ABSTRACT
A study on rapid propagation of cassava through tissue culture was conducted with three elite cassava genotypes: Slicass 6, Slicass 11 and Cocoa from Sierra-Leone. They showed slow growth in Murashige & Skoog (MS) basal medium which was proven to be optimal for a vast number of cassava accessions. Prior to mutation induction, a large population needs to be produced for mutagen susceptibility test and for mutant population development. The ultimate objective of this study was to investigate the effects of plant growth regulators on the shoot development of three cassava genotypes. In vivo shoot tips were sterilized and sub-cultured on MS media supplemented with six combinations of plant growth regulators (PGRs) at different concentrations. The results showed that from all media used, the MS medium with 1.0 mg/L α-naphthalene acetic acid (NAA) showed the best response for rooting (5.50), fresh weight (0.29 g), root number (10.00) and plantlet height (3.81 cm), while 0.1 mg/L 6-benzylaminopurine (BAP) was found to be more favourable to shoot development of leaves (6.38). The highest plant height and fresh weight were 3.81 cm and 0.29 g, respectively for Cocoa at 1.0 mg/L α-naphthalene acetic acid (NAA), 10.00 roots for Slicass 6 at 1.0 mg/L, 6.37 leaf numbers for Slicass 11 at 0.1 mg/L 6-benzylaminopurine (BAP) and 5.6 at 1.0 and 1.5 mg/L α-naphthalene acetic acid (NAA). These observations indicate that a supplement of 0.1 mg/L 6-benzylaminopurine (BAP) in MS medium can be useful in propagation of recalcitrant cassava and low concentration of α-naphthalene acetic acid (NAA) will be beneficial in root induction prior to acclimatization with promotion in recovery of the ex vitro plants before field assessment.
Key words: Cassava, Manihot esculenta, propagation, shoot tip, 6-benzylaminopurine (BAP), α-naphthalene acetic acid (NAA).
Cassava (Manihot esculenta Crantz) is one of the major food crops for over 800 million people in sub-Saharan Africa (Taye, 2009) and the second most important staple food in Sierra Leone (Olsen and Schaal, 2001). In tropical countries like Sierra Leone, cassava constitutes the most important source of energy in the diet of people (Burns et al., 2010; Perez et al., 2011). In addition, about 70% of the world cassava root is used for human consumption and the remaining is used for animal feed and industrial products, such as starch, glucose and alcohol (El-Sharkawy, 2004). In many African countries of which Sierra Leone is not exempted, the young leaves of cassava are also consumed as a vegetable to provide proteins, calcium, iron and vitamins, supplementing predominantly starchy diets in poor communities (Fregene et al., 2000). The world annual production of cassava is estimated at 241 million tonnes of fresh roots mostly from smallholder farmers in Africa and Asia (Bull et al., 2011). According to the Food and Agricultural Organization/Ministry of Agriculture Forestry and Food Security Crop Survey Report of 2003, land area under cassava cultivation in Sierra Leone was 99,484 ha yielding a total of 479,458 metric tonnes with average yield of 4.8 mt/ha (FAO/MAFFS, 2003). In Sierra Leone, one of the major constraints responsible for low production of cassava is the widespread cultivation of inherently low-yielding local varieties that are also highly susceptible to diseases (African cassava mosaic disease) and high pest incidence (Grasshopper) (Samura et al., 2017; Mansaray et al., 2012). Recently, the government of Sierra Leone has given top research priority with special emphasis on cassava promotion to develop superior cultivars and their production expansion. Hence, there is urgent need for disease free and high-quality cassava planting materials in the production system. To achieve this aim, the Sierra Leone Agricultural Research Institute (SLARI) released fourteen cassava varieties, but limitations are the low propagation of the crop due to conventional methods of production and systemic infections that prevent the progress in substituting susceptible varieties.
