Full Length Research Paper
ABSTRACT
Bio-synthesised silver nanoparticles are effective in controlling several micro-organisms. They are correspondingly environmentally friendly, affordable, and easy to synthesise when compared with chemically synthesised silver nanoparticles. This study investigated the efficacy of biosynthesized silver nanoparticles against the fungus Sporisorium scitamineum, the causal agent of sugarcane smut. The reduction of silver nitrate upon mixing with the plants’ crude extracts was evidenced by the change in colour of the mixture to dark brown. Optimization of the mixtures using ultraviolet-visual spectroscopy showed peaks in the range of 340 to 450 nm. The Fourier transform infrared spectroscopy analysis identified proteins to be essential capping agents, and reducing sugars were responsible for the reduction of silver nitrate to nanoparticles and stabilizing the nanoparticles. The transmission electron microscope analysis showed the sizes of the nanoparticles to vary between 3 and 70 nm. Carissa spinarum and Melia azedarach had the most antifungal activity against S. scitamineum as observed from the inhibition-zone assay. Silver nanoparticles were successfully synthesized using the selected botanicals. All the synthesized nanoparticles showed varying antifungal effects against the S. scitamineum. C. spinarum and M. azedarach exhibited the highest antifungal activity, while Azadirachta indica showed the least.
Key words: Sporisorium scitamineum, Acacia nilotica, Carissa spinarum, Senna didymobotrya, Warburgia ugandensis, Melia azedarach, Azadirachta indica, bio-synthesised silver nanoparticles, antifungal activity.
INTRODUCTION
Traditionally, silver has been known and used for its antimicrobial activity (Jamiu and Bello, 2018). When reduced to their nano-form, silver nanoparticles (AgNPs) possess novel and more efficient antimicrobial properties, owing to their large surface to volume ratio, size, shape and structure (Rafique et al., 2017).
Metal nanoparticles have been traditionally produced by physio-chemical methods that include ion sputtering or pulsed laser ablation, reduction, solvothermal synthesis, hydrothermal and sol-gel methods. Recently, there have been environmentally friendly synthesis methods that use natural products that have been termed the “green synthesis” or “biosynthesis” of nanoparticles (Chouhan, 2018; Vala et al., 2021).
Chemical and physical methods are generally expensive, harmful and inflammable, unlike the biosynthesis method which is cost-effective, energy-saving and environmentally benign as it uses microorganisms and plant extracts. The phytochemicals such as lipids, proteins, polyphenols, carboxylic acids, saponins, amino acids, polysaccharides, and enzymes present in biological material are used as reducing, capping and stabilizing agents. The use of agricultural waste helps in reducing the cost of producing the AgNPs as well as limiting the need of using hazardous chemicals and therefore encourages the “green synthesis” production (Chouhan, 2018; Hemlata et al., 2020). The use of plant extracts has been proven to be affordable, easy to bulk up, simple and environmentally friendly (Sanchooli et al., 2018; Raza et al., 2021).
Biosynthesised silver nanoparticles (b-AgNPs) have been found to have antibacterial, antifungal, and antiviral properties, with no environmental concerns and development of microbial resistance. These characteristics have ignited increasing interest in the synthesis of silver nanoparticles (Velu et al., 2017; Hemlata et al., 2020).
Upon synthesis of the AgNPs are synthesised, it is essential to characterise them to understand their physicochemical properties which could have an impact on their biocompatibility during use. The characterization is aimed at understanding the size, size distribution, shape, surface area, stability and aggregation of the particles (Zhang et al., 2016). Characterizing can be done through various analytical techniques which include ultraviolet-visible (UV-Vis) spectroscopy, Fourier transform infrared (FTIR) spectroscopy, X-ray diffractometry (XRD), dynamic light scattering (DLS), transmission electron microscopy (TEM) and atomic force microscopy (AFM) (Khatoon et al., 2017; Al-zubaidi et al., 2019; Jamiu and Bello, 2018; Khan and Javed, 2021).
Upon characterising the b-AgNPs, they can then be used for various purposes which include using them as an antifungal treatment. Overcoming fungal diseases is difficult, especially because of the limitation of several antifungal remedies as well as the environmental impacts that are caused by chemical treatments (Zhang et al., 2016).
