ultraviolet absorbance K232 and K270, fatty acid composition, chlorophylls and total phenols) of Tunisian “Chemlali” olive oil. Water and oil content increased with increasing rainfall and temperature respectively. The seasonal variations of climatic factors within the growing region did not affect the main legal quality indices of all oil samples. K232, total phenol contents and oxidative stability increased at high temperature, while K270 and chlorophyll content decreased.
Key words: Analytical quality parameters, chlorophyll, fatty acid composition, Olea europaea L., rainfall, temperature, total phenols.
Mediterranean climatic conditions are favourable to the olive tree cultivation. The Tunisian olive trees number about 57 million units covering 16000 ha. “Chemlali” is the main variety cultivated in Tunisia, covering 60% of the olive growing surface, spread from the north-east of the country to the extreme south. This cultivar, appreciated for its sensory characteristics, contributes to 80% of the national olive oil production (Baccouri et al., 2007). It is a productive variety that is well resistant to severe environmental conditions. In fact, into the large agricultural areas of this region, the olive is primarily dry farmed and orchard density is tuned to average precipitation.
Seasonal factors such as rainfall and temperature vary year to year influencing the composition and quality of virgin olive oil. Considering that virgin olive oil (VOO) is obtained from the olive fruit solely by mechanical and physical means, it retains more health beneficial phytochemicals as compared to other commercially available refined vegetable oils, which are generally solvent extracted (Visioli and Galli, 1998). Its potential beneficial effects to human health (Krichene et al., 2007) are due to the balanced fatty acid composition, rich in monounsaturated fatty acid (MUFAs), which are more
resistant to lipid peroxidation than polyunsaturated fatty acid (PUFAs) (Kris-Etherton et al., 2002) coupled with the presence of polyphenols (Visioli et al., 2000), powerful antioxidant. The unique chemical and organoleptic characteristics of VOO depend on several factors, such as environmental (climate, soil), agronomic (irrigation, fertilization, harvesting time and methods), technological factors (fruit storage, extraction procedure) (Aparicio and Luna, 2002; Di Giovacchino, 2000; Salvador et al., 2000) and fruit health. Among these factors, the climate (humidity, rainfall, temperature) is a determinant parameter for fatty acid composition (Ben Temime et al., 2006; Aparicio and Luna, 2002; Stefanoudaki et al., 1999; Ranalli et al., 1997) and total phenol contents (Boskou, 2000).
Also the harvesting time can influence the VOO fatty acid from year to another probably because of the differences in the amount of summer rainfall in the growing region and temperature during olive fruit ripening and oil biosynthesis (Beltran et al., 2004).
Changes in chemical composition (fatty acid composition and polyphenols) of extractable olive oil according to macroclimatic factors have been studied by several authors in Spanish (Aranda et al., 2004) and other Tunisian cultivar (Ben Temime et al., 2006). Some reports include information about fatty acid composition (Angerosa et al., 1996; Montedoro et al., 1993) and changes of antioxidant compounds according to macroclimatic conditions (Ben Temime et al., 2006). Yet only a few studies took into account the effect of the macro and meso-climatic environments in olive water and oil contents (Mailer and Beckingham, 2006) and oil quality parameters (Spyros et al., 2004; Gutierrez et al., 1999, 2000), fatty acid profile, polyphenols and induction time (Tura et al., 2007; Ranalli et al., 1999). Studies on the effects of seasonal climatic factors variations on “Chemlali” olive oil quality are scarce.
The aim of this work was to investigate the effect of the seasonal climatic variations on the quantity and the quality characteristics (free acidity, specific ultraviolet absorbance K232 and K270, fatty acid composition, chlorophylls and total phenols) of Tunisian “Chemlali” olive oil.
Experimental orchard and olives sampling
The trial was carried out in 2003-2004, 2004-2005 and 2006-2007 on olive trees (Olea europaea L.) of “Chemlali” variety at the experimental farm of “El Hajeb” in the region of Sfax (34° 43 N, 10° 41 E), Centre East of Tunisia. In order to determine the influence of the seasonal climatic variations on “Chemlali” virgin olive oil composition and quality.
