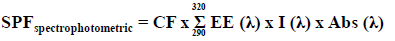ABSTRACT
Simira gardneriana is a Rubiaceae species commonly found in the Brazilian Northeast region, presented several therapeutic and biotechnological applications. In this paper, the antioxidant and photoprotective properties of extracts from the seeds of S. gardneriana were highlighted. The antioxidant activity of ethanol and methanol extracts (Si-EtOH and Si-MeOH, respectively) was determined, using 1,1-Diphenyl-2-picryl-hydrazyl (DPPH) free radical scavenging assay. The photoprotective activity of the extracts was evaluated using a spectrophotometric method. Total phenolic and flavonoid content was determined. In addition, a qualitative analysis of phytochemical markers and a high-performance liquid chromatography-diode-array detector (HPLC-DAD) analysis were also performed for both extracts. Concerning the antioxidant activity in vitro, Si-MeOH (EC50 = 70.94±2.17 µg/ml) showed greater activity in comparison to Si-EtOH (EC50 = 138.60±7.39 µg/ml). Once the sun protection factor spectrophotometric (SPFspectrophotometric) of samples was calculated, it was demonstrated that the extracts show a similar photoprotective effect on all concentrations tested. Si-EtOH and Si-MeOH tested on a concentration of 100 mg/l, exhibited SPF values equal to 3.37±0.006 and 3.36±0.007, respectively. HPLC fingerprints was recorded and showed the presence of characteristic peaks for phenolic compounds. The extracts showed significant phenols and flavonoids content according to the quantification methods used. According to the results, it was concluded that Si-EtOH and Si-MeOH have significant antioxidant and photoprotective activities. These activities are probably related to the profile of flavonoids and phenolic compounds found in this species.
Key words: Phenolic and flavonoids compounds, photoprotective, oxidative stress, plant, Rubiaceae.
Exposure to UV radiation promotes a range of damaging effects on the body which include production of reactiveoxygen species (ROS) in skin. In particular, ultraviolet B (UVB) (290 to 320 nm) can reach the skin and cause erythema, burns, local inflammation, DNA damage and early aging. In addition, ultraviolet A (UVA) radiation (320 to 400 nm) penetrates deeper into the epidermis and dermis and stimulates ROS production (O2. and OH. for example), which can modify proteins, lipids and DNA structure (Stevanato et al., 2014; Surget et al., 2015). An alternative that has been used to combat the damage caused by solar exposition is the use of natural products.
Rubiaceae family comprises 637 genera and about 13,000 species mainly distributed in tropical and subtropical regions (Rogers, 2005). In America, this family represents 229 genera and 5,200 species (Delprete, 1999), while in Brazil, Rubiaceae is represented by about 1,500 species, making this one of the main families of Brazilian vegetation (Souza and Lorenzi, 2005). Some Rubiaceae species have described biological properties, such as the species Uncaria tomentosa (Willd) D.C., popularly known as "unha-de-gato", being widely used in folk medicine for various indications: arthritis, asthma, cancer, gastric ulcer, inflammation and bleeding (Heitzman et al., 2005). Moreover, pharmacological studies also demonstrated that other species of this family have anti-inflammatory (Zhu et al., 2012), antinociceptive (Déciga-Campos et al., 2006), antibacterial (Comini et al., 2011), antitumor and antioxidant activities (Dreifuss et al., 2010).
In relation to family phytochemistry, some alkaloids were mentioned as important chemical markers (Moraes et al., 2009). In addition to these compounds, the presence of flavonoids, benzenoid derivatives, anthraquinones, coumarins, saponins, lignoids, terpenoids, cucurbitacines, amides and pheophytins has also been reported in Rubiaceae species (Rudrapaul et al., 2014; Mongrand et al., 2005; Moreno et al., 2014; Ferreira-Júnior et al., 2012).