As a result, tissue culture techniques could be a viable option to resolve these difficulties. Santana et al. (2009) reported that plant tissue culture technique has been acknowledged as a powerful tool for studying and solving basic and applied problems of cassava production and productivity. Moreover, Loyola-Vargas et al. (2006) indicated that plant tissue culture technique is quicker and requires less space as compared to conventional methods of preparing cassava cuttings. Similarly, Le et al. (2007) had long established the tissue culture technique to be one of the realistic and efficient means for supplying large volumes of true-to-type clean planting materials of cassava within limited period. This is also an advantage for the production of large size population as required in mutation breeding. The multiplication of shoots requires optimum concentration of plant growth regulators (PGRs) in Murashige & Skoog (MS) medium, which appears to vary among materials with different botanical origins (including 2008). Acedo (2009) and Konan et al. (2006) showed that multiplication of cassava shoot could be enhanced with a relatively higher concentration of cytokinins, while rooting is boosted by the use of auxin. Kane (2005) also reported cytokinins, 6-benzylaminopurine (BAP) and kinetin (Kin), and auxin, α-Naphthalene acetic acid (NAA) as the most widely used and effective PGRs for shoot multiplication and root induction. Despite these good reports, no work has so far been done to develop an in vitro mass propagation procedure for these selected cassava genotypes (Slicass 6, Slicass 11 and Cocoa) in Sierra Leone. Thus, the development of optimal protocol is needed to ensure fast mass propagation prior to improvement through mutation induction and spreading of the improved cassava genotypes to increase cassava production in the country. This study aimed to investigate the responses of the three cassava genotypes to plant growth regulators on shoot propagation.
Three selected most desirable cassava genotypes (Slicass 6, Slicass 11 and Cocoa) were acquired from Sierra Leone Agricultural Research Institute (SLARI). The two improved Slicass genotypes were selected due to the following characteristics: early maturity, high yielding and resistance to pests and diseases in comparison with other genotypes tested, while the Cocoa (Local) genotype was chosen due to its palatability, poundability and farmer's preference across Sierra Leone. Each of the three cassava genotypes with 20 cm long stem cuttings possessing 7 to 8 nodes were transferred and grown in the glasshouse of FAO/IAEA Plant Breeding and Genetics Laboratory (PBGL), Seibersdorf, in Austria to provide shoots as donor material to culture.
Establishment of donor plant
About 20 cm long stem cuttings with 2 to 4 nodes of all three varieties were collected from the Njala Agricultural Research Center research fields and taken to the IAEA Seibersdorf glasshouse for establishment. The cuttings were planted in plastic containers of 4 L volume filled with surface sterilized soil mixture of manure, Seibersdorf soil and sand. The donor plants were established in the glasshouse of the Plant Breeding and Genetic Laboratory at a temperature of 25±2°C.
Media preparation
A liter of the medium was prepared by weighing 4.4 g of Murashige and Skoog (1962), MS basal powder in a beaker of 700 ml distilled water stirred on hot plate with a string ball, 1 ml/L MS vitamin and 3% sucrose for which six media combinations were supplemented with 0.05 mg/L BAP, 0.1 mg/L BAP, 1 mg/L NAA, 1.5 mg/L NAA, 0.1 mg/L BAP + 1.5 mg/L NAA + 1.0 mg/LGA3 and control MS without PGR. As gelling agent, 1.8 g/L gelrite was used and pH adjusted to 5.8 before autoclaving. The autoclaved media were kept in the cold room before use.
Surface sterilization and initiation of explants
Sprouted shoots over 2 cm long were harvested from the three cassava genotypes. The de-leaved buds were washed thoroughly using running tap water in order to clean off debris and taken to lamina flow bench for surface sterilization and initiation. The explants were initiated in vitro after surface sterilization with 70% ethanol for 30 s and instantly rinsed with sterile distilled water, 20% commercial bleach (Clorox) for 20 min, shaken intermittently and at least rinsed three times with sterile distilled water. The dead edges were excised for Clorox damage. The sterilized explants were cut into 1 to 2 nodes and transplanted into test tube containing 12 ml of solid propagation medium, Murashige and Skoog (1962) medium supplemented with 3% sucrose (w/v), 1.8% gelrite (w/v) and different concentrations and combinations of plant growth regulators. The cultured explants were incubated in a controlled growth room at 22±2°C for 16 h photoperiod and sub-cultured at 4 weeks on the same initiation medium.