Biosynthesised silver nanoparticles have shown exceptional antifungal activity against several phytopathogenic fungi including Rhizoctonia solani, Alternaria alternata, Sclerotinia sclerotiorum, Botrytis cinerea, Macrophomina phaseolina and Curvularia lunata at the concentration of 15 mg (Zhang et al., 2016).
Several plants have been found to possess medicinal and antimicrobial properties. These properties are attributed to the presence of phytochemicals such as limonoids, flavonol glycosides, saponins, steroids, terpenes, and tannins (Ibrahim et al., 2010; Jafari et al., 2013; Okello and Kang, 2019). The leaf extracts may possess variable effects on pathogens, but have been found to have a higher efficacy when they are used as b-AgNPs (Chouhan, 2018).
The objective of this study is to develop, characterise, and evaluate AgNPs synthesised from the leaf extracts of known antimicrobial plants against Sporisorium scitamineum (Syd) M. Piepenbr., M. Stoll & Oberw., the fungal pathogen that causes sugarcane smut (Bhuiyan et al., 2021). Hitherto, only Azadirachta indica and Melia azedarach, among the selected botanicals, have been used to synthesize AgNPs, yet their antifungal efficacy has not been evaluated against S. scitamineum.
MATERIALS AND METHODS
Sourcing plant extracts
This study was done in Kenya and Eswatini from 2020 to 2021. The selection of the plants to be used was influenced by their known medicinal properties as well as availability (Abdel-Rahim et al., 2016; Berhanu and Babele, 2020; Carpinel and Alonso, 1999; Hasan et al., 2019; Jeruto et al., 2016; Okello and Kang, 2019). The selected plants were Warburgia ugandensis, Carissa spinarum, Acacia nilotica, A. indica, M. azedarach and Senna didymobotrya. The M. azedarach extracts were sourced from Eswatini and the other five plant leaves were sourced from the Jomo Kenyatta University of Agriculture and Technology’s (JKUAT) botanical garden, in Kenya.
Plant extract preparation
The plant crude extract preparation was done by cleaning the leaves with sterile water, drying them and cutting them into small pieces using a blender. Then 50 g of each leaf sample was heated at 80°C in 250 ml of sterile water in a 500 ml Erlenmeyer flask for 30 min. The crude leaf extracts were then filtered using Whatman No. 1 and stored at 4°C (Velu et al., 2017).
Biosynthesis of nanoparticles
To synthesize the AgNPs, 1 mM of silver nitrate was formulated by adding 0.167 g of silver nitrate into 1 L of distilled water. The mixture of the silver nitrate and the plant’s crude extract was made
at an optimised ratio and kept in darkness, to prevent photo-reduction of the silver, at 28oC in a 150rpm shaking incubator. The intensity of the colour which is indicative of nanoparticle formation was recorded between 200 and 800 nm on a UV-Vis spectrophotometer using the Flight Deck, Jenway Model 6800 Spectrophotometer (Velu et al., 2017).
Optimizing the nanoparticles
The b-AgNPs were optimised under different reaction conditions which included leaf extract reaction volume (2, 3, 4, 5, 6, 7, 8, and 9 ml) and the duration of incubation of the AgNPs in darkness which was varied at 0, 2, 4, 12, 24, 48 and 72 h (Houllou et al., 2019). While optimizing each parameter, the other parameters were kept constant.
The b-AgNPs were isolated from the optimized mixture by centrifugation at 12 000 rpm for 20 min. The pellet was then purified using distilled water and washed twice to ensure better separation of free entities from the AgNPs. The b-AgNPs were kept at -20°C for 24 h, moved to -80°C to be kept for 48 h, and then they were lyophilized and used for further characterization (Velu et al., 2017).
Characterizing the nanoparticles
UV-Vis Spectra analysis
A sample (1 ml) of the suspension was collected periodically to monitor the completion of bio-reduction of Ag+ in an aqueous solution. The UV-Vis spectrum of the solution was measured between wavelengths 200 and 800 nm using the Jenway Model 6800 Spectrophotometer Flight Deck with a resolution of 1 nm (Sanchooli et al., 2018).