The Simira genus is an important genera of Rubiaceae and comprises about 45 species, predominantly found in neotropical regions, of which 16 occur in Brazil (Sampaio et al., 2002). Some of these species are used in folk medicine as natural remedies. In fact, studies describe their phototoxic activity, justified by the presence of bioactive secondary metabolites (Araújo et al., 2012; Arnason et al., 1983). In Caatinga, a biome is located in the Northeast region of Brazil. There are six Simira species, among which S. gardneriana M. R. V. Barbosa and Peixoto is the only one endemic (Sampaio et al., 2002).
S. gardneriana is known as "pereiro-de-tinta" or "pereiro-vermelho” and is used as forage during the dry season (Sampaio et al., 2002). However, there have been no reports on the phytochemical profile and biological properties of this species until now. Thus, considering that several Rubiaceae species are promising sources of bioactive molecules, this study aimed to evaluate the antioxidant and photoprotective activities of extracts from the seeds of S. gardneriana and to investigate its phytochemical profile through high-performance liquid chromatography-diode-array detector (HPLC-DAD) analysis.
Plant material
The seeds of S. gardneriana were collected in the city of Afrânio, State of Pernambuco, Brazil, in February 2012 (coordinates 08°28’40.60” S and 40°56’10.60” W). A voucher specimen of the plant (13949) was deposited in the Herbarium Vale do São Francisco (HVASF) of the Universidade Federal do Vale do São Francisco.
Preparation of extracts
Initially, the dried and pulverized plant material (1.938 g) was subjected to maceration with 95% ethanol. Five extractions were performed and the solvent was replaced every 72 h. The extraction solution obtained was filtered and concentrated in a rotary evaporator apparatus oven at 50°C, providing 115 g of ethanol extract (Si-EtOH, 5.93%).
Subsequently, the maceration was continued with absolute methanol. Three extractions were carried out and the solvent was replaced every 72 h. The extraction solution obtained was concentrated under the same conditions as Si-EtOH, resulting in 162 g of methanol extract (Si-MeOH, 8.36%).
Qualitative analysis of phytochemicals
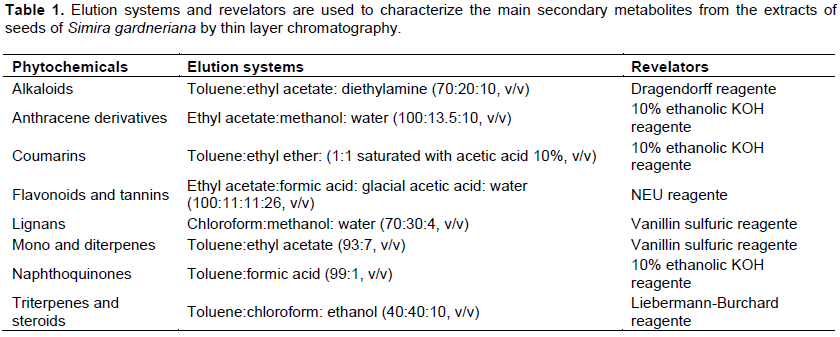
Extracts solutions (1 mg/ml) were evaluated on thin layer chromatographic plates of silica gel 60 F254 aluminum supports, applied with a micropipette and eluted in different solvent systems as previously described (Wagner and Bladt, 1996), seeking to highlight the main groups of secondary metabolism (Table 1).
HPLC-DAD analysis
Solutions of Si-EtOH and Si-MeOH extracts (1 mg/ml, in methanol) were individually analyzed by High Performance Liquid Chromatography (HPLC), following parameters which is previously described (Cai et al., 2003). The solvents used were of analytical grade from Merck®. A Milli-Q System® (SMART, China) was used to purify the water. Analysis was performed on a liquid chromatograph Shimadzu® equipped with a quaternary pump system (LC-20ADVP), a SPD-20AVP Diode-Array Detector (DAD), and an SIL-20ADVP auto sampler.