Data collection
After a period of 4 weeks, various growth parameters such as number of leaves/plantlet (nodes), plantlet height (cm), fresh weight/plantlet (g), root induction and number/plantlet were evaluated.
Data analysis
Data was subjected to analysis of variance using Genstat 12.1 statistical package and means were separated according to Student-Newman-Keuls multiple-range test (SNK) at 5% level of probability.
Five culture media supplemented with cytokinin or auxin were used in the improvement of tissue culture initiation of the three Sierra Leonean cassava cultivars in comparison with MS medium used by Owoseni and Ogunnusi (2006) in over 20 cassava accessions from Nigeria. The values from analysis of variance of the hormones and genotypes indicated that the F-probability of all the five parameters studied were highly significant with regards to the different media used. The interactive effects of genotype with hormone was significant for plantlet height, number of roots, root induction and formation and plantlet weight, while number of leaves (or number of nodes) was significantly different. However, genotype showed significant difference for root number, weight of plantlet and leaf number.
Effect of hormones on in vitro plantlet height of the different cassava genotypes
The MS medium supplemented with BAP and NAA either alone or in combination with GA3 showed that there was significant (P<.001) difference between treatments used. Among the various concentrations, treatment 1.0 mg/L NAA showed the greatest plant height of 3.67, 3.20 and 3.81 cm, respectively, for Slicass 6, Slicass 11 and Cocoa. Whereas, the analysis of variance showed that MS medium with NAA (1.0 and 1.5 mg/L) alone produced better results in response to plant height when compared with the other treatments (Table 1). 0.1 mg/L BAP exhibited the second better growth for Slicass 6 and Cocoa with respectively, 3.13 and 3.30 cm as plantlet height as compared to other plant growth regulators. However, the Slicass 11 observed second better plantlet height of 2.65 with 0.1 mg/L BAP and 1.5 mg/L NAA. The combinations of BAP + NAA + GA3 cultured in MS medium elicited optimal responses with an average mean plant height of 2.13 cm as compared to only 0.05 mg/L BAP (1.11 cm). On the contrary, MS medium supplemented with 0.0 mg/L (control) concentrations produced greater plant height than 0.05 mg/L BAP which produced the shortest plant height.
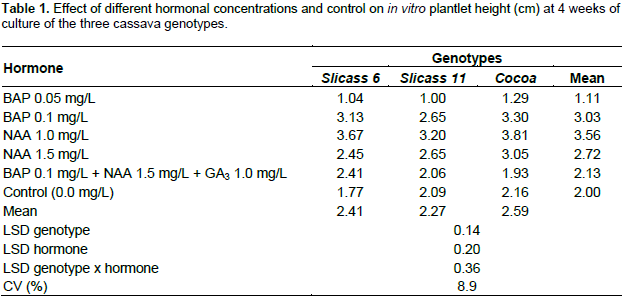
This improvement in plantlet height could be attributed to addition of the phytohormone. Cytokinin and auxin (alone or together) are known to stimulate cell division and reduce lateral bud dormancy which is relevant to the plant tissue culture (Davies, 2004). The increase in plant height is also due to the effects of phytohormone influencing initiation of cell division with cell growth and expansion. The results of the present experiment were found to corroborate the findings of Davies (2004), that cytokinin influences cell division in order to broaden the area of the tissues and plantlet height. However, medium without phytohormone (control) produces taller plant height as compared to 0.05 mg/L BAP media. Similar results were reported by Mapayi et al. (2013) where MS medium (without growth regulator) showed better growth for three cassava genotypes evaluated for micro-propagation. Figure 1 is visual appearance of lower root number, stunted stem and fewer leaves in the control treatment (A); NAA 1.0 and 1.5 mg/L (Figure 1B and D) showed maximum root number and turgid stem growth. Furthermore, BAP 0.05 mg/L (Figure 1C) produced callus roots, thin stem and tiny yellowish green leaves. Thus, BAP 0.1 mg/L produced greater number of leaves and larger stem, but inhibited root formation.