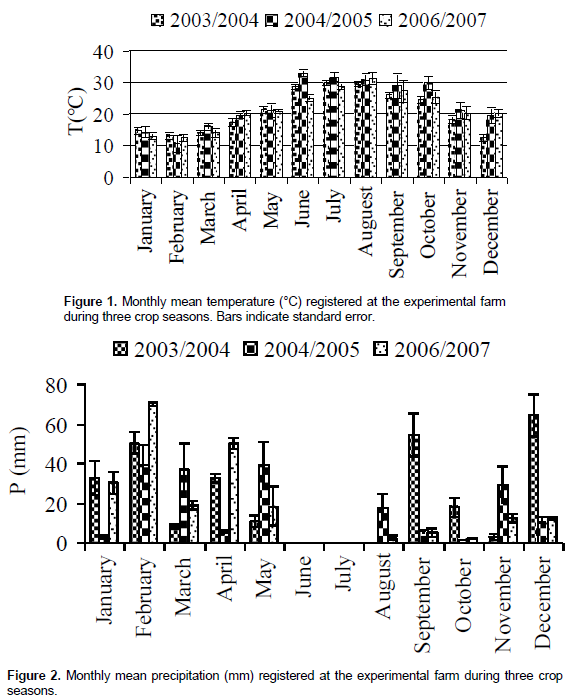
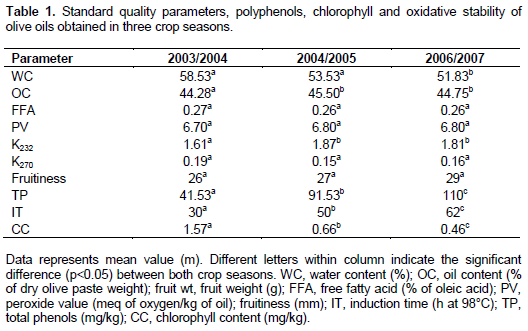
The sandy soil had an organic matter content of 1.3%; 10.5% CaCO3; 1.2% N and a pH of 7.8. The experimental orchard consisted of thirty mature olive trees planted at spacing of 24 m x 24 m and irrigated with drip irrigation system by well water (WW). Irrigation with WW was started in spring (April-May) and in autumnal period (October-December) when olive tree need a water supply for vegetative growth and fruit development. The amount of water received for each crop year was equal to 5.000 m3 ha-1. The experimental plot was divided into three completely randomized blocks. Climatic conditions of the experimental site were obtained from a meteorological station installed within the experimental orchard. Averages of rainfall and air temperature were recorded. The monthly values of mean temperatures and rainfalls registered for the studied year were reported in Figures 1 and 2.
Healthy fruit samples were harvested in triplicate from all the olive trees belonging to each block at the same ripening degree. The olive samples were brought to the laboratory for oil extraction. Fifty olives fruits were taken from each olive sample in order to determine water and oil contents.
Fruit water and oil yield estimation
Samples of approximately of 100 g (5 g × 20 replications) of olive flesh from fruits were weighed and then dried for 24 h at 105°C, cooled for 30 min in desiccators and reweighed. The oil content was determined by Soxhlet extraction and was expressed as a percentage of dry olive paste weight.
Oil extraction
The oils were extracted using a laboratory olive mill (Sohxlet Analyzer, MC2 Ingenierias y Sistemas, Servilla, Spain) equipped with a metal crusher, a mixer whose internal partition is in rustproof steel, having a vertical or horizontal axis and a basket centrifuge. The crushed fruits were mixed for 30 min at 25°C, and then the oil was separated by a vertical centrifugation, collected and left to decant. The obtained oil was filtered and stored at -4°C in darkness using amber glass bottles until analyses.
Olive oil analyses
Quality indices
The free fatty acids (FFA), peroxide value were measured following the analytical methods described in European Economic Community regulation EEC 2568/91 (EEC Regulation 2568/91 and subsequent modifications ECC/796/02 and ECC/1991/03). K232 and K270 extinction coefficients, calculated from absorption at 232 and 270 nm respectively with UV spectrophotometer.