FTIR analysis
The nanoparticle characterization included ascertaining the active biomolecules responsible for the reduction; capping and stabilising by Fourier transform infrared (FTIR) spectrometer model 8400, Shimadzu. For the FTIR analysis, the dried b-AgNPs were added to FTIR-grade potassium bromide (KBr) in 1: 100 ratios and observed in the range of 4000 to 400 cm-1 (Mondal et al., 2020).
TEM analysis
Analysis to determine the morphology, size and shape of the nanoparticles was done using the JEM-2100 Electron Microscopy. The TEM sample grid with a continuous silicon oxide film was prepared. The sample grid was then derivatized by exposing the silicon oxide to 10 µl of aminopropyldimethylethoxysilane solution. The b-AgNPs were then citrate-stabilized for them to have a negative charge to be attracted to the positively charged TEM surface grid (Bonevich and Haller, 2010).
Collection and identification of the fungus
The smut-infected plants were identified at the Eswatini Sugar Association’s experimental plots at Nsoko. Visible sori were cut from the infected sugarcane plants and bagged to prevent any spread to healthy plants. These spores were rinsed three times with distilled water and cultured in potato dextrose agar (PDA). The plates were incubated for fourteen days in darkness at 28°C (Cui et al., 2020; Singh et al., 2005). To purify the cultures, single colonies were transferred onto new plates and incubated in darkness at 28°C (Que et al., 2014).
Fungal genomic DNA was extracted using a Zymo Fungal/Bacterial Genomic DNA Extraction Kit (Inqaba Biotech, South Africa) following the manufacturer’s instructions. The quality and concentration of DNA were analysed by 1% agarose gel electrophoresis and by using a nanodrop spectrophotometer.
To verify the identity of the fungus, the extracted DNA was amplified in conventional PCR using the bE4 (5’- CGCTCTGGTTCATCAACG - 3’) and bE8 (5’- TGCTGTCGATGGAAGGTGT - 3’) primers that are specific for S. scitamineum (Zhang et al., 2015).
Conventional PCR amplification was carried out in a 25 ?L volume containing 1 ?L DNA, 12.5 ?L of 2x OneTaq master mix, 0.5 ?L of each of the upstream and downstream primers and 10.5 ?L of water. The PCR amplification was performed following a thermal cycling programme of 95°C for 5 min; 35 cycles of 95°C for 30 s, 50°C for 30 s, and 68°C for 40 s; and a final extension at 72°C for 5 min.
The PCR amplicons were checked for quality on a 1% agarose gel electrophoresis and then documented. A negative control sample only contained the master mix with no DNA template.
Screening of b-AgNP for antifungal activity
The silver nanoparticles that were produced from the crude extracts from the different plants were evaluated to select the b-AgNPs that had the highest antifungal activity against S. scitamineum. Fungal spores suspended in sterile water were spread onto PDA media using a sterile swab and then incubated for 24 h at 28°C. The disc method was used, with the standard antifungal nystatin (100 µg) as a positive control and distilled water as a negative control (Alyousef et al., 2019; Al-zubaidi et al., 2019; Hameed et al., 2015; Khan and Javed, 2021; Medda et al., 2015). The synthesised b-AgNPs were dissolved in distilled water and each b-AgNP treatment was evaluated at 2.5, 5 and 10 mg/ml in three replicates.
Data analysis
The antifungal efficacy of the various b-AgNPs was evaluated by measuring the zones of inhibition. Data were subjected to ANOVA and means were separated at P = 0.05.
RESULTS
Biosynthesis of silver nanoparticles
The reduction of the silver nitrate, upon mixing it with the crude extract, was seen by the colour change from light pale into dark brown (Figure 1).
