ABSTRACT
A demyelinating sickness is any ailment of the nervous system in which the myelin casing of neurons is injured. This harm weakens the transmission of signals in the pretentious nerves. Demyelinating diseases, like multiple sclerosis (MS) and Charcot-Marie-Tooth (CMT) disease, are categorized on the basis of the scratch of the myelin covering around neurons, because of swelling and gliosis in the central nervous system (CNS) and peripheral nervous system (PNS), respectively. In this current research, an amalgam approach of comparative modeling and molecular docking pursued by inhibitor recognition and structure modeling was used. Existing treatments mark anti-inflammatory ways to hinder or slow disease sequence. The recognition of a means to improve axon myelination would present innovative remedial approaches to restrain and probably turn around disease progression. A computational ligand-target docking method was applied to investigate structural composites of the Leucine Rich Repeat and Ig Domain Containing 1 (LINGO1) with three ligands to understand the structural foundation of this protein goal specificity. The following ten residues were conserved for all the three ligands interaction LEU133, ILE134, Pro135, LEU136, ILE155, ILE157, LEU159, ASP160, TYR161, and MET162 which are present in Leucine Rich Repeat 3 and 4 domains. Therefore, these three ligands can be utilized as the potential inhibitors to prevent various neurological disorders and the axonal neuropathies especially the CMT disease. Docking analysis showed that the two important drugs which are widely used have the potential to block the Rho-Rock pathways. Here, we report inhibitors which showed maximum binding affinity for the three most important axonal regeneration inhibitors. However, further studies are required to find the applications of these drugs.
Key words: Demyelination, RTN4, CMT1A, spinal cord injury, ROCK inhibition, neurite growth inhibitors.
Abbreviation:
LINGO1, Leucine rich repeat and Ig domain containing 1; MS, multiple sclerosis; MAG, myelin-associated glycoprotein; CMT, Charcot-Marie-Tooth disease; NgR, Nogo-66 receptor; SCI, spinal cord injury; MAIFs, myelin-associated inhibitory factors.
A demyelinating disorder is a state of the nervous system in which the myelin casing of neurons is wounded (Perveen et al., 2015). This devastation deteriorates the broadcast of signals in the affected nerves. Sequentially, the decrease in transmission ability causes deficit in sense, movement, thought, or other functions related to nerves. Demyelinating diseases, like multiple sclerosis (MS), are categorized by the damage of the myelin covering around neurons, due to swelling and gliosis in the central nervous system (CNS) and Charcot-Marie-Tooth (CMT) disease (Choi et al., 2015). MS is the supreme common demyelinating disease of the CNS. MS and further demyelinating diseases usually end in vision loss, muscle weakness, muscle stiffness and spasms, loss of coordination, loss of sensation, pain, and changes in bladder and bowel function. Spinal cord injury (SCI) results in damage to axonal tracts that control motor and sensory function. Axons in the spinal cord restore imperfectly, restraining practical retrieval. Wounded neurons in developed organisms are incapable to efficiently regrow their axons after CNS injury. (Filbin, 2003). At least three of these constituents, Nogo-66, myelin-associated glycoprotein (MAG), and oligodendrocyte myelin glycoprotein, either independently or mutually, have been shown to be powerful barrier of neurite outgrowth (Filbin, 2003; He and Koprivica, 2004). All three motion inhibition through the Nogo receptor complex, poised of the ligand-binding Nogo-66 receptor (NgR) and two complementary co-receptors p75 and Lingo-1 that action as a signal-transducing pair on an axon's cell membrane (Barker, 2004; Carimâ€Todd et al., 2003). Although both NgR and p75 nerve growth factor receptors have fine recognized roles in the context of myelin inhibition; information discovering the role of Lingo-1 are more recent.