Effect of hormones on in vitro rooting percentage/plantlet of the different cassava genotypes
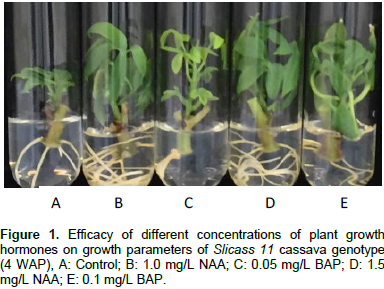
The analysis of variance indicated that in vitro rooting percentage was influenced considerably by different concentrations of culture medium used in the experiment (Table 2). The results showed that there was increasing formation on rooting percentage at various hormonal concentrations which was significant at P<0.001 level of significance. The percentage of rooting was remarkably highest on MS media supplemented with 1.0 and 1.5 mg/L NAA hence, best for rooting in the cassava genotypes studied, followed by hormone free medium (control) than the other treatments (Table 2). The results of the present study conformed to the findings of Demeke et al. (2014) that regenerated cassava shoots produced an average of 6.14 roots within a 0.5 mg/l NAA. Furthermore, Chen et al. (2003) reported that in Dioscorea zingiberensis, half strength MS with NAA (2.0 mg/L) was the best rooting medium. Similar results in yam were also obtained by Behera et al. (2010). From this study, smaller rooting was observed among the genotypes Slicass 6, Slicass 11 and Cocoa in the 0.1 and 0.05 mg/L BAP medium during 4 weeks of culture. Thus, the addition of BAP, especially at high doses in the MS medium deduced strongly, the formation of roots (Faye et al., 2015). The experimental results on NAA 1.0 (E) and 1.5 mg/L (A) produced relatively higher number of roots, percent rooting, bigger stems and lower number of leaves (Figure 2). In addition, BAP 0.1 mg/L (Figure 2B) and 0.05 mg/L (Figure 2D) showed greater number of leaves, lower rooting percentage and root number. Non-plant regulators media (Figure 2C) produced higher number of secondary roots, normal stem growth and profuse leaves.
Effect of hormones on in vitro root number per plantlet of the different cassava genotypes
The effect of different concentrations of BAP, NAA and in combination with GA3 on the number of roots/plantlet was found to be statistically significant at P<0.001 level of significance (Table 3). The number of roots varied with various concentrations of BAP, NAA and in combination with GA3 (Table 4). A good number of roots were achieved at 1.0 mg/L NAA with an average mean of 7.72 followed by only 1.5 mg/L NAA (6.39) which was superior to the other treatments. But the highest number of roots was significantly produced by 1.0 mg/L NAA (10.00) with the Slicass6 cultivar followed by Cocoa cultivar with 7.03 roots per plantlets. The use of NAA (1 to 1.5 mg/L) relatively produced higher number of roots as compared to BAP (0.05 to 0.1 mg/L). These results confirm the report of Fan et al. (2011) that NAA (0 to 2.0 mg/L) proved to be effective on root development in cassava. Also, Kane (2005) similarly reported that NAA (0.01 to 10 mg/L) is the most widely used and effective plant growth regulators for root induction. Cacai et al. (2012) has shown that kinetin induces more roots than BAP. On the contrary, 0.00 mg/L (control) produced higher number of roots as compared to BAP (0.1 and 0.05 mg/L) in all three cultivars together with the results of the three cassava genotypes assessed by Mapayi et al. (2013). The use of BAP (0.1 and 0.05 mg/L) showed no significant difference on root number as compared to the use of NAA (1.0 and 1.5 mg/L) among the three cassava genotypes. The lowest number of roots was produced by 0.1 mg/L BAP treatment. This indicates that the addition of cytokinin like BAP in growth medium has suppression effects, whereas auxin (NAA) promoted the root induction in comparison with control condition. The present results corroborate the findings of Gubbuk and Pekmezci (2001).