Organoleptic evaluation
Sensory analysis (fruitiness) of virgin olive oil samples were carried out by an analytical panel of 12 assessors according to European Official Methods of Analysis ECC/1991/03, and a sensory laboratory were used. Test Room was consistent to the standard COI/T.20/Doc. no. 6. All assessors had more than 8 years of experience in evaluating virgin olive oil. Oil samples were heated at 30 °C by a thermostat before sensory analyses and were presented fully randomized to the tasters. Dark-blue glasses were used as no colour evaluation was to be made. Each taster on the panel melled and then taste the oil under consideration. They then entered the intensity with which they perceive each of the negative and positive attributes on the 10 cm scale in the profile sheet provided. Regarding the fruity taste, each oil sample was graded on a scale from 1 to 10 cm, where 1 is a low intensity of perception of positive attributes.
Total chlorophylls
Total chlorophyll compounds were determined by the method described by Minguez et al. (1991). Chlorophyll pigments were determined by measuring the absorbance at 630, 670 and 710 nm with spectrophotometer (Perkin-Elmer UV/VIS Spectrophotometer, Norwalk, CT). The results were expressed in ppm and obtained from the following formula (Wolf, 1958).
Where L is vat thickness (1 cm) and 0.10786 is the variable coefficient according to the spectrophotometer used.
Total phenols
Total phenols were extracted three times from an oil-in-hexane solution using water: methanol buffer (60:40) according to the method described by Vazquez-Roncero et al. (1973). The amount of total phenols was determined colorimetrically according to the Folin-Ciocalteau procedure (Singleton and Rossi, 1965). The Folin-Ciocalteau reagent was added to a suitable aliquot of the combined extracts, and the absorption of the solution was measured at 725 nm with a spectrophotometer (Perkin-Elmer UV/VIS Spectrophotometer, Norwalk, CT). The results are expressed as mg of caffeic acid per kilogram of oil.
Oxidative stability
The oxidative stability was measured using a Rancimat apparatus (model 743 Metrohmn CO., Basel, Switzerland). Stability was expressed as the oxidation induction time (Hours) using 3.5 g of oil. The temperature was set at 102°C, and the air flow rate was 10 L per hour (Oueslati et al., 2009).
Fatty acid composition
Fatty acids were converted to fatty acid methyl esters (FAMEs) before analysis by shaking a solution of 0.2 g of oil and 3 ml of hexane with 0.4 ml of 2N methanolic potassium hydroxide and analysed by Shimadzu 17 Autosystem gas chromatograph equipped with flame ionisation detector (FID) and a fused silica capillary column (15 m length × 0.25 mm × 0.15 μm film thickness). Nitrogen was used as carrier gas with a flow through the column of 1 ml/min. The temperature of the injector was 220°C; detector and oven temperatures were 250 and 180°C respectively. Fatty acids were identified by comparing retention times with those of standard compounds. The results are expressed as relative area percentage of the total.
Statistical analysis
ANOVA analyses were performed for the obtained results, considering the temperature and rainfall as independent variables; results are presented as means of three repetitions. All statistical analyses were carried out with the program SPSS 10 for Windows, statistical software (SPSS Inc., Chicago, IL, USA). LSD test was used to determine significant difference for each parameter among seasonal weather variations.
Climatic conditions
Figures 1 and 2 show the trends of annual temperatures and rainfalls. The crops seasons (2003/2004, 2004/2005, 2006/2007) are characterized by relatively mild winters with abundant precipitation and by hot and dry summers. The rainfall amount was significantly (p < 0.01) high at autumnal period in 2003-2004 crop season as compared to both other crop seasons (10 to 25 mm at 2004/2005 and 5 to 20 mm at 2005/2006). The summer and autumnal periods of 2004-2005 crop season (May-September) were characterized by a significant high temperature (p < 0.01).
Fruit water and oil contents
The mean values of fruit water content ranged from 51.83 to 58.53%, for 2006 to 2007 and 2003 to 2004 crop seasons respectively (Table 1), coherent with the values reported by Mailer and Beckingham (2006), which reported that the fruit moisture content is often about 55% or higher in developing fruit. The significant difference (p < 0.05) in water content between crop seasons is ascribable to higher rainfall values (75.40 mm) at autumnal period in 2003-2004 crop season as compared to other crop seasons, involving an increase of water in fruits.