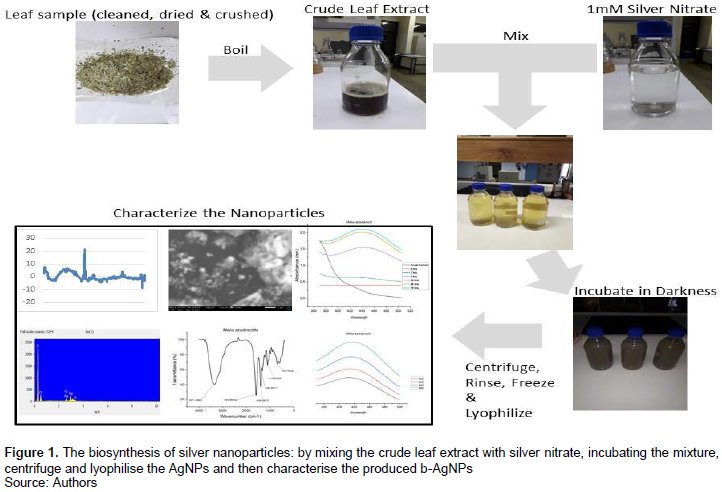
Characterization of the b-AgNPs
UV-Vis spectroscopy
The analysis showed absorption peaks for the b-AgNPs that were made from the different botanicals at a range between 340 and 450 nm. To optimise the amount of crude leaf extract that was added to the 1 mM silver nitrate (AgNO3) to reduce it to AgNPs; 5 ml was added for A. indica and Melia azedarach, while 4 ml was added for A. nilotica and S. didymobotrya, 3 ml was added for C. spinarum and W. ugandensis and the plasmon peaks were observed at 430, 435, 385, 385, 335 and 385 nm, respectively (Figure 2). When optimizing for the required incubation period of the mixture; A. indica, A. nilotica, M. azedarach and W. ugandensis required to be incubated for 72 h, while S. didymobotrya required to be incubated for 48 h and C. spinarum required 24 h, and the plasmon peaks were observed at 390, 385, 440, 390, 395, and 390, respectively (Figure 3).
.png)
Fourier transform infrared (FTIR) analysis
The FTIR analysis of the b-AgNPs (Figure 4) shows the bands that correspond with the biomolecules responsible for the reduction of AgNO3 to nanoparticles. The bands were observed at 3355 to 3402 cm-1, 1595 to 1605 cm-1, 1404 cm-1, 1200 to 1300 cm-1, 995 to 1118 cm-1, and at 500 to 720 cm-1.
.png)
TEM analysis
The sizes of the b-AgNPs that were synthesized using
the various botanicals varied between 3 and 70 nm. C. spinarum produced AgNPs with the size range of 3 to 33 nm, M. azedarach produced 9 to 70 nm, while A. indica, A. nilotica, S. didymobotrya and W. ugandensis produced 14 to 53 nm, 14 to 52 nm, 6 to 35 nm and 12 to 53 nm, respectively (Figure 5 and Table 1). The shape and surface texture of the b-AgNPs was consistently spherical and smooth for all the nanoparticles (Table 1).
.png)
Verification of the fungi
To verify the identity of the fungus, the collected samples were screened by conventional PCR using the S. scitamineum specific primers bE4 and bE8. The samples produced an amplicon of 459 bp which corroborated the results by Izadi and Moosawi-jorf (2007).
Screening of nanoparticles for antifungal activity
The evaluation of the antifungal activity of the different b-AgNPs showed that C. spinarum and M. azedarach had higher inhibition zones, but C. spinarum was shown to be superior at both 5 and 10 mg/ml (Figure 6). A. indica recorded the least antifungal activity at all the concentrations, while the other b-AgNPs had a moderate effect.
.png)
DISCUSSION
The colour change upon mixing the silver nitrate with the crude extract is indicative of the formation of the nanoparticles by the reduction of the AgNO3 by the crude leaf extract to form b-AgNPs (Alyousef et al., 2019; Sharma et al., 2014; Velu et al., 2017). The colour change is due to the occurrence of the Surface Plasmon Resonance (SPR) phenomenon which is caused by the interaction of the conduction electrons of the silver nanoparticles (Sharma et al., 2014; Vala et al., 2021). The phytochemicals (lipids, proteins, polyphenols, carboxylic acids, saponins, amino acids, polysaccharides and enzymes) present in plants are used as reducing, capping and stabilising agents (Chouhan, 2018).