The data was acquired and processed using Shimadzu® LC solution 1.0 software. The extracts were analyzed using a reverse-phase HPLC column: Ascentis® C18 (250 x 4, 6 mm, 5 µm) column (Supelco®). The mobile phase was composed of solvent (A) H2O/trifluoroacetic acid 0.1% and solvent (B) MeOH. The solvent gradient was composed of A (100 to 90%) and B (0 to 10%) for 0 to 7 min, A (90 to 60%) and B (10 to 40%) for 7 to 20 min, A (60 to 100%) and B (40 to 0%) for 20 to 25 min, and finally A (100 to 90%) and B (0 to 10%) for 25 to 40 min. A flow rate of 1.0 ml/min was used in an oven at 37°C, and 20 μl of each sample was injected. The procedure was repeated three times for each sample. Samples and mobile phases were filtered through a 0.22 μm Millipore filter prior to HPLC injection. Spectra data were recorded from 200 to 400 nm during the entire run and the chromatograms of extracts obtained at a wavelength of 254 nm were selected for analysis of its components.
Total phenolic content
Total phenolic contents were performed using the Folin-Ciocalteu reagent, based on a method previously reported, in which only the volumes were reduced (Slinkard and Singleton, 1977). Si-EtOH and Si-MeOH were diluted (1000 mg/l), an aliquot (40 μl) was added to 3.16 ml of distilled water and 200 μl of the Folin-Ciocalteu reagent. The mixture was shaken and allowed to stand for 6 min. Then, 600 μl of sodium carbonate solution was added and shaking to mix. The solutions remained at room temperature for 2 h and the absorbance of each sample was determined at 765 nm against the blank (Spectrophotometer Quimis, Brazil).
Total phenolic contents were expressed as mg gallic acid equivalents per gram (mg GAE/g) through the calibration curve with gallic acid (50 to 1000 mg/l, R2 = 0.997). All samples were performed in triplicates.
Total flavonoid content
Total flavonoid content was determined according to a colorimetric method described previously (Santos and Blatt, 1998; Marques et al., 2012). Si-EtOH and Si-MEOH extracts were diluted (1000 mg/l) and 0.20 ml of extracts or quercetin standard solution were mixed with 3.80 ml of distilled water, in a test tube followed by the addition of 200 μl of a 2.5% AlCl3 solution.
After 30 min of reaction at room temperature, the absorbance was measured against the blank at 408 nm using a spectrophotometer (QUIMIS, Brazil), in comparison with the standards prepared similarly with known quercetin concentrations. The results were expressed as mg of quercetin equivalents per gram of extracts (mg QE/g) through the calibration curve with quercetin (1 to 20 mg/l, R2 = 0.995). All assays were performed in triplicate.
Antioxidant activity in vitro - DPPH free radical scavenging assay
The free radical scavenging activity was measured using the 2, 2-diphenyl-1-picrylhydrazyl radical (DPPH) assay (Falcão et al., 2006). Sample stock solutions (1.0 mg/ml) of extracts were diluted to final concentrations of 243, 81, 27, 9, 3 and 1 μg/ml, in ethanol. One millilitre (1 ml) of 50 μg/ml DPPH ethanol solution was added to 2.5 ml of sample solutions of different concentrations, and allowed to react at room temperature. After 30 min of reaction, the absorbance values were measured at 518 nm and converted into the percentage antioxidant activity (AA) using the following formula: AA % = [(absorbance of the control – absorbance of the sample) / absorbance of the control] × 100. Ethanol (1.0 ml) plus plant extracts solutions (2.5 ml) were used as blank and DPPH solution (1.0 ml) plus ethanol (2.5 ml) was used as negative control. Ascorbic acid, BHA (butylated hydroxyanisole) and BHT (butylated hydroxytoluene) were used as positive controls. Assays were carried out in triplicate.
Photoprotective activity in vitro – determination of the maximum absorption wavelength and sun protection factor spectrophotometric (SPF)
For determining the maximum absorption wavelength (λmax), the extracts were diluted in absolute ethanol, obtaining concentrations of 5, 25, 50 and 100 mg/l. Subsequently, spectrophotometric scanning was performed at wavelengths between 260 to 400 nm, with intervals of 5 nm. The readings were performed using 1 cm quartz cell, and ethanol used as blank (Mansur et al., 1986). Calculation of SPF was obtained according to the equation:
Where: EE (λ) = erythemal effect spectrum; I (λ) = solar intensity spectrum; Abs (λ) = absorbance of sunscreen product; CF = correction factor (=10). The values of EE x I are constants. They were previously determined (Sayre et al., 1979). Benzophenone-3 (10 mg/l) was used as a positive control.