Effect of hormones on in vitro leaf number/plantlet of the different cassava genotypes
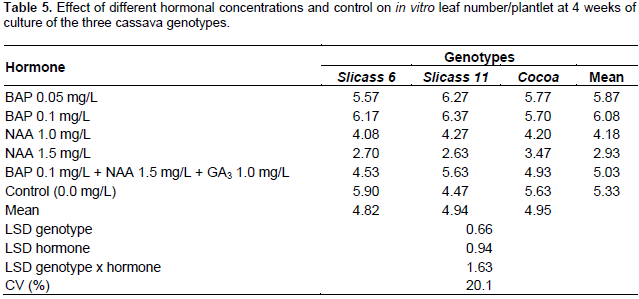
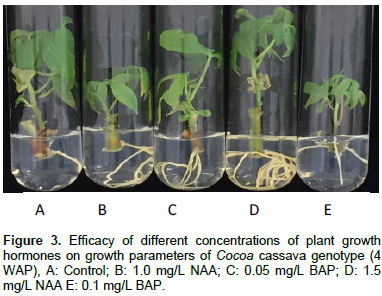
As shown in Table 5, plantlets cultured in medium with BAP produced more leaves (or also corresponded to the visible nodes) than those cultured in medium containing either NAA only or in combination with BAP and GA3. Significant differences in number of leaves were observed among genotypes grown on different hormonal treatments. Comparison of effects of different culture media on leaf number after 4 weeks revealed that 0.1 mg/L BAP showed the highest (6.37) number of leaves in Slicass 11 cultivar followed by 0.05 mg/L BAP (6.27), whereas, 0.1 mg/L BAP was effective with the Slicass 6 cultivar. However, no significant difference was observed in two concentrations of BAP with response of Cocoa cultivar. These results are confirmed by Ahanhanzo et al. (2008) who reported that explants cultured in media containing BAP produced highest number of leaves in all the cassava genotypes. On the contrary, NAA appeared to be unfavourable for the development of leaves in all genotypes. Therefore, number of leaves per plantlet was only 2.63 after four weeks of culture in the medium, 1.5 mg/L NAA in the Slicass 11 cultivar. The responses of Cocoa cassava genotype cultured on MS media supplemented with BAP 0.05 mg/L (Figure 3A) produced little root with some callosity at the base of the stem but had greater number of leaves (Figure 3). For BAP 0.1 mg/L (B) concentrations, few leaf number and short internodes was produced, thus it will be difficult to subculture. The hormone concentrations of NAA 1.5 (Figure 3C) and 1.0 mg/L (Figure 3D) produced maximum number of roots, rooting percentage, turgid stems but fewer leaved number. Control had stunted growth with few numbers of root and leaves (Figure 3E).
Effect of hormones on in vitro fresh weight/plantlet of the different cassava genotypes
The data indicated that increase in fresh weight/plantlet was significantly (P < 0.05) better at higher concentrations (Table 6). Treatments with 1.0 mg/L NAA showed the best results in terms of fresh weight followed by treatments with1.5 mg/L NAA. The interactive effect of BAP 0.1 + NAA 1.5 + GA3 1.0 mg/L significantly (P<.001) resulted in high fresh weight as compared to 0.05 mg/L BAP and control (0.00 mg/L). Similar results were reported by Hussein (2012), whereas, supplementing NAA to increasing concentration of BA (from 0.2 to 0.4 mg/L) resulted in higher fresh weight. This could be attributed to increased cytosolic calcium concentration resulting from enhanced uptake from the media due to the use of higher amount of NAA (Ngomuo et al., 2013).
Supplementing the growth medium with various hormonal concentrations and control each with Murashige & Skoog basal medium, showed in vitro responses of plantlet height, root induction, root number, leaf number, and fresh weight among three recalcitrant cultivars, Slicass 6, Slicass 11 and Cocoa. In all the media, the MS medium with 1.0 mg/L NAA showed the best response to percent rooting, fresh plantlet weight, root number and plantlet height, while 0.1 mg/L BAP was found to be more favourable for normal development of the leaves which is beneficial in shoot multiplication. Therefore, the addition of cytokinin like BAP at 0.1 mg/L is recommended for the propagation of recalcitrant cassava genotype, whereas, the auxin, NAA is optimal for root development prior to plantlets acclimatization, to improve the recovery of ex vitro plantlets for field evaluation.