During the synthesis of the b-AgNPs, the amount of crude extract that was added as well as the incubation period were optimised by UV-Vis spectroscopy. The optimization process produced absorption peaks for the b-AgNPs at a range between 340 and 450 nm and confirms the formation of the nanoparticles, which is consistent with the findings of Alyousef et al. (2019), Sanchooli et al. (2018) and Masum et al. (2019). Namratha and Monica (2013) reported the range of the peaks that indicate the formation of nanoparticles to be observed between 350 and 550 nm. The various botanicals required varying amounts of the leaf extract as well as incubation periods to achieve the optimum formation of the b-AgNPs. This variation could be caused by the changeable levels of phytochemicals that are contained in the different botanicals. This variation of phytochemicals could have resulted in a variable reduction rate of the silver nitrate as well as the variable capping and stabilizing of the nanoparticles (Chouhan, 2018).
The FTIR analysis of b-AGNPs validates the activity of biomolecules that are in charge of the reduction and stabilization of the b-AgNPs (Khatoon et al., 2017; Mondal et al., 2020). The analysis shows the bands between 3355 and 3402 cm-1 which correspond to N-H stretching of the proteins’ secondary amide. The peaks at 1596-1605 cm-1 indicate stretch vibrations for the –C=C- bond, whilst the Benzene ring C=C and C-C are shown by peaks at 1404 cm-1. The C-H bond in the pyridine ring appears at 1200-1300 cm-1 and the C-OH phenols appear at 995-1118 cm-1. The peaks at 500-720 cm-1 show the presence of the AgNPs (Al-zubaidi et al., 2019). The synthesised nanoparticles were surrounded by proteins and other functional groups such as terpenoids. These results indicate the strength of the carbonyl groups from the proteins and amino acids to bind with metal, thereby capping the AgNPs. The presence of the reducing sugars could indicate their responsibility in reducing the AgNO3 to AgNPs and stabilizing the AgNPs (Khatoon et al., 2017).
The TEM analysis was able to determine the sizes, shapes and texture of the b-AgNPs. Nanoparticles, by their definition, should range between 1 and 100 nm (Vala et al., 2021; Mondal et al., 2020). Their nano-scale size, morphological substructure and shape are of great importance as they give the AgNPs the physicochemical properties that suit them for their multiple applications (Chouhan, 2018; Khatoon et al., 2017). The size and shape of the A. indica AgNPs were shown to be consistent with the sizes that are documented by Firdhouse and Lalitha (2015), Khatoon et al. (2017) and Namratha and Monica (2013) (Table 1). The synthesis and characterisation of AgNPs made from other botanicals had not been documented before this study.
The fungal isolate was positively verified by conventional PCR using the S. scitamineum specific primers bE4 and bE8 (Zhang et al., 2015). All the b-AgNPs had an inhibitory effect on the growth of the fungus S. scitamineum, but with varying efficacies which could be due to the difference in phytochemicals that reduce the silver nitrate to nanoparticles among the botanicals (Hussain et al., 2019). This was observed by the formation of inhibition zones in all the b-AgNP treatments. The positive inhibition of the growth of the fungus in-vitro warrants further in-vivo studies.
CONCLUSION
In this study, biosynthesized silver nanoparticles (b-AgNPs) of varying sizes were successfully synthesized using A. nilotica, C. spinarum, S. didymobotrya, W. ugandensis, M. azedarach and A. indica. The synthesized nanoparticles were all spherical in shape, smooth in texture, and had a size range between 3 and 70 nm as indicated by the TEM, FTIR and UV-Vis characterization. All the biosynthesized nanoparticles showed antifungal effects against the fungus S. scitamineum in-vitro. C. spinarum and M. azedarach exhibited the highest antifungal activity, while A. indica showed the least.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
ACKNOWLEDGEMENTS
The authors thank the African Union for funding this research through the Pan African University, Institute of Basic Science, Technology and Innovation. They are grateful to the Eswatini Sugar Association for providing fungal isolates.
