The study was conducted to evaluate in vitro probiotic properties of lactic acid bacteria (LAB) isolated from fermented Shamita and Kocho. Sixteen samples, 8 each of Shamita and Kocho, were collected from Arat-Kilo and Merkato sites in Addis Ababa, respectively. The average pH values of Shamita and Kocho samples were 3.52 and 3.44, respectively. A total of 140 LAB were isolated, of which 101 isolates (72%) were found to inhibit one or more of the sensitive test organisms Shigella boydii and Salmonella typhimurium but none of them had antimicrobial activity against Staphylococcus aureus. The inhibition diameters on agar medium ranged from 8.5 to 17.5 mm. The 101 isolates having antagonistic effects against the test organisms were evaluated for their bile tolerance. Thirty six isolates (36%) tolerated 0.3% bile salts for 48 h with 55 to 93% survival. The 36 bile tolerant isolates were evaluated for their acid tolerance and 25 (69%), 30 (83%) and 34 (94%) tolerated pH 2, pH 2.5 and pH 3 for 3 h, respectively. Further extension of the incubation period for 6 h reduced the number of isolates to 21 (58%) and 33 (92%) at pH 2 and pH 3. Thirty of the bile tolerant isolates (83%) showed 80 to 94% survival at pH 2.5 for 6 h. These isolates were selected as LAB candidates with probiotic potential. Based on their phenotypic characteristics, the 30 isolates were identified as Lactobacillus (17 isolates), Leuconostoc (6 isolates) and Pediococcus (4 isolates) and Lactococcus (3 isolates). Antibiotic resistance patterns of the 30 isolates showed 100% resistance against oxacillin but lower resistance to levofloxacin; 57% of the isolates were resistant to penicillin. However, all the isolates were sensitive to erythromycin and gentamicin. Multiple drug resistance patterns were observed in two isolates one each from Shamita Lactobacillus (S9) and Kocho Lactobacillus (K64) having multiple resistances to penicillin, levofloxacin and oxacillin. Nine isolates (30%) were selected as probiotic candidates for further test on different fermented foods.
Key words: Lactic acid bacteria; Lactobacillus, Leuconostoc, Pediococcus, Lactococcus spp., acid tolerance, antimicrobial activity, bile tolerance.
Fermentation is an ancient widely practiced technology and fermented foods are an essential part of diets in all parts of the world. Traditional fermented foods are indigenous to a particular area and have been developed by the local people using age-old techniques and locally available raw materials (Rose, 1977). Traditional fermen-tation processes are increasingly attracting the attention of scientists and policy makers as a vital part of food security strategies and for their commercial value (Van de Sande, 1997).
Fermentation is a process in which raw substrates are converted into fermented food products by the action of microorganisms or their enzymes to desirable compounds that result in new aroma, flavor, taste and texture. Fermentation helps to increase the sensory quality, palatability and acceptability of the products (Campbell-Platt, 1987). Some of these processes involve ethanol production by yeasts or organic acids by lactic acid bacteria (LAB). Natural or spontaneous fermentation is considered as a means of improving the nutritional quality and safety of foods. It also helps to extend the shelf-life of foods by preventing the outgrowth of spoilage microorganisms and foodborne pathogens (Motarjemi, 2002).
LAB are well-known for their capacity to produce a variety of inhibitory substances including metabolic end products such as organic acids like lactic acid or acetic acid, carbon dioxide, hydrogen peroxide, diacetyl, reuterin, reutericyclin, antifungal peptides and bacteriocins. These substances are very important in food preservation (Holzapfel et al., 1995; El-Ziney et al., 2000). The application of LAB strains and/ or their antimicrobial products to inhibit unwanted bacteria in food was introduced to the concept of preservation. The majority of foodborne contaminants either pathogenic or non-pathogenic are sensitive to the organic acids accumulating that result in a low pH value of the growth environment and other antimicrobial substances produced by LAB. Therefore, the interest in the application of LAB and their metabolites in the prevention of food spoilage and the extension of the shelf-life of foods has increased during the last decade (Stiles, 1996). LAB also plays a role to maintain and promote human health. Some species of LAB reportedly have a beneficial role in health and well-being of the host which is defined as the probiotic effect (Guarner and Schaafsma, 1998).
Traditional preparation of fermented foods generally depends on naturally occurring LAB. However, the use of defined starter cultures is becoming popular in modern fermentation technology. The use of LAB strains as defined starters is desirable to improve more the safety and quality of fermented foods. Given the generally poor sanitary condition of traditional fermented foods, the use of selected LAB with high antimicrobial activity against the most frequent foodborne pathogenic bacteria could be an affordable way to improve the safety of fermented foods (Omar et al., 2006).