The components of myelin with axonal growth inhibitory properties include
Nogo-A (Chen et al., 2000; Prinjha et al., 2000), MAG (McKerracher and Rosen, 2015), oligodendrocyte-myelin glycoprotein (OMgp) (Mukhopadhyay et al., 1994), and ephrin-B3 (Benson et al., 2005).
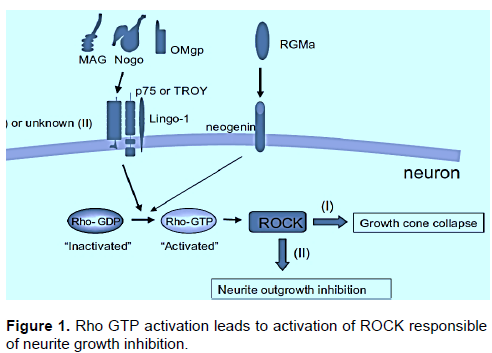
Earlier, Leucine-Rich Repeat and Ig Domain-Containing Nogo receptor-interacting protein (LINGO-1) has been recognized as an in vitro and in vivo adverse controller of oligodendrocyte distinction and myelination (Mi et al., 2007). LINGO-1 is a negative regulator of neuronal persistence, oligodendrocyte differentiation and axonal outgrowth and renewal, because it relates with assorted growth factor receptors hindering or preventing their action (Figure 1). Steady discoveries achieved in vitro and in animal models propose that anti-LINGO-1 therapy may be valuable in neurodegenerative disorders such as MS, Parkinson’s disease or essential tremor (ET), and CMT. Furthermore, hereditary and pathological indication deliver a healthy link between LINGO-1 and ET (Agúndez et al., 2015). Myelin-associated inhibitory factors (MAIFs) are inhibitors of CNS axonal renewal following damage. The Nogo receptor complex composed of the Nogo-66 receptor 1 (NgR1), neurotrophin p75 receptor (p75), and LINGO-1, represses axon regeneration upon binding to these myelin components (Shao et al., 2005).
Although neurons in CNS have the capability to restore their axons after damage, but they fail partially since renewal is restricted by growth inhibitory proteins present in CNS myelin (McKerracher and Rosen, 2015). Damage to the CNS in MS seems to be primarily due to continual swelling of the CNS with superimposed bouts of inflammatory activity by the adaptive immune system. The immune arbitrated injured can be augmented by neurodegenerative means in damaged axons as well as anterograde or retrograde axonal or transynaptic deterioration, synaptic pruning and neuronal or oligodendrocyte death. As such, it is highly unlikely that CNS damage can be prevented using only immuno-modulatory drugs (Villoslada, 2016).
In MS, CNS damage is shaped by a complex inflammatory procedure. Even though in the past it was supposed that in the relapsing-remitting phase CNS damage was due only to the presence of inflammatory infiltrates within the MS plaques; in the last decade it has been clearly shown that MS is a disperse disease with swelling, demyelination and axonal loss both in the grey and white matter (Mahad et al., 2015; Ransohoff et al., 2015).
Current treatments therefore target anti-inflammatory mechanisms to impede or slow disease progression. The identification of a means to enhance axon myelination would present new therapeutic approaches to inhibit and possibly reverse disease progression.
Accession of target protein and structure prediction
In the present project, structure forecast, sequence investigation, docking analysis, and relative proteomics investigation were executed on Samsung Electronics Workstation Core-i-5 (Samsung Company, Seoul, Korea). The amino acid sequence of LINGO1 was retrieved for homology modeling. The amino acid sequence was rescued in FASTA arrangement from the Uniprot with the accession number Q96FE5 for previously described gene. The mechanical protein modeling online program swiss modeller was utilized to calculate the 3D structure of LINGO-1 fulfilling spatial restraints. Swiss Modeller and Phyre2 was use to model the structure.
Evaluation of predicted model
Four evaluation tools were used: Rampage (Lovell et al., 2003), 47 ProCheck (Laskowski et al., 1993), 48 Anolea (Colovos and Yeates, 1993), and ERRAT were utilized to evaluate the forecast models. Lastly, the deprived Ramachandran outliers and rotamers were detached by employing WinCoot tool.