REFERENCES
|
Abdel-Rahim A, Wafa Y, Idris FA (2016). Antifungal Activity of the Extracts of Garad (Acacia nilotica L). Gezira Journal of Engineering and Applied Sciences 7(2):1-13 |
|
|
Alyousef AA, Arshad M, Alakeel R, Alqasim A (2019). Plant extract: biosynthesis, characterization and antibacterial activity. Biotechnology and Biotechnological Equipment 33(1):931-936. |
|
|
Al-zubaidi S, Al-ayafi A, Abdelkader H (2019). Biosynthesis, Characterization and Antifungal Activity of Silver Nanoparticles by Aspergillus Niger Isolate. Journal of Nanotechnology Research 1(1):23-36. |
|
|
Berhanu G, Babele DA (2020). A Review of the Medicinal and Antimicrobial Properties of Carissa spinarum L. American Journal of Biomedical Research 8(2):54-58. |
|
|
Bonevich JE, Haller WK (2010). Measuring the Size of Nanoparticles Using Transmission Electron Microscopy (TEM). Alliance for Nanotechnology in Cancer. https://tsapps.nist.gov/publication/get_pdf.cfm?pub_id=854083 |
|
|
Carpinel C, Alonso R (1999). Antifungal activity of Melia azedarach fruit extract. Fitoterapia 70(3):296-298. |
|
|
Chouhan N (2018). Silver Nanoparticles: Synthesis, Characterization and Applications. In Intech (P 13). |
|
|
Cui G, Yin K, Lin N, Liang M, Huang C, Chang C, Xi P, Deng YZ (2020). Burkholderia gladioli CGB10: A Novel Strain Biocontrolling the Sugarcane Smut Disease. Microorganisms 8(12):1943. |
|
|
Firdhouse MJ, Lalitha P (2015). Biosynthesis of Silver Nanoparticles and Its Applications. Journal of Nanotechnology (1):1-18 |
|
|
Hameed IH, Fadhil L, Kamal SA (2015). Analysis of bioactive chemical compounds of Aspergillus niger by using gas chromatography-mass spectrometry and fourier-transform infrared spectroscopy. Journal of Pharmacognosy and Phytotherapy 7(8):132-163. |
|
|
Hasan R, Ahmed R, Paul AC, Raha RK (2019). Antifungal efficacy of neem leaves (Azadirachta indica) and mahagoni fruit bark (Swietenia mahagoni) extracts on leather shoes. Bangladesh Journal of Scientific and Industrial Research 54(3):257-262. |
|
|
Hemlata MPR, Singh AP, Tejavath KK (2020). Biosynthesis of silver nanoparticles using Cucumis prophetarum aqueous leaf extract and their antibacterial and antiproliferative activity against cancer cell lines. ACS Omega 5(10):5520-5528. |
|
|
Houllou LM, Souza RAD, Malafaia CB, Carlos G, Duarte S (2019). Green synthesis of silver nanoparticles using leaf extract from Tabebua roseoalba and T. pentaphylla. Journal of Environmental Analysis and Progress 3:216-222. |
|
|
Hussain A, Alajmi MF, Khan MA, Pervez SA, Ahmed F, Amir S, Hussain FM, Khan MS, Shaik GM, Hassan I, Khan RA, Rehman MT (2019). Biosynthesized Silver Nanoparticle (AgNP) from Pandanus odorifer Leaf Extract Exhibits Anti-metastasis and Anti-biofilm Potentials. Frontiers in Microbiology 10:8. |
|
|
Ibrahim H, Oyi RA, Ehinmidu JO, Musa KY, Bright NT (2010). Antimicrobial activity of the water extracts of the leaves and fruits of Carissa edulis Vahl (Apocynaceae). Journal of Medicinal Plants Research 4(11):1028-1032. |
|
|
Jafari S, Saeidnia S, Hajimehdipoor H, Reza M, Ardekani S (2013). Cytotoxic evaluation of Melia azedarach in comparison with, Azadirachta indica and its phytochemical investigation. Journal of Pharmaceutical Sciences 21(1):1-7. |
|
|
Jamiu AT, Bello S (2018). Biosynthesis of Silver Nanoparticles using Azadirachta indica Leaf Extract and Assessment of its Antibacterial Activity on some Pathogenic Enteric Bacteria. International Journal of Research and Development in Pharmacy and Life Sciences 5(2):25-31. |
|
|
Jeruto P, Akenga T, Nyunja R (2016). In-vitro antifungal activity of methanolic extracts of different Senna didymobotrya (Fresen) HS Irwin and Barneby plant parts. African Journal of Traditional, Complementary and Alternative Medicines 13(6):168-174. |