The authors have not declared any conflict of interests.
This research was supported by the Technical Cooperation Project of the International Atomic Energy Agency (IAEA) and Njala University in partnership with Njala Agricultural Research Center (NARC). The authors thank and appreciate the staff of FAO/IAEA Plant Breeding and Genetic Laboratory (PBGL), Seibersdorf and Njala Agricultural Research Centre (NARC), Freetown, particularly, Dr Bado Souleymane.
REFERENCES
|
Acedo VZ (2009). Meristem cultured and micropropagation of cassava. J. Root Crops 4(5):65-78.
|
|
|
|
Ahanhanzo C, Agbangla C, Agassounon DTM, Cacaï GHT, Dramane K (2008). Comparative study of the influence of growth' regulators on morphogenesis in vitro of some cassava varieties (Manihot esculenta) in Benin. Rev. CAMES-Ser. A, 07:47-52.
|
|
|
|
Behera TK, Behera S, Bharathi LK, John KJ, Simon PW, Staub JE (2010). Bitter Gourd: Botany, horticulture, and breeding. Hort Rev. 3(7):101-141.
Crossref
|
|
|
|
Bull ES, Ndunguru J, Gruissem W, Beeching JR, Vanderschuren H. (2011). Cassava: constraints to production and the transfer of biotechnology to African laboratories. Plant Cell Rep 30:779-787.
Crossref
|
|
|
|
Burns A, Gleadow R, Cliff J, Zacarias A, Cavagnaro T (2010). Cassava: The drought, war and famine crop in a changing world. Sustainability 2(3):572-3607.
Crossref
|
|
|
|
Cacai GHT, Ahanhanzo C, Dangou JS (2012). Effect of different hormonal combinations on organogenesis in vitro of some local improved varieties of cassava (Manihot esculenta-Euphorbiaceae) cultivated in Benin. Int. J. Biol. Chem. Sci. 6(4):1593-1607.
|
|
|
|
Chen Y, Fan J, Yi F, Luo Z, Fu Y (2003). Rapid clonal propagation of Dioscorea zingiberensis. Plant Cell Tissue Organ Cult. 7(3):75-80.
Crossref
|
|
|
|
Davies PJ (2004). The plant hormones: Their Nature, Occurrence and Function In: Davies PJ (Ed.) Plant Hormones Biosynthesis, Signal Transduction, Action. Kluwer Acad. Press. pp. 1-15.
|
|
|
|
Demeke Y, Tefera W, Dechassa N, Abbie B (2014). Effects of plant growth regulators on in vitro cultured nodal explants of cassava (Manihot esculentaCrantz) clones. Afr. J. Biotechnol. 13(28):2830-2839.
Crossref
|
|
|
|
El-Sharkawy MA (2004). Cassava biology and physiology. Pl. Mol. Biol. 5(6):481-501.
Crossref
|
|
|
|
Fan M, Liu Z, Zhou L, Lin T, Liu Y, Luo I. (2011). Effect of plant growth regulators and saccharides on in vitro plant and tuberous root regeneration of cassava (Manihot esculanta Crantz). J. Plant Growth Regul. 30:11-19.