The oil content constitutes a determinant element from an economic point of view, in fact, an increment in oil content determines an increase of the yield. As shown in Table 1, oil content, expressed as a percentage of dry matter, ranged from 44.28 to 45.50%. The mean values of fruit flesh were comparable to those reported by Abaza et al. (2003) in the same cultivar. Results showed that oil content varies year to year. This variation is probably due to the differences in temperature in the growing region during olive fruit ripening which may be stimulating some enzymes responsible for oil biosynthesis. Data showed a significant increase of oil content with increasing autumnal temperature (p < 0.05) and rainfall decrease. All the oil content increments occurred simultaneously to a decrease in water content of fruit (r2 = 0.86) (Table 1).
Free fatty acid, peroxide value and specific ultraviolet absorbance (K232 and K270)
In all the examined samples, the free fatty acid (FFA) contents were much lower than the upper limit of 0.8% established for the best commercial olive oil quality, designated as “extra” virgin (EC Regulation 2568/91 and subsequent modifications ECC/796/02 and ECC/1991/03). The peroxide values (PV) of the oils analyzed were below the limit of 20 meq O2 kg-1 of oil, which is accepted as the limit for extra-quality virgin olive oil (International Olive oil Council, 2003). As shown in Table 1, the analytical parameters (FFA and PV) are not significantly influenced by the seasonal climatic variations. This findings on “Chemlali” cultivar behaviour are in contrast with previous results reported by Gutierrez et al. (1999, 2000) on Spanish cultivars (Picual and Hojiblanca). In fact, they found that FFA is a parameter negatively correlated to the temperature into an olive orchard characterized by a conventional cropping system. Indeed, the analytical parameters depend on several factors, such as olive fly attacks or improper systems of harvesting, transport and storage of olives.
In the studied crop seasons K232 and K270 ranged from 1.61 to 1.87 and from 0.15 to 0.19 respectively (Table 1). The values were lower than the limits established for extra virgin olive oil (2.5 and 0.22 for K232 and K270, respectively). The high K232 value was associated to high autumnal temperature, while minimum K270 value was found in oils obtained from fruits grown at dry conditions. Data showed that the high autumnal temperature values determined an increase of K232 (2004 to 2005 crop season), while a high amount of autumnal rainfall caused an increment of K270 value (2003-2004 crop season).
Fatty acid composition
Table 2 shows the results of fatty acid methyl esters (FAME) contents for all the VOO samples collected during three crop seasons (2004 to 2006). The most abundant fatty acid was the oleic acid (C18:1) with contents varying from 57.49 to 59.22%. Furthermore, the levels of palmitic (C16:0) and palmitoleic (C16:1) acids ranged from 18.95 to 19.74% and from 2.57 to 2.61%. The variability of fatty acid composition of the oil samples covered the normal range expected for olive oils (Codex Stan, 1989). However, the oil samples had high monounsaturated fatty acid (MUFA) content and a moderate level of linoleic acid (18%), in accordance with the general observations for oils from the south of producing countries like Spain, Greece and Italy (Giansante et al., 2003; Psomiadou et al., 2003).
The palmitic, palmitoleic, linoleic and linolenic acid contents remained unchanged in three crop seasons. No significant differences were found in ANOVA, that is different to say the growing region have no effect in major fatty acid. Results are in contrast with the findings of previous studies in other varieties, which reported that olive fatty acid composition changed in relation to climatic factors variations (Allalout et al., 2009; Ben Temime et al., 2006; Beltran et al., 2004; Stefanoudaki et al., 1999). Differences can be explained by cultivar which an essential factor determining olive fatty acid composition (Baccouri et al., 2007; Ben Temime et al., 2006).
The stearic acid level was lower than the upper limit 5% established for high extra virgin olive oil (EEC, 2003). At 2004-2005 crop season, the stearic acid content significantly increased, whereas oleic acid content showed an opposite trend (Table 2). The decrease of oleic acid content is due to the transformation of oleic acid into stearic acid by stearoyl-ACP Δ9 desaturase activity which is active during triacylglycerol biosynthesis accentuated by high summer rainfall (August).
The percentages of saturated, monounsaturated and polyunsaturated fatty acids (PUFA) and the oleic acid/linoleic acid ratio (O/L) in the studied olive oils were also evaluated. It was observed that “Chemlali” oil was rich in total saturated fatty acids (SFA) (21.98%) essentially due to its higher content in palmitic acid which represents the major acid of the SFA fraction. The total monounsaturated fatty acids (MUFA) are low for olive oil, considering the most abundant varieties, such as Picual. The olive oil was rich in total polyunsaturated fatty acids (18.82%) because of its high contents in linoleic acid representing the major fatty acid component of that fraction. The oleic acid/linoleic acid ratio varies between 3.23 and 3.38 according to crop season. This ratio can be useful to characterize olive cultivars and to have a marked relationship with stability (Ouselati et al., 2009).