Ligand selection
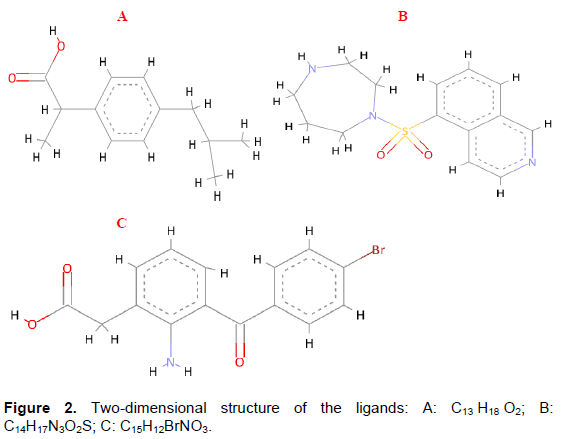
The chemical structures of Ibuprofen, Fasudil and Formanac were obtained from PubChem compound database (Figure 2). It was prepared by ChemBioDraw and MOL SDF format of this ligand was converted to PDBQT file using PyRx tool to generate atomic coordinates.
Target and ligand optimization
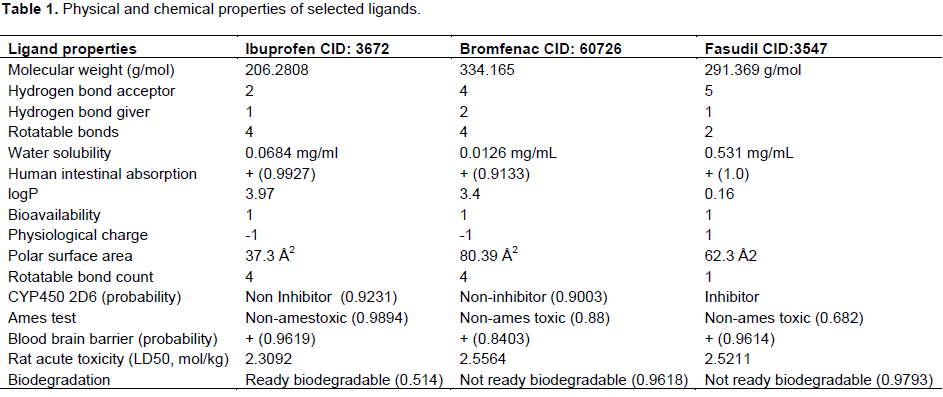
For docking investigation, PDB coordinates of the objective protein and ligand molecules were improved by Drug Discovery Studio version 3.0 software and UCSF Chimera tool, respectively. These coordinates had minimum energy and stable conformation (Wass et al., 2010). The chemical structures were estimated to be an efficient drug-like complex subjected to Lipinski’s rule of five55 and for their oral bioavailability, supply, metabolism, secretion, and toxicity characteristics of compound and was investigated by applying dmetSAR online server. Eleven mathematical models (Ames toxicity, blood-brain barrier penetration, fish toxicity, aqueous solubility [LogS], honey bee toxicity, carcinogens, acute oral toxicity, Caco-2 permeability, cytochrome P450 2D6 inhibition, rat acute toxicity, and human intestinal absorption) were employed to predict the ADMET possessions of nominated compound. Several poisonous-ness are expected often and are used in drug designing (Table 1).
Molecular docking analysis
Molecular docking procedures are extensively used for forecasting the binding attractions for a number of ligands. The present effort was to inspect the likelihood of an existing relationship between the investigational bioactivities of the inhibitors under study and the docking scores. In order to get precise results, all the docking trials were completed with the default parameters. PatchDock device was used for docking studies, and crown composites of gene having lowly binding energies were chosen for additional analysis. Dissimilarity was experienced in all the complexes examined with lowest binding energies.