Statistical analysis
The data obtained were analyzed using the GraphPad Prism® version 5.0 and expressed as mean ± S.D. The EC50 values were obtained by interpolation from non-linear regression analysis with 95% confidence level.
EC50 is defined as the concentration sufficient to give 50% of maximumeffect estimated at 100%. Statistically significant differences were calculated by the application of Student’s t-test. Values were considered significantly different at p < 0.05.
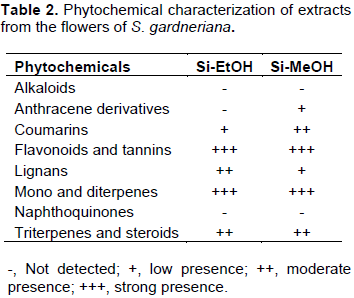
Qualitative analysis of phytochemicals
The phytochemical profile of the extracts was characterized by thin layer chromatography. In general, the extracts showed positive results for the presence of several classes of secondary metabolites, especially flavonoids, mono and diterpenes, coumarins, lignans, triterpenes and steroids, as shown in Table 2. The intensity scale of the identified phytochemicals was defined by comparison with standard samples whenever possible.
HPLC-DAD analysis
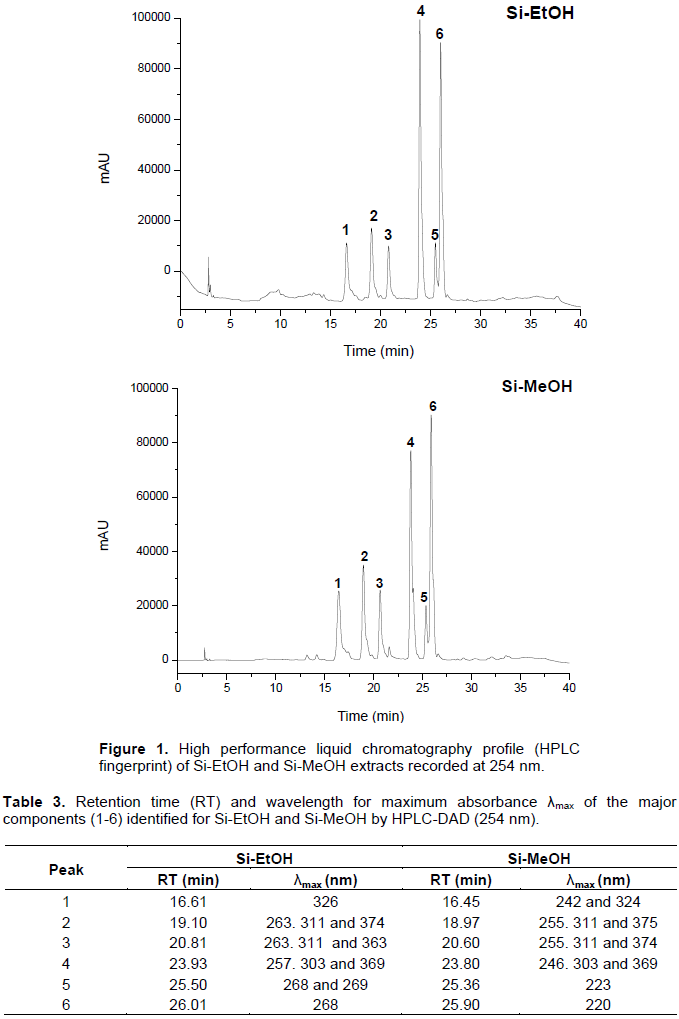
HPLC fingerprint for Si-EtOH and Si-MeOH are presented in Figure 1. The chromatogram shows the presence of six majority peaks for both extracts with different retention times. Furthermore, the λmax values observed for compounds 1 to 6 are characteristic of phenolic constituents for the analyzed wavelength (254 nm). Based on their UV-Vis spectral data and their retention time, the compounds have UV band characteristic for phenolic acids and flavonoid derivatives (Table 3). These compounds are under investigation.