As shown in Table 1, the olive fruit from cooler areas contained oil with more unsaturated fatty acids than the fruit from warm areas. This finding agrees the result reported by Ben Temime et al. (2006).
Organoleptic evaluation
All the virgin olive oils obtained in the three crop seasons were characterized by the absence of defects and a typical “fruity” flavour particularly preferred by consumers. All the oil samples were classified as ‘extra virgin’. Table 1 shows the organoleptic evaluations made by an IOOC (International Olive Oil Council) recognized olive oil taster panel, which reveal no significant differences were found in the fruity flavour of all oil samples
Chlorophyll contents
The colour of olive oil, even if it is not a parameter legally established, is an important quality characteristic which can play a key role as a factor of acceptability among consumers. In fact, many consumers associate a deep green colour with the idea of a good quality virgin olive oil (Baccouri et al., 2007). The mean values of chlorophyll contents in all oil samples obtained during the crop seasons are reported in Table 1. Chlorophyll values were lower than the mean contents previously reported by Allalout et al. (2009) in Arbequina and Koroneiki cultivars. Differences into the pigment contents can be attributed both to the cultivar (Psomiadou and Tsimidou, 2001) and the ripening degrees at the harvesting date.
Chlorophyll contents decreased from 1.57 mg kg-1 (2003 to 2004) to 0.46 mg kg-1 in 2005 to 2006 crop season. This significant decrease of chlorophyll contents could be due to the peroxidase activity stimulation by high autumnal temperature. In fact, the maturation of fruit is associated to the chlorophyll degradation (Gandul-Rojas et al., 2004; Johnson-Flanagan and Spencer, 1996). Simultaneously with the decrease of chlorophyll contents, a photosynthetic activity decrease was observed (data not shown). The highest chlorophyll contents, reported at the beginning of the study period, was positively correlated with a high rainfall that could inhibit the peroxidase activity.
Total phenols
Total phenols (TP), the main antioxidants present in VOO, are major responsible of the product stability and taste (Servili et al., 2003; Abaza et al., 2002; Kellie and Petter, 2002). TP contents presented a great variability depending on the crop season ranging from 41.53 to 110 mg kg-1 (as caffeic acid). As reported by different authors, the amount of total phenols is ranging between 50 and 1000 mg kg-1, depending on various factors such as cultivar (Salas et al., 1997; Bruni et al., 1994), climate (Salvador et al., 1998), location, degree of maturation, type of crushing machine and oil extraction procedures among others (Aguilera et al., 2005).
The oils from the last crop year (2005-2006) had a higher mean TP contents in comparison to the first and the second crop years. The latter is probably related to low rainfall accumulation of that year, as it is known that water shortage tends to generate a stress situation in the olive tree that induces phenol production in the olive fruit (Tovar et al., 2002). The higher TP content found in oils obtained in 2004 to 2005 crop season was associated to a low fruit water content which during ripening, while the lowest oil total phenol contents at 2003 to 2004 crop season was associated to the highest fruit water content (58.53%) This finding is in agreement with results reported by Salvador et al. (2001) for some Spanish cultivar.
A significant increase of TP content was reported in oils coming from fruits grown at high autumnal temperature (Table 1), as described previously by Ben Temime et al. (2006). The increase could be probably due to L-phenylalanine ammonia-lyase activity stimulation by high autumnal temperature.
Oxidative stability
The oxidative stability of VOO samples was evaluated by the Rancimat test. Results are reported in Table 1. The mean values of oxidative stability ranged from 30 to 62 h in 2003 to 2004 and 2006 to 2007 crop seasons, respectively. The induction time was lower than those reported by several authors for others varieties (Aguilera et al., 2005; Beltran et al., 2005). The major reason can be a lower oleic acid in Chemlali, regarding the widespread varieties.