In Ethiopia, a wide range of traditional fermented foods and beverages are produced from different raw materials such as cereals, Enset (false banana), honey, milk, etc. (Kebede et al., 2002; Mogessie, 2006). Some of the most popular Ethiopian traditional fermented foods and beverages are Injera, Dabo, Ambasha, Kocho, Bulla, Ergo, Siljo, Tella, Tej, Arekie, Borde, Shamita and Kineto (Mogessie, 2006). Most of the customs and rituals involving the Ethiopian traditional fermented foods and beverages are still popular in urban areas, village communities and rural households. Shamita is a traditional Ethiopian plant fermented beverage of roasted barley which contains low alcohol with a thick consistency. It is consumed as a meal replacement commonly by those people who cannot afford a reasonable meal (Ketema et al., 1999). Kocho is a traditional Ethiopian fermented product which is prepared from the starchy pulp separated from the fibers of Enset plant (Enset ventricosum) corm and pseudo-stem and left to ferment spontaneously at ambient temperature in an earthen pit (Berhanu, 1987).
In a recent review the potential of probiotics in African fermented foods was suggested to have an impact on both nutrition and health (Franz et al., 2014). There are several studies that show the importance of LAB to improve food safety and quality of fermented food/beverage products in Ethiopia (Ketema et al., 1999; Asnake and Mogessie, 2010; Anteneh et al., 2011). There has been at least one report for search of isolates with desirable characters as probiotics from the traditional Ethiopian fermented product Shamita (Ketema et al., 1999). More studies are needed on probiotic properties of LAB of indigenous fermented foods that may help to obtain products with effective probiotic cultures for future application. This study was aimed at in vitro evaluation of desirable characteristics as probiotic properties of LAB isolated from Shamita and Kocho.
Description of Kocho preparation processes
According to Berhanu (1987), the scrapings from pseudo-stems and pulverized corms of four to eight mature Enset plants (Enset ventricosum) are decorticated and pounded pulp (from which the fiber is removed) mixed and kneaded into a mash. The mash is rolled into a ball, then covered with fresh Enset leaves and kept in an earthen pit lined with fresh leaves of Enset. This is covered with discarded plant parts and left to ferment for 2-3 days. The fermented dough well mixed, kneaded and covered with fresh Enset leaves and placed in a pit lined with fresh Enset leaves, and top-pressed with heavy materials (like large stone) to ensure the airtight condition in the pit which leads to anaerobic condition. This is left to ferment spontaneously and produce the final product after a few weeks to several months or years depending on ambient temperature and the need of the family. The final product is usually squeezed, mashed on chopping board with knife to shorten the fiber. Kocho is an intermediate product as a kind of traditional fermented dough for baking Kocho bread.
Description of Shamita preparation processes
According to Ketema et al. (1999), Shamita is prepared by adding lightly roasted barley malt flour, salt, ground linseed and small amounts of spices and mixing the contents with water in air-dried and clean clay jar. The microorganisms responsible for the fermentation come mostly from back-slopping using a small amount of Shamita from a previous fermentation as well as from ingredients and equipment. Thereafter, the clay jar is sealed and left to ferment overnight.
Sample collection
Shamita and Kocho samples were selected based on the prediction of the presence of LAB in these fermented products. Based on this, a total of 16 samples, 8 each from Shamita (liquid suspension) and Kocho (solid mass) were collected between January and August 2012 from Arat-Kilo and Merkato sites in Addis Ababa, respectively. The samples were collected using sterilized 250 ml bottles and kept in a refrigerator until analysis.
pH measurement of samples of Shamita and Kocho
The pH of each sample was determined using a digital pH-meter (NIG 333, Naina Solaris Ltd, New Delhi, India), after mixing 10 ml of Shamita and 10 g of Kocho separately with 90 ml distilled water in a laboratory blender as suggested by Erkmen and Bozkurt (2004).
Isolation of lactic acid bacteria
For isolation of LAB, each of the 25 ml of Shamita and 25g of Kocho was separately mixed with 225 ml of buffered peptone water (0.1%, w/v) and homogenized using a laboratory blender. A volume of 0.1 ml of appropriate dilutions of Shamita and Kocho samples was each spread in duplicates on sterile MRS (de Man Rogosa Sharpe, Oxoid, London, England) agar plates. The plates were incubated at 32°C for 48 h in an anaerobic jar (Gas Pack Anaerobic System, BBL, New Delhi, India) (Vanden-Berg et al., 1993).
Purification and identification of lactic acid bacteria
Purification and identification of LAB were done using criteria as described by Kivanc et al. (2011), Harrigan and McCance (1990) and Wood and Holzapfel (1995).
Designation of the isolates
The isolates were designated with S for Shamita and K for Kocho, followed with different numbers.
Cell morphology
Cellular morphology of isolates was determined according to Tittsler and Sandholzer (1936).