Total phenolic and flavonoid content
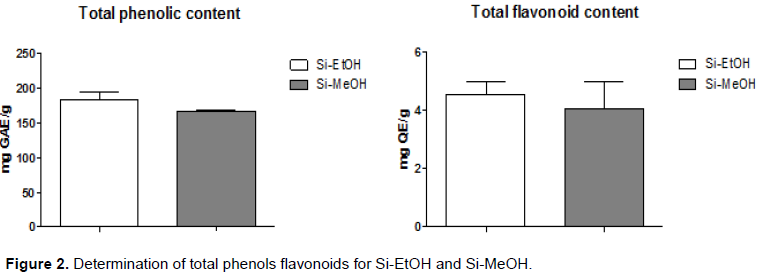
The total phenols and flavonoids contents for extracts were determined using different methods. Total phenol content was determined by the method of Folin-Ciocalteu reagent, where Si-EtOH and Si-MeOH showed 183.70±9.87 and 166.50±1.67 mgGAE/g, respectively. In relation to determination of total flavonoids, a colorimetric assay using quercetin was conducted as a standard.
In this method, Si-EtOH and Si-MeOH showed 4.55±0.73 and 4.05±1.60 mgEQ/g, respectively. However, the extracts showed no significant differences in total phenolic and flavonoids contents found (Figure 2). The results are expressed in mg of gallic acid equivalents per gram of sample (mg GAE/g) and in mg of quercetin equivalents per gram of sample (mg QE/g), respectively. The Student’s t-test was used for analysis of the results.
Antioxidant activity in vitro
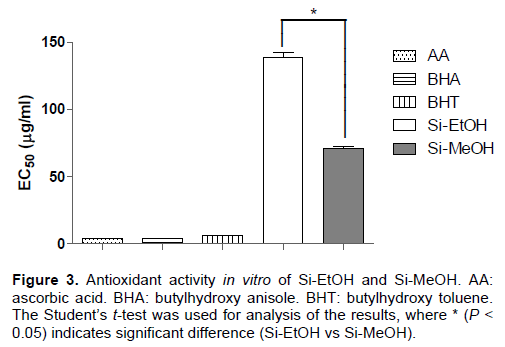
Concerning the antioxidant activity in vitro, Si-MeOH (EC50 = 70.94±2.17 µg/ml) showed better activity in comparison to Si-EtOH (EC50 = 138.60±7.39 µg/ml) in DPPH free radical scavenging assay. However, ascorbic acid, BHA and BHT proved more effective than both extracts, presenting EC50 of 3.65±0.04, 3.76±0.09 and 6.10±0.31 µg/ml, respectively (Figure 3).
Photoprotective activity in vitro
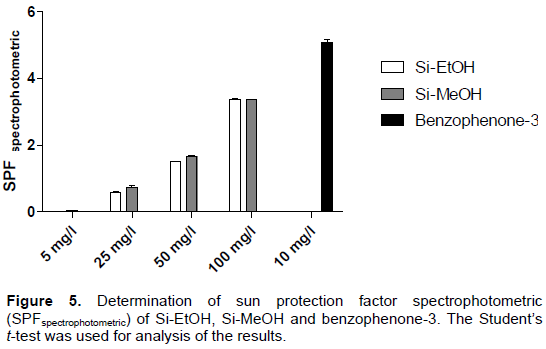
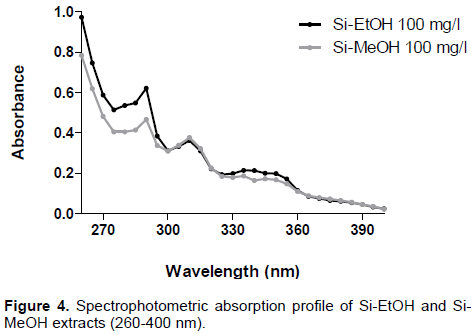
To evaluate the photoprotective effect of the extracts, the spectrophotometric method was adopted. This test is based on spectrophotometric absorption capacity of the sample in order to evaluate the ultraviolet region of the spectrum (100 to 400 nm) at which the sample shows a higher absorbance value. Accordingly, it was found that both extracts (100 mg/l) showed absorption bands in UVA (320 to 400 nm) and UVB (290 to 320 nm) regions, possibly suggesting photoprotective activity (Figure 4).