|
|
Khan K, Javed S (2021). Silver nanoparticles synthesized using leaf extract of Azadirachta indica exhibit enhanced antimicrobial efficacy than the chemically synthesized nanoparticles: A comparative study. Science Progress 104(2):1-15. |
|
|
Khatoon N, Mazumder JA, Sardar M (2017). Biotechnological Applications of Green Synthesized Silver Nanoparticles. Journal of Nanosciences: Current Research 2(107):2572-0813. |
|
|
Masum MI, Siddiqa MM, Ali KA, Zhang Y (2019). Biogenic Synthesis of Silver Nanoparticles Using Phyllanthus emblica Fruit Extract and Its Inhibitory Action Against the Pathogen Acidovorax oryzae Strain RS-2 of Rice Bacterial Brown Stripe. Frontiers in Microbiology 10:820. |
|
|
Medda S, Hajra A, Dey U (2015). Biosynthesis of silver nanoparticles from Aloe vera leaf extract and antifungal activity against Rhizopus sp. and Aspergillus sp. Applied Nanoscience 5(7):875-880. |
|
|
Mondal AH, Yadav D, Mitra S, Mukhopadhyay K (2020). Biosynthesis of Silver Nanoparticles Using Culture Supernatant of Shewanella sp. ARYI and their Antibacterial Activity. International Journal of Nanomedicine 15:8295-8307. |
|
|
Namratha N, Monica PV (2013). Synthesis of Silver Nanoparticles using Azadirachta indica (Neem) extract and usage in water purification. Asian Journal of Pharmacy and Technology 3(4):170-174. |
|
|
Okello D, Kang Y (2019). Ethnopharmacological Potentials of Warburgia ugandensis on Antimicrobial Activities. Chinese Journal of Integrative Medicine 27(8):633-640. |
|
|
Que Y, Xu L, Wu Q, Liu Y, Ling H, Liu Y, Zhang Y, Guo J (2014). Genome sequencing of Sporisorium scitamineum provides insights into the pathogenic mechanisms of sugarcane smut. BMC Genomics 15(1):1-20. |
|
|
Rafique M, Sadaf I, Rafique MS, Tahir MB (2017). A review on green synthesis of silver nanoparticles and their applications. Artificial Cells, Nanomedicine, and Biotechnology 45(7):1272-1291. |
|
|
Raza S, Ansari A, Siddiqui NN, Ibrahim F, Abro MI, Aman A (2021). Biosynthesis of silver nanoparticles for the fabrication of cytotoxic and antibacterial metallic polymer-based nanocomposite system. Scientific Reports 11(1):1-15. |
|
|
Sanchooli N, Saeidi S, Barani HK, Sanchooli E (2018). In vitro antibacterial effects of silver nanoparticles synthesized using Verbena officinalis leaf extract on Yersinia ruckeri, Vibrio cholera and Listeria monocytogenes. Iranian Journal of Microbiology 10(6):400-408. |
|
|
Sharma AK, Kumar A, Yadav SK, Rahal A (2014). Studies on Antimicrobial and Immunomodulatory Effects of Hot Aqueous Extract of Acacia nilotica L. Leaves against Common Veterinary Pathogens. Veterinary Medicine International. |
|
|
Singh N, Somai B M, Pillay D (2005). In vitro screening of sugarcane to evaluate smut susceptibility. Plant Cell, Tissue and Organ Culture 80(3):259-266. |
|
|
Vala AK, Trivedi H, Gosai H, Panseriya H, Dave B (2021). Biosynthesized silver nanoparticles and their therapeutic applications. Comprehensive Analytical Chemistry 94:547-584. |
|
|
Velu M, Lee JH, Chang WS, Lovanh N, Park YJ, Jayanthi P, Palanivel V, Oh BT (2017). Fabrication, optimization, and characterization of noble silver nanoparticles from sugarcane leaf (Saccharum officinarum) extract for antifungal application. 3 Biotech 7(2):1-9. |
|
|
Zhang XF, Liu ZG, Shen W, Gurunathan S (2016). Silver nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. International Journal of Molecular Sciences 17(9):1534. |
|
|
Zhang Y, Huang N, Xiao XH, Huang L, Liu F, Su WH (2015). Molecular variation of Sporisorium scitamineum in Mainland China revealed by internal transcribed spacers. Genetics and Molecular Research 14(3):7894-7909. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0