KOH-test (test on lipopolysaccharide)
Gram reaction of the isolates was detected using 3% KOH method outlined by Gregersen (1978).
Catalase-test
Presence of catalase was determined by transferring colonies from 48 h old culture on MRS agar plate to a clean microscopic glass slide using a sterile wire loop and followed by adding two drops of 3% solution of hydrogen peroxide (H2O2) (Kovacs, 1956).
Cytochrome oxidase-test
This test was conducted following the method outlined by Kovacs (1956).
Acid and gas production from glucose
A colony of each LAB isolate grown for 48 h was inoculated into 5 ml MRS broth tubes containing 5% glucose and 0.01% phenol red and adjusted to pH 7.4. The tubes were incubated for 48 h at 32°C. The presence of free air space just above the broth in the inverted Durham tube was recorded as heterofermentative and the absence as homofermentative LAB. The change in color of the medium from red to yellow was considered as an indicator of acid production (Mueller, 1990).
Growth of the isolates at different temperatures
Growth of the isolates at 15°C, 30°C and 45°C was measured using the method described by Barbu (2008).
Growth of the isolates at different salt concentrations
Growth of the isolates at increasing salt concentrations (2, 4, and 6.5%) was tested following the protocol given by Ahmed and Kanwal (2004).
Test microorganisms
The test organisms (Staphylococcus aureus ATCC25923, Salmonella typhimurium ATCC13311 and Shigella boydii ATCC9289) were obtained from the Ethiopian Health and Nutrition Research Institute (EHNRI), Addis Ababa, Ethiopia, and used to evaluate the antimicrobial activity of LAB isolated from Shamita and Kocho.
Determination of antimicrobial activity of LAB isolates using agar well diffusion method
The antimicrobial activities of LAB isolates against selected foodborne pathogens were determined using the agar well diffusion method described by Saranya and Hemashenpagam (2011). Hundred (100) µl of the cell free supernatants were used.
Bile and acid tolerance test
LAB isolates with antagonistic activity towards the test organisms were examined for bile tolerance following the procedure described in Dunne et al. (2001). Bile tolerant isolates were used for acid tolerance test following the procedure described by Hyronimus et al. (2000).
Antibiotic resistance test of LAB isolated from Shamita and Kocho
Clinical and Laboratory Standards Institute (CLSI, 2012) methods were applied. The antibiotic discs used in this study were penicillin (6 μg), erythromycin (15 μg), gentamicin (10 μg), levofloxacin (5 μg) and oxacillin (1 μg).
Statistical data analysis
The average pH samples of Shamita and Kocho from the duplicates of independent experiments were statistically analyzed using SPSS version 20. Variation in pH between Shamita and Kocho was compared using independent samples t-test at 0.05 p-values and the coefficient of variation (%, CV) value was calculated to determine if significant variation occurred in pH within the samples.
In this study, a total of 16 samples, 8 each of Shamita and Kocho were collected and analyzed aseptically for presence of LAB. The average pH values of Shamita and Kocho samples were 3.52 and 3.44, respectively (Table 1). The variation in pH values within the samples of Shamita and Kocho was not significant (CV<10%) (Table 1). Similarly, there was no significant difference in pH values between Shamita and Kocho samples (p>0.05).
A total of 160 different colonies were selected and purified, of which 140 colonies were confirmed as LAB isolates. Out of these, 71 isolates (51%) and 69 isolates (49%) were from Shamita and Kocho samples, respectively (Table 1). The isolates were Gram positive, catalase negative, rod or cocci shaped and appeared in
single, pairs, chains or tetrad cellular arrangement.
The pH value of Kocho in this study was much lower than the ones reported by Berhanu (1987) where the pH value of Kocho samples was around 4.2. Ayele and Berhanu (1998) reported that the pH of commercial fermented Kocho was 4.3, a higher pH than found in the present study. Mogessie and Tetemke (1995) also reported that the pH of ready to consume Shamita was around 4.2. Similarly, Ketema et al. (1999) reported that the average pH value of Shamita samples collected from local brewers in Addis Ababa was around 4.22 which was higher than the result of the present study. The difference in the pH value of the present study and other related studies could be due to the duration of fermentation or type of microorganisms involved during the fermentation as the samples were collected from local producers in open markets.
Grouping of the lactic acid bacteria isolates to different genera
Grouping of the isolates were done according to the criteria described by Harrigan and McCance (1990) and Wood and Holzapfel (1995). Some of the criteria used for grouping of LAB are shown in (Table 2).
Based on their morphological, biochemical and physiological characteristics, the 30 selected isolates were grouped into 4 different genera belonging to Lactobacillus (17 isolates, 57%), Leuconostoc (6 isolates, 20%), Pediococcus (4 isolates, 13%) and Lactococcus (3 isolates, 10%) as shown in Table 3. Lactobacilli were the most frequently isolated genus from Shamita and Kocho samples followed by Leuconostoc isolates from Kocho.