The achievement of the human genome mission has caused a growing amount of new therapeutic targets for drug discovery. At the same time, high-throughput protein distillation, crystallography and nuclear magnetic resonance spectroscopy methods have been recognized and donated to many structural details of proteins and protein-ligand complexes. These advances allow the computational strategies to permeate all feature of drug discovery today (Gohlke and Klebe, 2002; Jorgensen, 2004; Kitchen et al., 2004; Langer and Hoffmann, 2001), such as the virtual screening (VS) techniques (Bajorath, 2002) for hit identification and methods for lead optimization.
Modeling
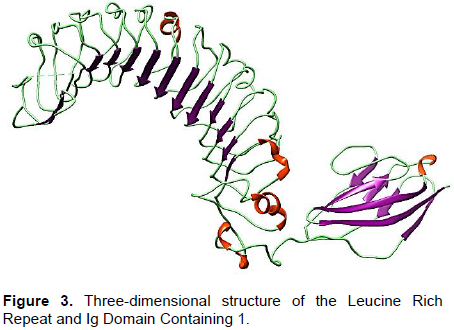
The purpose of this study was based on the relativeness of LINGO-1 protein with ligands and its bioinformatics analysis to investigate the interactions for curing the axonal neuropathies. The three dimensional structure of the LINGO1 was predicted by using two online tools Swiss modeller and phyre2 (Figure 3).
Evaluation
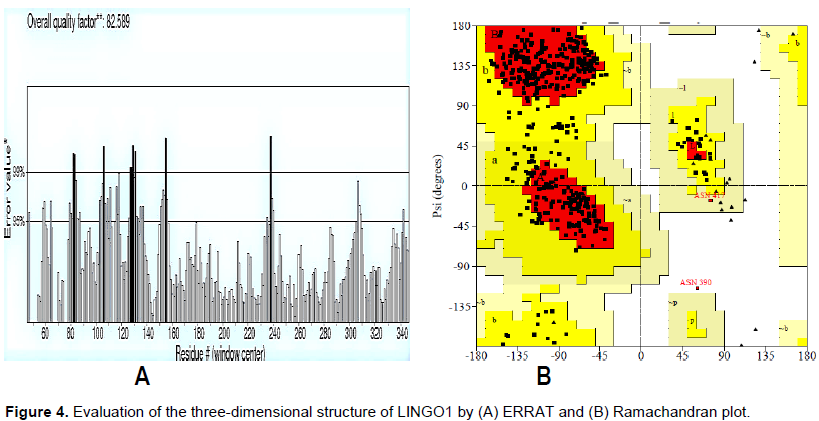
Estimate tools revealed the consistency and effectiveness of LINGO1 three-dimensional predicted structures (Figure 4). Regions of favored and outlier predicted models were spotted in Ramachandran plot. Only one residue was found in outlier regions. Quality of the expected structure was evaluated by Ramachandran plot and ERRAT. The overall quality factor was 83% and only one residue was in the outlier region.
Docking
Drug designing process is not only time taking but also expensive. Therefore, we applied bioinformatics approaches for drug finding procedure. This up-and-coming inclination has huge importance in declining the time essential and amplifying the elected compound with better biological activity and minimal side effects for precise illness targets. The chemical structures are assessed to be an effective drug-like compound subjected to Lipinski’s rule of five55 and for their oral bioavailability. Various toxicities are predicted often and are used in drug designing.