A significant increase of oxidative stability was reported in 2006-2007 crop season. In accordance with many other authors (Amirante et al., 2006; Andrews et al., 2003; Gomez-Alonso et al., 2002), a positive correlation between TP contents and oxidative stability was recorded (r2 = 0.98), showing that the oil rich in TP are characterized by the higher stability. The higher stability was also into relation with fatty acid composition. In fact, samples characterized by the lower content in polyunsaturated fatty acids showed a high oxidative stability (Peres et al., 2004).
The study of the effect of the seasonal climatic variations on the composition and quality of “Chemlali” virgin olive oil is of great interest to the local industrial sector, the international olive oil business, and the final consumer since, despite the economic importance of this olive oil variety in Tunisia, complete and reliable data are still no available. The preliminary results revealed that the high autumnal temperature affected positively the olive oil content, with a positive increment of economical profits. No substantial differences in FFA and PV were noticed, while the K232 and K270 values were significantly different. Regard to the organoleptic evaluation and the other legally required parameters, all the VOO samples were defined as extra virgin olive oil. The seasonal climatic variations within the region influenced strongly total phenol and chlorophyll contents. A significant decrease of chlorophyll contents was found in oils coming from fruits grown at high temperature. However, the total phenol contents and the oxidative stability increased. Further studies of the effects of macroclimatic environments on “Chemlali” virgin olive oil composition are under investigation.
The authors have not declared any conflict of interest.
REFERENCES
|
Abaza L, Ben Temime S, Msallem M, Daoud D, Zarrouk M (2003). Etude comparative de la lipogenèse chez quelques variétés d'olivier cultivées en Tunisie. Riv. Ital. Sost. Grasse 80:297-306.
|
|
|
|
Aguilera MP, Beltran G, Ortega D, Fernandez A, Jimenez A, Uceda M (2005). Characterization of virgin olive oil of Italian olive cultivars: Frantoio and Leccino, grown in Andalusia. Food Chem. 89:387-391.
Crossref
|
|
|
|
|
Allalout A, Krichene D, Methenni K, Taamalli A, Oueslati I, Daoud D, Zarrouk M (2009). Characterization of virgin olive oil from Super Intensive Spanish and Greek varieties grown in northern Tunisia. Sci.
Crossref
|
|
|
|
|
Amirante P, Clodoveo ML, Dugo G, Leone A, Tamborrino A (2006). Advance technology in virgin olive oil production from traditional and de-stoned pastes: Influence of the introduction of a heat exchanger on oil quality. Food Chem. 98:797-805.
Crossref
|
|
|
|
|
Andrews P, Busch JL, De Joode T, Groenewegen P, Alexandre H (2003). Sensory properties of virgin olive oil, polyphenols: Identification of Deacetoxy-ligstroside aglycon as a key contributor to pungency. J. Agric. Food Chem. 51:1415-1420.
Crossref
|
|
|
|
|
Angerosa F, Di Giacinto L, Basti C, Serraiocco A (1996). Influenza della variabile "ambiente" sulla composizione degli oli vergini di oliva. Riv. Ital. Sost. Grasse 73:461-467.
|
|
|
|
|
Aparicio R, Luna G (2002). Characterisation of monovarietal virgin olive oils. Eur. J. Lipid Sci. Technol. 104:614-627.
Crossref
|
|
|
|
|
Aranda F, Gomez-Alonso S, Rivera Del Alamo RM, Salvador MD, Fregapane G (2004). Triglyceride, total and 2-position fatty acid composition of Cornicabra virgin olive oil: Comparison with others Spanish cultivars. Food Chem. 86:485-492.
Crossref
|
|
|
|
|
Baccouri B, Ben Temime S, Taamalli W, Daoud D, Msallem M, Zarrouk M (2007). Analytical characteristics of virgin olive oils from two new varieties obtained by controlled crossing on Meski variety. J. Food Chem. 14:19-34.
Crossref
|
|
|
|
|
Beltran G, Aguilera MP, Del Rio C, Sanchez S, Martinez L (2005). Influence of fruit ripening process on the natural antioxidant content of hojiblanca virgin olive oils. Food Chem. 89:207-215.
Crossref
|
|
|
|
|
Beltran G, Del Rio C, Sanchez S, Martinez L (2004). Influence of harvest date and crop yield on the fatty acid composition of virgin olive oils from cv. Picual. J. Agric. Food Chem. 52:3434-3440.