Morphological, biochemical and physiological characterization of LAB isolates
Of the total 140 LAB isolates, 30 isolates were selected for further identification based on their probiotic potential, such as antimicrobial activity, acid and bile tolerance. The 30 isolates were tested for their motility, oxidase activity, acid and gas production from glucose, growth at various temperatures and NaCl concentrations. All the 30 isolates were found to be non-motile in stab cultures, oxidase negative but positive for acid production from glucose.
Out of the 30 isolates, 20 isolates (67%) were homofermentative, and the other 10 isolates (33%) were considered as heterofermentative based on their glucose fermentation profile (Table 3). Among the homo-fermentative isolates, 8 isolates (40%) were obtained from Shamita and 12 isolates (60%) from Kocho. All the heterofermentative isolates were from Kocho. All the isolates were able to grow at 10°C and 15°C but unable to grow at 45°C. Likewise, 7 isolates (23%), 1 isolate (3%) and 4 isolates (13%) were not able to grow at 37°C, 4% and 6.5% of NaCl concentrations, respectively. Whereas, 23 isolates (77%), 29 isolates (97%) and 26 isolates (87%) were able to grow at 37°C, 4% and 6.5% of NaCl concentrations, respectively (Table 3).
This was in line with Kebede (2007) who indicated that all the strains isolated from Borde (another traditional Ethiopian cereal beverage) showed growth at 10°C, 15°C and 37°C incubation but reduction in growth rate was observed at 45°C. Similarly, Evelyne and Laksmi (2011) reported that all LAB isolated from Indonesian fermented Sayur Asin were able to grow at 10°C and 6.5% NaCl, but unable to grow at 45°C.
Similarly, Abdulkadir et al. (2011) reported that lactobacilli were dominant, from Ergo (traditional fermented milk) samples. Eyassu et al. (2012) also reported that Lactobacillus species isolated from Ititu (fermented camel milk) was the dominant genus which comprised about 58% of the total LAB isolates followed by Lactococcus species which accounted for 25%. Additionally, Girum et al. (2005) reported that LAB isolated from ready to consume Borde and Shamita were tentatively grouped into Lactobacillus (60 isolates), Leuconostoc (15 isolates), Pediococcus (18 isolates) and Streptococcus (25 isolates).
All the lactobacilli isolated from Shamita were homofermentative. However, in a microbiological study of Shamita fermentation by Ketema et al. (1999), found that the most dominant lactic flora comprised of both heterofermentative and homofermentative Lactobacillus spp. and that homofermentative LAB predominated after 24 h of Shamita fermentation. In a related work, Asnake and Mogessie (2010) reported that about 94% of the LAB isolated from Awaze, Qotchqotcha and Tef dough (all fermented Ethiopian foods) was homofermentative while 6% of the isolates were heterofermentative.
Antimicrobial activity of LAB isolates against selected foodborne pathogens
All the 140 isolates were subjected to antimicrobial activity test against 3 test organisms of which 101 isolates (72%) were found to inhibit one or more of the sensitive test organisms Shigella boydii ATCC9289 and S. typhimurium ATCC13311 (Table 4). No isolate showed antimicrobial activity against S. aureus ATCC25923. Out of these isolates (101), 76 isolates (75%) showed inhibitory effects against both S. boydii and S. typhimurium compared to 9 isolates (9%) and 16 isolates (16%) against S. boydii and S. typhimurium alone, respectively (Table 4).
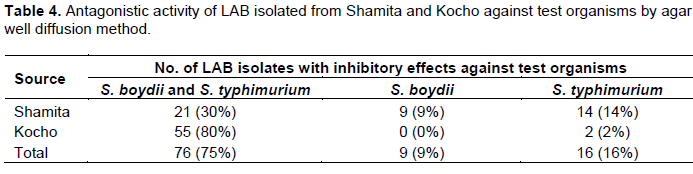
The isolates from different sources showed variations in their inhibitory activity against the test organisms S. boydii and S. typhimurium. Eighty percent (80%) of the isolates from Kocho showed inhibitory activity against both test organisms, whereas only 30% from Shamita were active against the two test organisms. Among the test organisms, S. typhimurium was found to be the most sensitive. It was inhibited by 92 isolates (91%), whereas 85 isolates (84%) inhibited S. boydii.