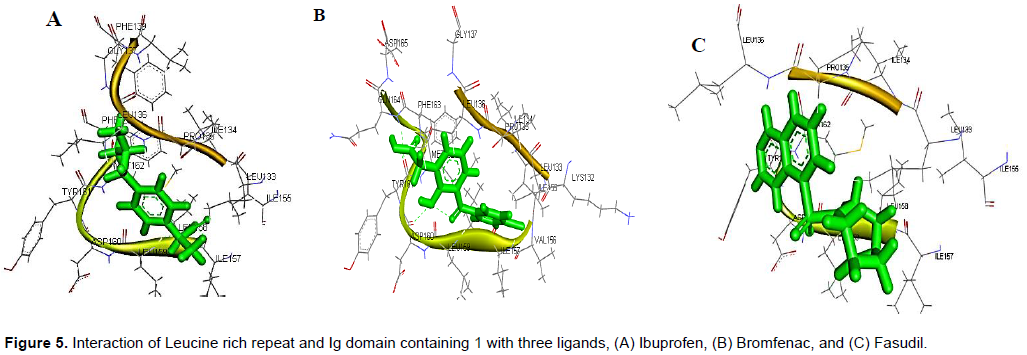
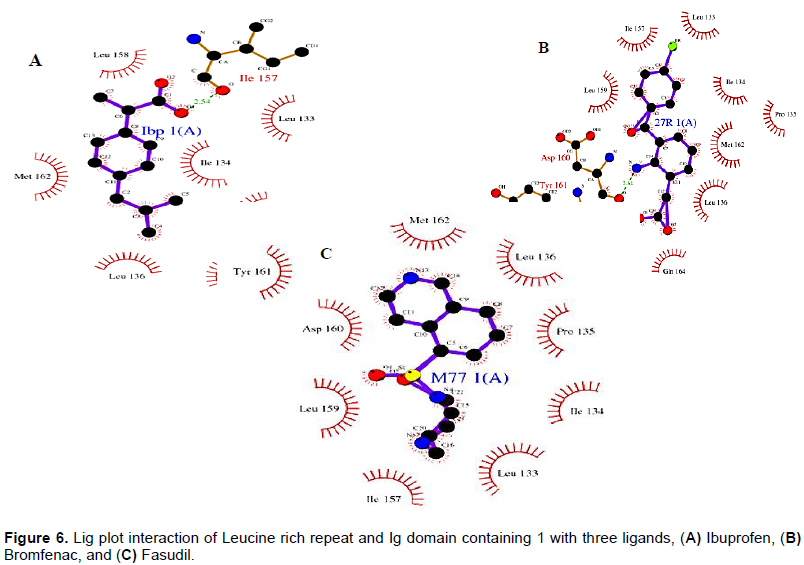
Docking of best poses (ligands binding to active sites) of Tirofiban and Amotosalen hydrochloride to CD-61 were done through Patchdock online docking softwares. PatchDock tool was utilized for docking analysis and top complexes of gene having lowest binding energies were selected for further analysis. From docking analysis, it was observed that the binding residues remains constant in all three ligands which strongly suggest that LINGO1 can be strong candidate to inhibit the RHO/ROCk pathway to enhance the regeneration ability of the axons (Figure 1). Ibuprofen showed the strong binding with LINGO1 at the residues LEU133, ILE134, Pro135, LEU136, GLY137, ILE157, LEU158, and MET162, while Bromfenac showed the binding interaction with LYS132, LEU133, ILE134, PRO135, LEU136, GLY137, ILE155, ILE157, LEU158, LEU159, TYR161, MET162, PHE163, GLN164, and ASP165. The third ligand shows the interaction with the residues at position of LEU133, ILE134, Pro135, LEU136, ILE155, ILE157, LEU158, ASP160, and TYR161.
Deviation was experienced in all the complexes investigated with the lowest binding energies. Possibly, the constancy of the ligand may be because of the allowing of binding likeness. Though, the residues remain steady for production of the complexes with the drugs more preserved. It was observed that Leucine Rich Repeat 3 and 4 was the main domain to show interaction with the ligands (Table 2 and Figure 5).
These are the conserved residues found in the favored region. In an effort to understand the better relations experiential between ligand and amino acid remainder in the active site of protein, a plot of amino acids-ligand contacts were created employing Ligplot as shown in Figure 6.
