ABSTRACT
The growing levels of antimicrobial resistance have triggered interest in the search for alternative compounds to wage the war against it. Plants have been shown to harbor endophytic fungi that produce bioactive idiolites, which have potential lead compounds in the development of new drugs. This study evaluated the antimicrobial activity of endophytic fungi isolated from leaves of Dacryodes edulis. Endophytic fungi associated with healthy leaves of D. edulis were isolated using standard methods. The fungi were subjected to solid-state fermentation on rice media at 28°C for 21 days and the idiolites extracted using ethylacetate. The extracts of the endophytic fungi were evaluated for antimicrobial properties using agar well diffusion method against selected human pathogens. The endophytic fungi isolates were also characterized using macroscopy and microscopy (photomicrograph). A total of six (6) endophytic fungi (LB2, LB3, MR1, MR2, MR3, and MR4) were isolated from leaves of D. edulis. The extracts of isolated endophytic fungi displayed varying antimicrobial activities, with inhibition zone diameters ranging from 4 to 12 mm. The MR3 extract was the most effective metabolite and showed broad spectrum antimicrobial activity against Staphylococcus aureus, Salmonella typhi, Escherichia coli, Pseudomonas aeruginosa and Candida albicans. Endophytic fungi isolated from the leaves of D. edulis have antimicrobial activity and could be a hopeful source antimicrobial agent.
Key words: Endophytic fungi, antimicrobial, idiolites, Dacryodes edulis.
The initial discovery of antimicrobials transformed humans, resulting in improved survival for both humans and their livestock. However, this health achievement was immediately dominated by the abilities of bacteria to modify themselves to resist against antimicrobials and spread these resistance traits among others. Bacterial resistance to clinically applied antibiotics has been recognized as a serious public threat worldwide and necessitates the search for new drugs to counter the problems. Thus, there is a constant and high demand for new drugs that might help to overcome this alarming scenario of resistance development and spread. Endophytic fungi constitute a relatively unstudied and promising source for such new antibacterial agents (Nwobodo et al., 2020a; Debbab et al., 2011). Endophytic fungi are polyphyletic microorganisms that establish mutualistic associations with their host plants without causing disease symptoms (Nicoletti and Fiorentino, 2015). They are found in virtually all plants and have multiple activities on the host plants and their survival, which are exerted via varied pathways. Such pathway is via the production of idiolites, also known as secondary metabolites.
The mutual relationships between endophytes and their host plants are believed to result in survival benefits for both partners (Khare et al., 2018). The endophytes may provide protection and survival conditions to their host plant by producing a plethora of substances which, once isolated and characterized, may also have potential for use in industry, agriculture, and medicine (Verma et al., 2016). This group of microorganisms has been regarded as one of the most creative groups of secondary metabolites that play important biological roles and are potential sources of novel natural agents for exploitation in the pharmaceutical industry (Nwobodo et al., 2017; Selim et al., 2012). It is estimated that about 5% of the fungi population have been studied and the rest remain unexplored for their contribution to human welfare (Qadri et al., 2013).
Natural products from endophytic fungi have been reported to inhibit or kill a wide variety of pathogenic microorganism (Nwobodo et al., 2020a; Chirlei et al., 2012). Endophytic fungi isolated from Nigerian medicinal plants have been reported to possess broad spectrum activity against pathogenic fungi and bacteria (Nwobodo et al., 2020b; Akpotu et al., 2017; Okezie et al., 2017). The plant Dacryodes edulis has a long history in African traditional medicine for the treatment of various ailments (Okwu and Nnamdi, 2008). Olasunkanmi and Adeniyi (2017) reported that the leaf extract possesses broad spectrum antibacterial activity. The leaves are employed to cure skin diseases, disorders of the digestive tract, toothache and ear ache (Hassan-Olajokun et al., 2020). In the present study, crude metabolites produced by endophytic fungi isolated from sterile leaflet of D. edulis were evaluated for their antimicrobial activity.
Plant collection, isolation and maintenance of endophytic fungi
Fresh leaves of D. edulis were collected from Agulu, Anaocha Local Government Area of Anambra State, Nigeria in June, 2019. The plant was identified and authenticated at the Department of Pharmacognosy and Traditional Medicine, Nnamdi Azikiwe University, Awka, Nigeria and Voucher specimen (PCG/19/A/045) was deposited.