When calculating SPFspectrophotometric of samples, it was found that the extracts show a similar effect at all concentrations tested. Si-EtOH and Si-MeOH tested at a concentration of 100 mg/l, for example, SPF exhibit values equal to 3.37±0.006 and 3.36±0.007, respectively (Figure 5). Furthermore, it was found that the photoprotective activity of the extracts is directly proportional to the concentration used suggesting an effect of the concentration dependent type as described in previous studies, to extracts fractions of plants with photoprotective activity (Sônia et al., 2015; Serafini et al., 2014). Benzophenone-3 exhibited SPFspectrophotometric value, which is equal to 5.09 ± 0.147.
In this study, it was indicated that Si-EtOH and Si-MeOH have phenolic compounds, which are possibly responsible for their antioxidant and photoprotective properties. A HPLC fingerprint of phenolic compounds was developed and showed the presence of characteristic peaks for these compounds. The extracts showed significant phenols and flavonoids content through the quantification methods used. However, there was no statistically significant difference between them.
Flavonoids represent an important class of secondary metabolites that possesses photoprotective and antioxidant efficacy and tolerability greater than currently used synthetic filters. In general, flavonoids and other phenolic compounds have the ability to reduce the oxidative damage caused by short solar wavelengths and reduce the risk of generation of ROS (Stevanato et al., 2014).
The antioxidant ability of the S. gardneriana extracts was investigated through DPPH method, commonly used for screening antioxidants from plant extracts. DPPH is a stable free radical that reacts with compounds which can donate a hydrogen atom. This assay is based on the scavenging of DPPH through the addition of an antioxidant that decolorizes the DPPH solution (Lima-Saraiva et al., 2012).
In this model, Si-MeOH was more effective than Si-EtOH, with a minor EC50 value. Several publications with plant extracts have demonstrated linear correlations between the profile of phenolic compounds and antioxidant activity. However, it is possible that other compounds present in Si-EtOH and Si-MeOH act as antioxidants since the flavonoid and phenolic content was similar in both extracts.
The photoprotective activity was determined by the spectrophotometric method developed by Mansur et al. (1986) using UVB region, which is considered to be the region of greatest incidence during the day. Although this test has been performed in vitro, there is a relevant correlation with in vivo tests because it relates the absorbance of the samples with its photoprotective potential in combating an erythematogenic effect, caused by radiation at specific wavelengths between 290 and 320 nm (UVB region) (Violante et al., 2009).
Si-EtOH and Si-MeOH showed characteristic absorption bands in UVB and UVA regions, suggesting a possible photoprotective potential. The maximum absorption wavelength (λmax) for extracts was 225 (UVC), 290 (UVB), 310 (UVB) and 335 nm (UVA). Concerning the SPF values, the extracts showed an interesting photoprotective activity in a concentration dependent manner. These results can be justified by the presence of flavonoids in the extracts. Some reports correlate the concentration of flavonoids in plant extracts and fractions with their photoprotective activity. In fact, flavonoids have the ability to reduce the oxidative damage caused by short solar wavelengths and reduce the risk of generation of ROS by absorption and stabilization of the energy, emitted by UVB radiation on the skin (Stevanato et al., 2014).
According to the results shown, it was concluded that Si-EtOH and Si-MeOH have significant antioxidant and photoprotective activities. These activities are probably related to the profile of flavonoids and phenolic compounds found in this species.
This study provides the use of extracts of S. gardneriana in pharmaceutical preparations as sunscreens. However, other studies are needed to reach the isolation of the compounds responsible for the properties of the extracts.
The authors have not declared any conflict of interests.