Earlier, it was considered that CNS renewal of injured cells is impossible. Existing progress in our thoughtful aspects which limit CNS re-establishment and those which smooth PNS renewal have guide to treatments which let some degree of recover from brain and SCI in animal models. These results open the option of promoting renewal of the injured human CNS.
In wrapping up, the chosen ligands molecules are successful in handling of MS, essential tremer, CMT disease and other neurological disorders for targeting LINGO1. The in silico investigation of LINGO1 has higher likelihood and usefulness on the origin of binding energy and other applied parameters. Fasudil is an effective Rho-kinase inhibitor and vasodilator (Barker, 2004; Shibuya and Suzuki, 1993). From the time since it was exposed, it has been used for the treatment of cerebral vasospasm, which is frequently due to subarachnoid hemorrhage (Doggrell, 2005) as well as to pull through the cognitive deterioration seen in stroke victims. It has been found to be efficient for the treatment of pulmonary hypertension (Huentelman et al., 2009). It was verified in February 2009 that Fasudil have the ability to improve recall in normal mice, identifying the drug as a possible treatment for age related or neurodegenerative memory loss. Additional study and production of novel compounds considering these results can anticipate alike reaction and can heal the range of neurological disorders.
The authors have not declared any conflict of interests.
REFERENCES
|
Agúndez JAG, Jiménez-Jimenez FJ, Alonso-Navarro H, García-Martín E (2015). The potential of LINGO-1 as a therapeutic target for essential tremor. Expert Opin. Ther. Targets 19(8):1139-1148.
Crossref
|
|
|
|
Bajorath J (2002). Integration of virtual and high-throughput screening. Nat. Rev. Drug Discov. 1(11):882-894.
Crossref
|
|
|
|
|
Barker PA (2004). p75NTR is positively promiscuous: novel partners and new insights. Neuron 42(4):529-533.
Crossref
|
|
|
|
|
Carimâ€Todd L, Escarceller M, Estivill X, Sumoy L (2003). LRRN6A/LERN1 (leucineâ€rich repeat neuronal protein 1), a novel gene with enriched expression in limbic system and neocortex. Eur. J. Neurosci. 18(12):3167-3182.
Crossref
|
|
|
|
|
Chen MS, Huber AB, van der Haar ME, Frank M, Schnell L, Spillmann AA, Christ F, Schwab ME (2000). Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature 403(6768):434-439.
Crossref
|
|
|
|
|
Choi BO, Nakhro K, Park HJ, Hyun YS, Lee JH, Kanwal S, Jung SC, Chung KW (2015). A cohort study of MFN2 mutations and phenotypic spectrums in Charcot-Marie-Tooth disease 2A patients. Clin. Genet. 87(6):594-598.
Crossref
|
|
|
|
|
Colovos C, Yeates TO (1993). Verification of protein structures: patterns of nonbonded atomic interactions. Protein Sci. 2(9):1511-1519.
Crossref
|
|
|
|
|
Doggrell SA (2005). Rho-kinase inhibitors show promise in pulmonary hypertension. Expert Opin. Investig. Drugs 14(9):1157-1159.
Crossref
|
|
|
|
|
Filbin MT (2003). Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat. Rev. Neurosci. 4(9):703-713.
Crossref
|
|
|
|
|
Gohlke H, Klebe G (2002). Approaches to the description and prediction of the binding affinity of small-molecule ligands to macromolecular receptors. Angew. Chem. Int. Ed. Engl. 41(15):2644-2676.
Crossref
|
|
|
|
|
He Z, Koprivica V (2004). The Nogo signaling pathway for regeneration block. Annu. Rev. Neurosci. 27:341-368.