In a similar work, Esayas et al. (2008) reported that out of 112 LAB isolated from Ergo only 12 isolates belonging to the genera Lactobacillus, Lactococcus, Leuconostoc and Pediococcus showed antimicrobial activity against some pathogenic bacteria, including Salmonella typhi, Shigella flexineri, S. aureus and Escherchia coli with inhibition zone ranged from 7 to 12 mm in diameters. However, Girum et al. (2005) observed that all the LAB isolates (118 isolates) originated from Borde and Shamita belonging to the genera Lactobacillus, Lactococcus, Leuconostoc and Streptococcus were found to inhibit the growth of the test strains, such as S. aureus, Shigella flexneri, Salmonella spp. and E. coli O157:H7 with inhibition zone ranged from 15 to 17 mm in diameters.
Bile tolerance patterns of LAB isolates at 0.3% bile salt concentration
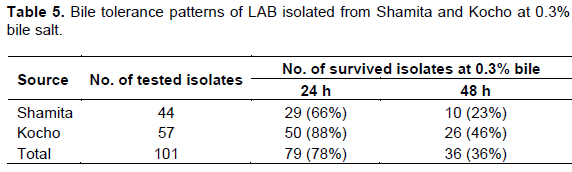
Among the isolates subjected to bile tolerance test from Shamita, 29 isolates (66%) and 10 isolates (23%) survived for 24 and 48 h, respectively. Whereas, 50 isolates (88%) and 26 isolates (46%) from Kocho tolerated incubation for 24 and 48 h, respectively (Table 5). A total of 101 LAB isolates having antagonistic effects were evaluated for their tolerance to bile salt. Out of the 101 isolates, 79 isolates (78%) and 36 isolates (36%) tolerated incubation in MRS broth supplemented with 0.3% bile salt for 24 and 48 h, respectively (Table 5).
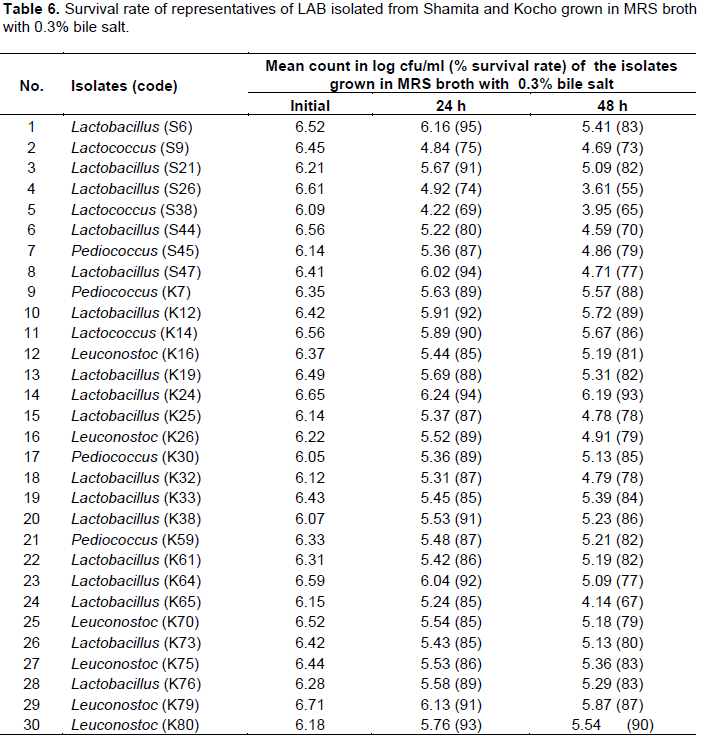
Survival rate of the isolates ranged from 69 to 95% for 24 h incubation period in MRS broth supplemented with 0.3% bile salt (Table 6). Lactobacillus (S6) isolate was the most tolerant with 95% survival rate at 24 h which was isolated from Shamita, followed by Lactobacillus (S47) from Shamita and Lactobacillus (K24) from Kocho with 94% survival rate at 24 h (Table 6). On the other hand, survival rate of the isolates ranged from 55 to 93% for 48 h incubation period. Lactobacillus (K24) isolated from Kocho was the most effective with 93% survival rate among the isolates for 48 h incubation, followed by Leuconostoc (K80) with 90% survival rate and Lactobacillus (K12) with 89% survival rate both from Kocho (Table 6).
In a related study, Asnake and Mogessie (2010) showed that 58% of the 257 tested LAB isolates belonging to the genus Lactobacillus, Lactococcus, Pediococcus and Leuconostoc survived bile concentration greater than 0.3% for 5 days incubation period. A study conducted by Evelyne and Laksmi (2011) indicated that, all the 25 isolates of LAB belonging to the genus Lactobacillus obtained from Indonesian fermented Sayur Asin survived in an environment containing 0.3% and 0.5% bile salt for 4 h. Similarly, Jacobsen et al. (1999) studied 47 Lactobacillus spp. isolated from Ghanian fermented maize and dairy products for their probiotic activities by in vitro techniques and reported that 46 isolates (98%) showed tolerance to 0.3% bile salt in MRS broth for 4 h of incubation. In addition, Anteneh et al. (2011) indicated that 9 (33%) out of the 27 LAB strains isolated from Ethiopian locally fermented products tolerated 0.3% bile for 48 h incubation.