REFERENCES
|
Araújo MF, Braz-Filho R, Carvalho MG, Vieira IJC (2012). Other compounds isolated from Simira Glaziovii and the 1H and 13C NMR chemical shift assignments of new 1-Epi-Castanopsol. Quim. Nova 35(11):2202-2204.
Crossref
|
|
|
|
Arnason T, Morand P, Salvador J, Reyes I, Lambert J, Towers N (1983). Phototoxic substances from Flaveria trinervis and Simira salvadorensis. Phytochemistry 22(2):594-595.
Crossref
|
|
|
|
|
Cai R, Hettiarachchy NS, Jalaluddin M (2003). High-Performance Liquid Chromatography Determination of Phenolic Constituents in 17 Varieties of Cowpeas. J. Agric. Food. Chem. 51:1623-1627.
Crossref
|
|
|
|
|
Comini LR, Montoya SCN, Páez PL, Argüello GA, Albesa I, Cabrera JL (2011). Antibacterial activity of anthraquinone derivatives from Heterophyllaea pustulata (Rubiaceae). J. Photochem. Photobiol. B 102:108-114.
Crossref
|
|
|
|
|
Déciga-Campos M, Guerrero-Analco JA, Quijano L, Mata R (2006). Antinociceptive activity of 3-O-β-D-glucopyranosyl-23, 24-dihydrocucurbitacin F from Hintonia standleyana (Rubiaceae). Pharmacol. Biochem. Behav. 83:342-348.
Crossref
|
|
|
|
|
Delprete PG (1999). Rondeletieae (Rubiaceae). Flora Neotropica. Mem. N. Y. Bot. Gard. 226 p.
|
|
|
|
|
Dreifuss AA, Bastos-Pereira AL, Ávila TV, Soley BS, Rivero AJ, Aguilar JL, Acco A (2010). Antitumoral and antioxidant effects of a hydroalcoholic extract of cat's claw (Uncaria tomentosa) (Willd. Ex Roem. & Schult) in an in vivo carcinosarcoma model. J. Ethnopharmacol. 130:127-133.
Crossref
|
|
|
|
|
Falcão DQ, Costa ER, Alviano DS, Alviano CS, Kuster RM, Menezes FS (2006). Atividade antioxidante e antimicrobiana de Calceolaria chelidonioides Humb.Bonpl. & Kunth. Braz. J. Pharmacogn. 16:73-76.
|
|
|
|
|
Ferreira-Júnior JC, Lemos RPL, Conserva LM (2012). Chemical constituents from Spermacoce verticillata (Rubiaceae). Biochem. Syst. Ecol. 44:208-211.
Crossref
|
|
|
|
|
Heitzman ME, Neto CC, Winiarz E, Vaisberg AJ, Hammond GB (2005). Ethnobotany, phytochemistry and pharmacology of Uncaria (Rubiaceae). Phytochemistry 66:5-29.
Crossref
|
|
|
|
|
Mansur JS, Breder MVR, Mansur MCA, Azulay RD (1986). Determinação do fator de proteção solar por espectrofotometria. An. Bras. Dermatol. 61:121-124.
|
|
|
|
|
Marques SG, Monteiro RPM, Leão WF, Lyra MAM, Peixoto MS, Rolim-Neto PJ, Xavier HS, Soares LAL (2012). Avaliação de procedimentos para quantificação espectrofotométrica de flavonoides totais em folhas de Bauhinia forficata link. Quim. Nova 35(3):517-522.
Crossref
|
|
|
|
|
Mongrand S, Badoc A, Patouille B, Lacomblez C, Chavent M, Bessoule JJ (2005). Chemotaxonomy of the Rubiaceae family based on leaf fatty acid composition. Phytochemistry 66:549-559.
Crossref
|
|
|
|
|
Moraes TMS, Barros CF, Silva-Neto SJ, Gomes VM, Cunha M (2009). Leaf blade anatomy and ultrastructure of six Simira species (Rubiaceae) from the Atlantic Rain Forest, Brazil. Biocell 33(3):155-165.
|
|
|
|
|
Moreno BP, Fiorucci LLR, Carmo MRB, Sarragiotto MH, Baldoqui DC (2014). Terpenoids and a coumarin from aerial parts of Psychotria vellosiana Benth. (Rubiaceae). Biochem. Syst. Ecol. 56:80-82.