Crossref
|
|
|
|
FAO/MAFFS (2003). Food and Agricultural Organization/Ministry of Agriculture Forestry and Food Security Crop Survey Report of 2003. 40p.
|
|
|
|
Faye A, Sagna M, Kane PMD Sane D (2015). Effects of different hormones on organogenesis in vitro of some varieties of cassava (Manihot esculentaCrantz) grown in Senegal. Afr. J. Plant Sci. 9(8): pp. 305-312. Fregene M, Bernal A, Duque M, Dixon AGO, Tohme J (2000). AFLP analysis of African cassava (Manihot esculenta Crantz) germplasm resistant to the cassava mosaic disease (CMD). Theor. Appl. Genet. 100:678-685.
|
|
|
|
Gubbuk H, Pekmezci M (2001). The effects of different hormone types and concentrations on propagation of different banana clones by meristem culture. Ziraat Fakultesi Dergisi, Akdeniz Universitesi 14(1):127-137.
|
|
|
|
Hussein N (2012). Effects of nutrient media constituents on growth and development of banana (Musa spp.) shoot tips cultured in vitro. Afr. J. Biotechnol. 11(37):9001-9006.
|
|
|
|
IITA (2009) Cassava processing and gene banking manual,
View
|
|
|
|
Kane ME (2005). Shoot culture procedure in plant development and biotechnology. In: R. N. Trigiano, and D. J. Gray, (eds.) CRC Press. P 332.
|
|
|
|
Konan NK, Sangwan RS, Sangwan-Norreel BS (2006). Efficient in vitro shoot-regeneration systems in cassava (Manihot esculenta Crantz). Plant breeding 113(3):227-236.
Crossref
|
|
|
|
Le BV, Anh BL, Soytong K, Danh ND, Anh Hong LT (2007). Plant regeneration of cassava (Manihot esculenta Crantz) plants. J. Agric. TechnoL. 3(1):121-127.
|
|
|
|
Loyola-Vargas VM, Vázques-Flota F (2006). Plant cell culture protocols. In: Loyola-Vargas, V. M. and Vázquez-Flota, F. (Eds). 2ndMethods in Molecular Biology 318. Humana Press Inc., New Jersey. Pp:3-8.
|
|
|
|
Mansaray A, Sundifu AJ, Samura AE, Massaquoi FB, Quee DD, Fomba SN, Moseray MT (2012). Cassava genotype evaluation for grasshopper Zonocerus variegatus (L.) (Orthoptera: Pyrgomorphidae) susceptibility in Southern Sierra Leone. Int. J. Agric. Forestry, 2(6):294-299.
Crossref
|
|
|
|
Mapayi EF, Ojo DK, Oduwaye OA, Porbeni JBO (2013). Optimization of invtro propagation of cassava (Manihot esculenta Crantz) genotypes. J. Agric. Sci. 5(3):261-269.
|
|
|
|
Ngomuo M, Mneney E, Ndakidemi P (2013). The Effects of auxins and cytokinin on growth and development of (Musa sp.) Var. "Yangambi" explants in tissue culture. Ame. J. Plant Sci. 4(2):174-2180.
|
|
|
|
Olsen KM, Schaal BA (2001). Microsatellite variation in cassava (Manihot esculenta, Euphorbiaceae) and its wild relatives: Further evidence for a southern Amanzonian origin of domestication. Am. J. Bot. 8(8):131-142.
Crossref
|
|
|
|
Owoseni AA, Ogunnusi T (2006). Antibacterial effects of three selected chewing sticks extracts on Lactobacillus sp. Int. J. Trop. Med. 1(3):103-106.
|
|
|
|
Perez JC, Lenis JI, Calle F, Morante N, Sanchez T, Debouck D, Ceballos H (2011). Genetic variability of root peel thickness and its influence in extractable starch from cassava (Manihot esculenta Crantz) roots. Plant Breeding 1(30):688-693.
Crossref
|
|
|
|
Samura AE, Lakoh KA, Nabay O, Fomba SN and Koroma JP C (2017). Effect of cassava mosaic disease (CMD) on yield and profitability of cassava and gari production enterprises in Sierra Leone. J. Agric. Sci. 9(2):2015-216.
Crossref
|
|
|
|
Santana MA, Romay G, Matehus J, Vicente-Villardon JL, Demey JR (2009). A simple and low-cost strategy for micropropagation of cassava (Manihot esculenta Crantz). Afr. J. Biotechnol. 8(16):3789-3897.
|
|
|
|
Taye B (2009). Cassava, Africa's Food Security Crop.
View
|