An acid tolerance pattern of LAB isolates at different pH values
Thirty-six LAB isolates that showed bile tolerance were
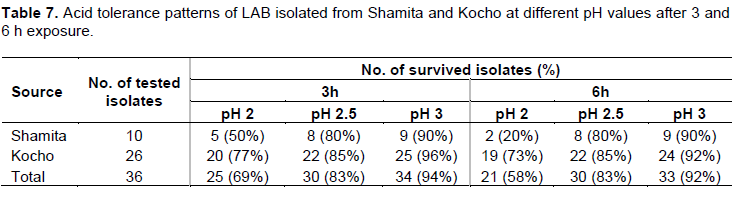
evaluated for their acid tolerance patterns. Testing of the acid tolerance of the isolates showed that, out of 36 isolates, 25 isolates (69%), 30 isolates (83%) and 34 isolates (94%) tolerated pH 2, pH 2.5 and pH 3 for 3 h, respectively. Further extension of the incubation period for 6 hours reduced the surviving isolates to 21(58%) and 33 (92%) at pH 2 and pH 3, respectively. But, the number of surviving isolates at pH 2.5 after incubation for 6 h was similar to the result obtained for incubation at the same pH for 3 h (Table 7).
In a similar study, Anteneh et al. (2011) reported that out of 27 LAB isolates tested for acid tolerance, 9 isolates (33%) showed a survival rate of ≥50% at pH 2.5 for 3 and also for 6 h. Asnake and Mogessie (2010) reported that out of 257 LAB isolates belonging to the genera Lactobacillus, Lactococcus, Pediococcus and Leuconostoc, only 10 isolates (4%) were tolerant to pH 2 for 3 h. However, all the LAB isolates did not survive at pH 2 for 6 h. With respect to pH 2.5, 41 isolates (16%) and 14 isolates (5%) were found to be tolerant for 3 and 6 h incubation time, respectively. The authors also pointed out that 198 isolates (77%) could survive at pH 3 for the first 3 h, but further incubation for 6 h decreased the number of surviving isolates to 172 (67%). According to Ketema et al. (2009), about 44% of the 99 LAB isolates had 100% survival rate for 3 h in MRS broth at pH 3 but only 7 isolates (7%) for 3 h, 2 isolates (2%) for 6 h had 100% survival rate at pH 2.5, respectively. The authors also added that about 4 isolates (4%) showed 60-75% survival rate at pH 3 for 3 h, whereas about 40 (41%) showed 90 to 100% survival rate for 6 h at pH 3.
Generally, survival rate of the isolates ranged from 77 to 97% at different pH values for 3 and 6 h incubation period (Table 8). Lactobacillus (K24) was the most tolerant at pH 2 for 3 h incubation period with 89% survival rate, followed by Lactobacilli isolates (K12, K25, K33), Lactococci isolates (K14, S9) and Leuconostoc (K70) with 88% survival rate. Similarly, Lactobacillus (K24) was the most tolerant at pH 2 for 6 h incubation period with 88% survival rate, followed by Lactobacillus (K12) and Leuconostoc (K26) with 87% survival rate (Table 8).
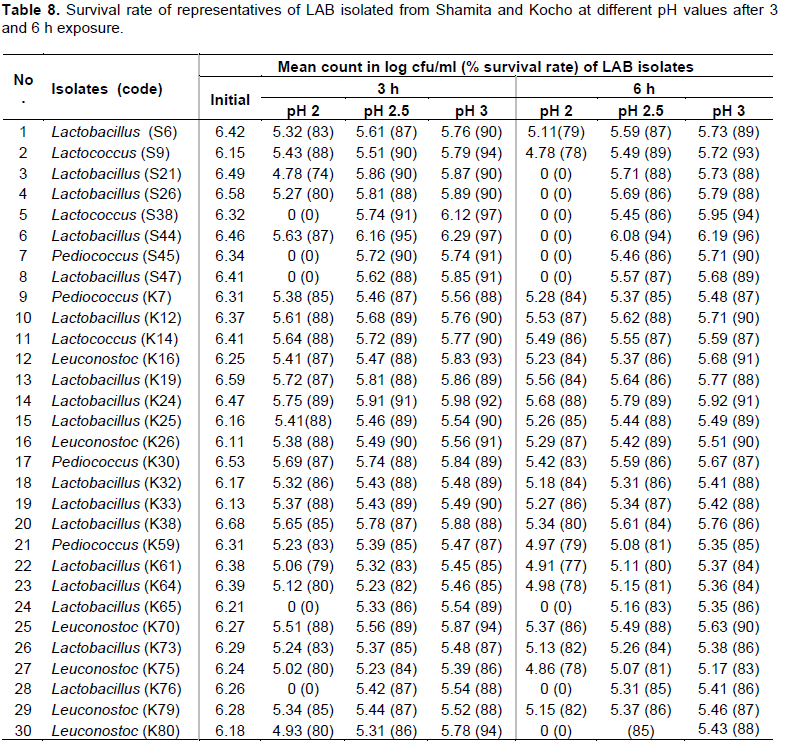
Among the isolates exposed to pH 2.5 for 3 h, Lactobacillus (S44) was the most tolerant with 95% survival rate, followed by Lactobacillus (K24) and Lactococcus (S38) with 91% survival rate. Lactobacillus (S44) was also the most effective at pH 2.5 for 6 h with 94% survival rate, followed by Lactobacillus (K24), Leuconostoc (K26) and Lactococcus (S9) with 89% survival rate. Similarly, Lactobacillus (S44) was found to be the most tolerant to pH 3 for 6 h with 96% survival rate, followed by Lactococcus (S38) with 94% survival rate (Table 9). The results revealed that 30 isolates had 80 to 94% survival rate at pH 2.5 for 6 h and these isolates were selected as LAB candidates with probiotic potential (Table 8).