Crossref
|
|
|
|
|
Rogers GK (2005). The genera of Rubiaceae in the southeastern United tates, part II. Subfamily Rubioideae and subfamily Cinchonoideae revisited (Chiococca, Erithalis, and Guettarda). Harvard. Papers Bot. 10:1-45.
Crossref
|
|
|
|
|
Rudrapaul P, Das N, De UC, Dinda B (2014). New 19a-hydroxyursane-type triterpenes from the leaves of Meyna spinosa (= Vangueria spinosa), Rubiaceae. Phytochem. Lett. 9:7-10.
Crossref
|
|
|
|
|
Sampaio EVSB, Giulietti AM, Virgínio J, Gamarra-Rojas CFL (2002). Vegetação e flora da caatinga. APNE, Recife.
|
|
|
|
|
Santos MD, Blatt CTT (1998). Teor de flavonoides e fenóis totais em folhas de Venusta miers de mata e cerrado. Braz. J. Bot. 21(2):135-140.
Crossref
|
|
|
|
|
Sayre RM, Agin PP, Levee GJ, Marlowe E (1979). Comparison of in vivo and in vitro testing of sunscreening formulas. Photochem. Photobiol. 29:559-566.
Crossref
|
|
|
|
|
Serafini, MR, Detoni CB, Menezes PP, Pereira-Filho, RN, Fortes VS, Vieira MJF, Guterres SS, Albuquerque-Junior RLC, Araújo AAS (2014). UVA-UVB Photoprotective Activity of Topical Formulations Containing Morinda citrifolia Extract. BioMed. Res. Int. 2014:1-10.
Crossref
|
|
|
|
|
Slinkard K, Singleton VL (1977). Total Phenol Analysis: Automation and Comparison with Manual Methods. Am. J. Enol. Viticulture 28:49-55.
|
|
|
|
|
Sônia CCC, Detoni CB, Branco CRA, Botura MB, Branco A (2015). In vitro photoprotective effects of Marcetia taxifolia ethanolic extract and its potential for sunscreen formulations. Braz. J. Pharmacogn. 25:413-418.
|
|
|
|
|
Souza VC, Lorenzi H (2005). Botânica Sistemática: Guia ilustrado para identificação das famílias de Angiospermas da flora brasileira, baseado em APGII. São Paulo: Nova Odessa, Instituto Plantarum.
|
|
|
|
|
Stevanato R, Bertelle M, Fabris S (2014). Photoprotective characteristics of natural antioxidant polyphenols. Regul. Toxicol. Pharm. 69:71-77.
Crossref
|
|
|
|
|
Surget G, Stiger-Pouvreau V, Lann KL, Kervarec N, Couteau C, Coiffard LJM, Gaillard F, Cahier K, Guérard F, Poupart N (2015). Structural elucidation, in vitro antioxidant and photoprotective capacities of a purified polyphenolic-enriched fraction from a saltmarsh plant. J. Photochem. Photobiol. B Biol. 143:52-60.
|
|
|
|
|
Violante IMP, Souza IM, Venturini CL, Ramalho AFS, Santos RAN, Ferrari M (2009). Avaliação in vitro da atividade fotoprotetora de extratos vegetais do cerrado de Mato Grosso. Braz. J. Pharmacogn. 19:452-457.
Crossref
|
|
|
|
|
Wagner H, Bladt S (1996). Plant drug analysis: a thin layer chromatography atlas. Berlim Heidelberg: SpringerVerlag.
Crossref
|
|
|
|
|
Zhu W, Pang M, Dong L, Huang X, Wang S, Zhou L (2012). Anti-inflammatory and immunomodulatory effects of iridoid glycosides from Paederia scandens (LOUR.) MERRILL (Rubiaceae) on uric acid nephropathy rats. Life Sci. 91:369-376.
Crossref
|
|