Crossref
|
|
|
|
|
Ben Temime S, Taamalli W, Baccouri B, Abaza L, Daoud D, Zarrouk M (2006). Changes in olive oil quality of Chetoui variety according to origin of plantation. J. Food Lipids 13:88-99.
Crossref
|
|
|
|
|
Boskou D (2000). Olive oil. In AP Simpopoulos and F Visioli, Mediterranean diets. World Revue Nutr. Diet 87:56-77.
Crossref
|
|
|
|
|
Bruni U, Cortesi N, Fiorino P (1994). Influence of agricultural techniques, cultivar and area of origin on characteristics of virgin olive oil and on levels of some of its minor components. Olivae 45:40-42.
|
|
|
|
|
Codex Stan (1989). Norme codex pour les huiles d'olive vierges et raffinées et pour l'huile de grignons d'olive raffinée Codex stan 33-1981 (Rév. 1-1989).
|
|
|
|
|
Di Giovacchino L (2000). Technological aspects. In Harwood J and R Aparicio. Handbook of olive oil. Gaithersburg: Aspens Publishers. pp. 17-59
Crossref
|
|
|
|
|
EEC (2003). Characteristics of olive and olive pomace oils and their analytical methods. EEC Regulation1989/2003. Offic. J. Eur. Commun. 295:57-66.
|
|
|
|
|
European Union Commission (1991). Regulation EC 2568/91 on the characteristics of olive oil and olive pomace and their analytical methods. Offic. J. Eur. Commun. 248:6-36.
|
|
|
|
|
Gandul-Rojas B, Roca M, Minguez-Mosquera MI (2004). Chlorophyll and carotenoid degradation mediated by thylakoid-associated peroxidative activity in olives (Olea europaea) cv. Hojiblanca. J. Plant Physiol. 161:499-507.
Crossref
|
|
|
|
|
Giansante L, Di Vincenzo D, Bianchi G (2003). Classification of monovarietal Italian olive oils by unsupervised (PCA) and supervised (LDA) chemometrics. J. Sci. Food Agric. 83:905-911.
Crossref
|
|
|
|
|
Gomez-Alonso S, Salvador MD, Fregapane G (2002). Phenolic compounds profile of Cornicabra virgin olive oil. J. Agric. Food Chem. 50:6812-6817.
Crossref
|
|
|
|
|
Gutierrez F, Jimenz B, Ruiz A, Albi MA (1999). Effect of olive ripeness on the oxydative stability of virgin olive oil extracted from the varieties Picual and Hojiblanca and on the different components involved. J. Agric. Food Chem. 47:121-127.
Crossref
|
|
|
|
|
Gutierrez F, Varona I, Albi MA.(2000). Relation of acidity and sensory quality with sterol content of olive oil from stored fruit. J. Agric. Food Chem. 48:1106-1110.
Crossref
|
|
|
|
|
International Olive Oil Council (2003). World olive oil consumption. Available at: http:www.internationaloliveoil.org/
|
|
|
|
|
Johnson-Flanagan AM, Spencer MS (1996). Chlorophyllase and peroxidase activity during degreening of maturing canola (Brassica napus) and mustard (Brassica juncea) seed. Plant Physiol. 97:353-359.
Crossref
|
|
|
|
|
Kellie LT, Peter JH (2002). Major phenolic compounds in olive oil: Metabolism and health effects. J. Nutr. Biochem. 13:636-644.
Crossref
|
|
|
|
|
Krichene D, Taamali W, Daoud D, Salvador MD, Fregapane G, Zarrouk M (2007). Phenolics compounds, tocopherols and other minor components in virgin olive oils of some Tunisian varieties. J. Food Biochem. 31:179-194.
Crossref
|
|
|
|
|
Kris-Etherton PM, Hecker KD, Bonanome A, Coval SM, Binkoski AE, Hilpert KF, Griel AE, Etherton TD (2002). Bioactive compounds in food: their role in prevention of cardiovasculair disease and cancer. Am. J. Med. 113:71-88.