As reported by Hyronimus et al. (2000), all LAB isolated from cattle feces did not survive at pH 2.5 for 3 h. Charteris et al. (1998) also reported a complete loss of viability of lactobacilli isolated from traditional Greek cheese at pH 2.5 for 3 h. Thirabunyanon et al. (2009) reported that 3 of 5 lactobacilli isolated from Thailand fermented dairy milk survived at pH 2.5 for 3 h. When compared to the weak tolerance to low pH seen in some LAB investigated in earlier works (Charteris et al., 1998; Hyronimus et al., 2000), isolates of this study had a higher survival rate at pH 3 and pH 2.5 as well as at pH 2. Therefore, it is possible to consider the isolates have the potential to be good as candidates for probiotics as they have shown better survival rate in in vitro selection criteria during the experiment. This could be also an indication of their possible survival in the acidic condition of the stomach of the human host before their transit to the small intestine.
Antibiotic resistance or susceptibility patterns of LAB isolated from Shamita and Kocho
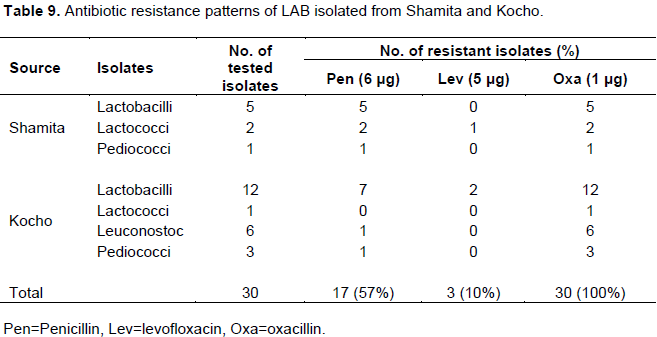
A total of 30 LAB isolates were tested for antibiotic resistance or susceptibility patterns. 100% resistance by isolates of Shamita and Kocho was observed against oxacillin. But, a relatively lower proportion of resistance for levofloxacin by isolates from Shamita and Kocho was observed. In addition, 17 isolates (57%) were resistant to penicillin (Table 9). None of them showed resistance to erythromycin and gentamicin (data not shown). Among the Lactobacillus isolates, 5 isolates (29%) from Shamita and 7 isolates (41%) from Kocho were resistant to penicillin. Likewise, 2 isolates (12%) of lactobacilli from Kocho were resistant to levofloxacin. Lactococcus, Leuconostoc and Pediococcus isolates showed varying degrees of resistance against penicillin and levofloxacin (Table 9).
Antibiotic resistance to penicillin by the LAB isolates was higher than of the work of Asnake and Mogessie (2010) who reported that only 43% of the isolates showed resistance to penicillin. On the contrary, Ketema et al. (2010) and Abdulkadir et al. (2011) reported that all their LAB isolates were sensitive to penicillin. Although susceptibility towards the inhibitors of cell wall synthesis such as penicillin and ampicillin has been observed in many species of LAB (Danielsen and Wind, 2003; Delgado et al., 2005; Zdolec et al., 2011), more than half
of the isolates tested in the current study were found to be resistant to penicillin.
Multiple drug resistance patterns were observed in two LAB isolates one each from Shamita Lactococcus (S9) and Kocho Lactobacillus (K64) with multiple resistances to penicillin, levofloxacin and oxacillin (data not shown).