Crossref
|
|
|
|
|
Huentelman MJ, Stephan DA, Talboom J, Corneveaux JJ, Reiman DM, Gerber JD, Barnes CA, Alexander GE, Reiman EM, Bimonte-Nelson HA (2009). Peripheral Delivery of a ROCK Inhibitor Improves Learning and Working Memory. Behav. Neurosci. 123(1):218-223.
Crossref
|
|
|
|
|
Jorgensen WL (2004). The many roles of computation in drug discovery. Science 303(5665):1813-1818.
Crossref
|
|
|
|
|
Kitchen DB, Decornez H, Furr JR, Bajorath J (2004). Docking and scoring in virtual screening for drug discovery: methods and applications. Nat. Rev. Drug Discov. 3(11):935-949.
Crossref
|
|
|
|
|
Langer T, Hoffmann RD (2001). Virtual screening: an effective tool for lead structure discovery? Curr. Pharm. Des. 7(7):509-527.
Crossref
|
|
|
|
|
Laskowski RA, Macarthur MW, Moss DS, Thornton JM (1993). {PROCHECK}: a program to check the stereochemical quality of protein structures. J. Appl. Cryst. 26:283-291.
Crossref
|
|
|
|
|
Lovell SC, Davis IW, Arendall WB, de Bakker PI, Word JM, Prisant MG, Richardson JS, Richardson DC (2003). Structure validation by Calpha geometry: phi,psi and Cbeta deviation. Proteins 50(3):437-450.
Crossref
|
|
|
|
|
Mahad DH, Trapp BD, Lassmann H (2015). Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol. 14(2):183-193.
Crossref
|
|
|
|
|
McKerracher L, Rosen KM (2015). MAG, myelin and overcoming growth inhibition in the CNS. Front Mol. Neurosci. 8:51.
Crossref
|
|
|
|
|
Mi S, Hu B, Hahm K, Luo Y, Hui ES, Yuan Q, Wong WM, Wang L, Su H, Chu TH, Guo J (2007). LINGO-1 antagonist promotes spinal cord remyelination and axonal integrity in MOG-induced experimental autoimmune encephalomyelitis. Nat. Med. 13(10):1228-1233.
Crossref
|
|
|
|
|
Mukhopadhyay G, Doherty P, Walsh FS, Crocker PR, Filbin MT (1994). A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron 13(3):757-767.
Crossref
|
|
|
|
|
Perveen S, Mannan S, Hussain A, Kanwal S (2015). Charcot-Marie-Tooth type 1A disease from patient to laboratory. J. Pak. Med. Assoc. 65(2):206-212.
|
|
|
|
|
Prinjha R, Moore SE, Vinson M, Blake S, Morrow R, Christie G, Walsh FS (2000). Inhibitor of neurite outgrowth in humans. Nature 403(6768):383-384.
Crossref
|
|
|
|
|
Ransohoff RM, Hafler DA, Lucchinetti CF (2015). Multiple sclerosis-a quiet revolution. Nat. Rev. Neurol. 11(3):134-142.
Crossref
|
|
|
|
|
Shao Z, Browning JL, Lee X, Scott ML, Shulga-Morskaya S, Allaire N, Thill G, Levesque M, Sah D, McCoy JM, Murray B (2005). TAJ/TROY, an orphan TNF receptor family member, binds Nogo-66 receptor 1 and regulates axonal regeneration. Neuron 45(3):353-359.
Crossref
|
|
|
|
|
Shibuya M, Suzuki Y (1993). Treatment of cerebral vasospasm by a protein kinase inhibitor AT 877. No to shinkei= Brain and nerve. 45(9):819-824.
|
|
|
|
|
Villoslada P (2016). Neuroprotective therapies for multiple sclerosis and other demyelinating diseases. Mult. Scler. Demyelinating Disord. 1(1):1.
Crossref
|
|
|
|
|
Wass MN, Kelley LA, Sternberg MJ (2010). 3DLigandSite: predicting ligand-binding sites using similar structures. Nucleic Acids Res. 38(suppl_2):W469-W473.
|
|