Crossref
|
|
|
|
|
Mailer R, Beckingham C (2006). Testing olive oil quality: chemical and sensory methods. Primefact. P 231.
|
|
|
|
|
Montedoro G, Servili M, Baldioli M, Selvaggini R, Miniati E, Maccioni A (1993). Simple and hydrolysable compounds in virgin olive oil. III. Spectroscopic characterization of the secoiridoid derivatives. J. Agric. Food Chem. 41:2228-2234.
Crossref
|
|
|
|
|
Oueslati I, Anniva C, Daoud D, Tsimidou MZ, Zarrouk M (2009). Virgin olive oil (VOO) production in Tunisia: The commercial potential of the major olive varieties from the arid Tataouine zone. Food Chem. 112:733-741.
Crossref
|
|
|
|
|
Psomiadou E, Karakostas KX, Blekas G, Tsimidou MZ, Boskou D (2003). Proposed parameters for monitoring quality of virgin olive oil (Koroneiki cv). Eur. J. Lipid Sci. Technol. 105:403-408.
Crossref
|
|
|
|
|
Psomiadou E, Tsimidou M (2001). Pigments in Greek virgin olive oils: Occurrence and levels. J. Sci. Food Agric. 81:640-647.
Crossref
|
|
|
|
|
Ranalli A, de Mattia G, Ferrante ML, Giansante L (1997). Incidence of olive cultivation area on the analytical characteristics of the oil. Note 1. La Rivista Italiana delle Sostanze Grasse 74:501-508.
|
|
|
|
|
Salas J, Pastor M, Castro J, Vega V (1997). Influenca del riego sobre la composicion y las caracteristicas organolépticas del aceite del oliva. Grasas Aceites 48:74-82.
Crossref
|
|
|
|
|
Salvador MD, Aranda F, Fregapane G (1998). Chemical composition of commercial Cornicabra virgin olive oil from 1995/96 and 1996/97 crops. J. Am. Oil Chem. Soc. 75:1305-1311.
Crossref
|
|
|
|
|
Salvador MD, Aranda F, Gomez-Alonso S, Fregapane G (2000). Quality characteristics of Cornicabra virgin olive oil. Res. Adv. Oil Chem. 1:31-39.
|
|
|
|
|
Salvador MD, Aranda F, Gomez-Alonso S, Fregapane G (2001). Cornicabra virgin olive oil: a study of five crops seasons. Composition, quality and oxydative stability. Food Chem. 74:267-274.
Crossref
|
|
|
|
|
Servili M, Selvaggini R, Taticchi A, Esposto S, Montedero GF (2003). Volatile compounds and phenolic composition of virgin olive oil: optimisation of temperature and time of exposure of olive pastes to air contact during the mechanical extraction process. J. Agric. Food Chem. 51:7980-7988.
Crossref
|
|
|
|
|
Singleton VL, Rossi JA (1965). Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 16:144-158.
|
|
|
|
|
Spyros A, Phillipidis A, Dais P (2004). Kinetics of diglyceride formation and isomerization of virgin olive oils by employing 31 PNMR spectroscopy. Formulation of a quantitative measure to asses olive oil storage history. J. Agric. Food Chem. 52:157-164.
Crossref
|
|
|
|
|
Stefanoudaki E, Kotsifaki F, Koutsaftakis A (1999). Classification of virgin olive oils of the two major cretan cultivars based on their fatty acid composition. J. Am. Oil Chem. Soc. 76:623-626.
Crossref
|
|
|
|
|
Tovar M, Romero M, Alegre S, Girona J, Motilva M (2002). Composition and organoleptic characteristics of oil from Arbequina olive (Olea europaea L.) trees under deficit irrigation. J. Sci. Food Agric. 82:1755-1763.
Crossref
|
|
|
|
|
Vazquez Roncero A, Janer del Valle C, Janer del Valle ML (1973). Determination of total polyphenolics in olive oil. Grasas Y Aceites 24:350-357.
|
|
|
|
|
Visioli F, Borsani L, Galli C (2000). Diet and prevention of coronary heart diseases: The potential role of phytochemicals. Cardiovasc. Res. 47:419-425.
Crossref
|
|
|
|
|
Visioli F, Galli C (1998). Olive oil phenols and their potential effects on human health. J. Agric. Food Chem. 46:4292- 4296.
Crossref
|
|
|
|
|
Wolf JP (1958). Manuel des Corps Gras, A. Azoulay, ed. Paris. P 55.
|
|