Probiotic potential patterns of the selected LAB isolates
A total of 30 LAB isolates were screened as candidates with probiotic potential based on in vitro evaluation of antimicrobial activity, bile and acid tolerance results. Screening was also done after ranking the results of antimicrobial activity, acid-bile tolerance and antibiotic resistance profile to identify isolates with the best probiotic potential among the 30 selected isolates. A ranking of the results was done from the overall sum calculated after standardizing the raw data to 5 point scale as indicated in (Table 10).
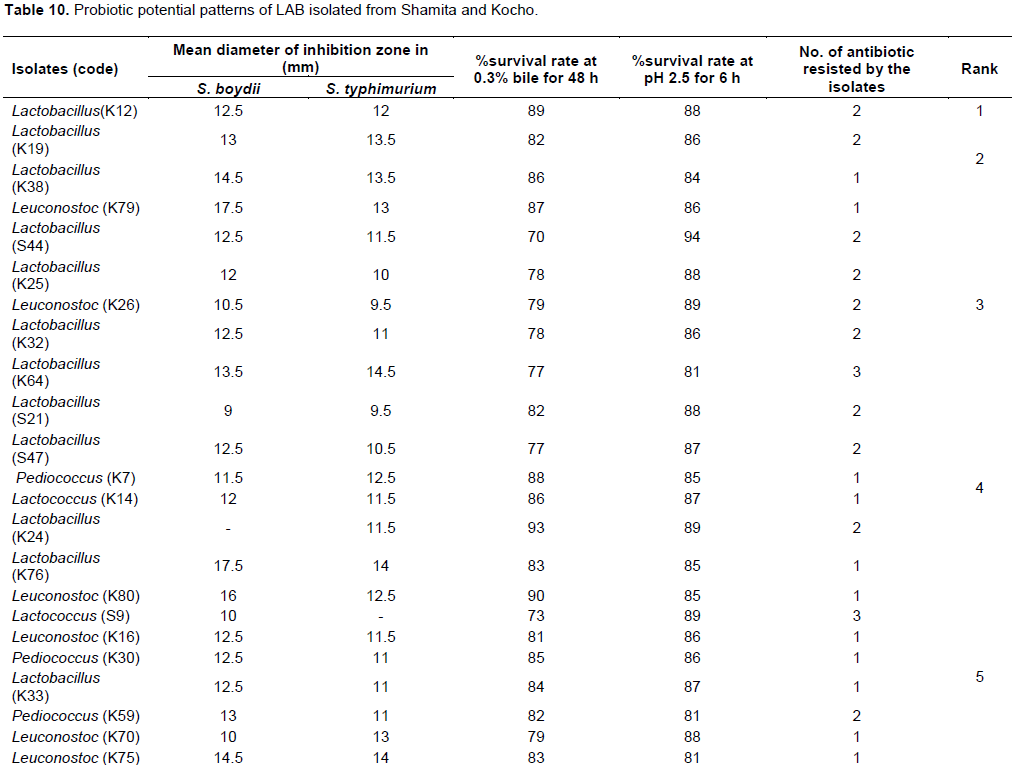
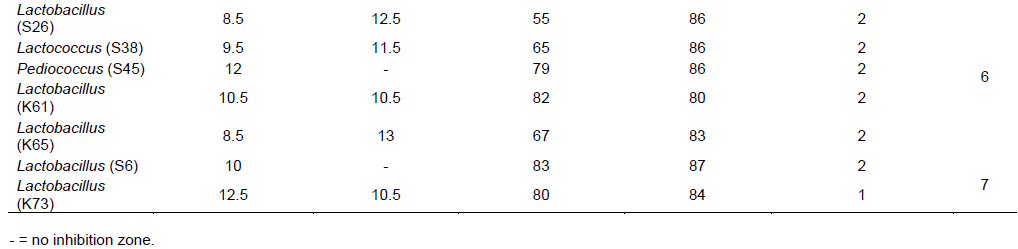
Out of 30 isolates, 9 isolates (30%) were selected as best probiotic candidate of LAB isolates based on their standardized probiotic potential results (Table 10). The 9 selected isolates were Lactobacillus (K12), Lactobacillus (K19), Lactobacillus (K38), Leuconostoc (K79), Lactobacillus (S44), Lactobacillus (K25), Leuconostoc (K26), Lactobacillus (K32) and Lactobacillus (K64). Among these isolates, Lactobacillus (K12) isolate showed the best overall probiotic property, followed by Lactobacillus (K19), Lactobacillus (K38) and Leuconostoc (K79) isolates (Table 10).Seven Lactobacillus and 2 Leuconostoc isolates were selected as the best LAB genera with probiotic potential. The results also show that LAB isolated from Kocho have the best overall probiotic potential compared to isolates from Shamita (Table 10).