Isolation of endophytic fungi from the leaves was carried out as described by Eze et al. (2018). The leaves were washed thoroughly in running tap water, and then cut into small fragments (about 1 cm2). The leaf fragments were surface-sterilized by immersion in 2% sodium hypochlorite solution for 2 min, 70% ethanol for nearly 2 min, before a final rinse in sterile water for 5 min. These leaf fragments were transferred into malt extract agar (MEA) plates, supplemented with chloramphenicol. The Petri plates were then incubated at 27°C for 7 days. Hyphal tips of fungal colonies emerging from the leaf segments were sub-cultured on fresh MEA plates, and then single pure colony was obtained by directly picking single spores using microscopic observation (Zhang et al., 2013).
Solid state fermentation and extraction of the fungal secondary metabolites
The solid-state fermentation and extraction of the fungal metabolites were carried out using methods previously described by Okoye et al. (2013) and Nwobodo et al. (2020a). Rice medium was prepared in 1000 ml Erlenmeyer flasks as follows: approximately 200 ml of distilled water was added to 100 g of rice, and then autoclaved at 121°C for 30 min. The flasks were inoculated individually with 4 agar blocks (3 mm diameter), cut from each pure endophytic fungal culture using a sterile cork borer, and then incubated at 28°C for 21 days. After the fermentation period, the culture media and the growing mycelia were extracted using ethyl acetate, and then separated by filtration. The organic phase was vacuum-concentrated at 40°C under reduced pressure, using a rotary vacuum evaporator to obtain the crude extracts.
Antimicrobial screening
Test isolates
The microbial cultures used in this study for the antimicrobial assay were laboratory strains obtained from the Department of Pharmaceutical Microbiology and Biotechnology laboratory, Faculty of Pharmaceutical Sciences, Nnamdi Azikiwe University, Awka Nigeria. The bacterial isolates were Staphylococcus aureus, Salmonella typhi, Pseudomonas aeruginosa, Escherichia coli, and a yeast, Candida albicans. The bacterial isolates were identified using the standard morphological and biochemical characteristics of the bacteria according to Bergey’s Manual of Determinative Bacteriology (Bergey and Holt, 2000).
Agar well diffusion assay
Antimicrobial activity of the endophytic fungal extracts was carried out using the agar well diffusion assay method as described by Akpotu et al. (2017). A stock concentration of 2 mg/ml of the fungal extracts was prepared by dissolving in dimethyl sulphoxide (DMSO 100% v/v). From the stock concentration, the working concentration of 1 mg/ml was prepared to determine the efficacy of the fungal extract. 0.1 ml standardized concentration (McFarland 0.5) of overnight cultures of the test bacteria and fungi were spread aseptically onto the surface of Mueller Hinton Agar (MHA) and Sabouraud dextrose agar (SDA) plates, for bacterial and fungal isolates, respectively. All plates were allowed to dry for about 5 min, and then agar wells were made by using a sterile cork-borer (6 mm in diameter). These wells were individually inoculated with 20 μl of each of the fungal extracts and the controls. The plates were then kept at room temperature for 1 h to allow the substances/ compounds to diffuse into the agar. The MHA plates were incubated at 37°C for 24 h, and SDA plates incubated at 25°C for 48 h. Ciprofloxacin (5 µg) and Miconazole (50 µg) served as the positive controls for the bacteria and fungi, respectively, while DMSO (100% v/v) served as the negative controls. The inhibition zones diameters (IZDs) were measured using a calibrating ruler. The assay was conducted in triplicates, and repeated twice.
Statistical analysis
Data obtained were presented as mean for experiments carried out in triplicates. The mean inhibition zones diameter of the fungal extracts against the various test microbes were compared using one-way ANOVA. Statistical significance was considered at p ≤ 0.05. Analysis of data and graph were made using Microsoft Excels 2013 software and SPSS version 20.
Multi-drug resistant pathogenic microorganisms have become a global health challenge. Endophytic fungi metabolites are indispensable as lead compounds in the search for novel antibiotics with new mechanisms of action and less toxic effects (Chirlei et al., 2012). Endophytes are known to be a rich source of idolites with several biological properties including antimicrobial activities (Nwobodo et al., 2020a; Eze et al., 2018; Kusari et al., 2008).
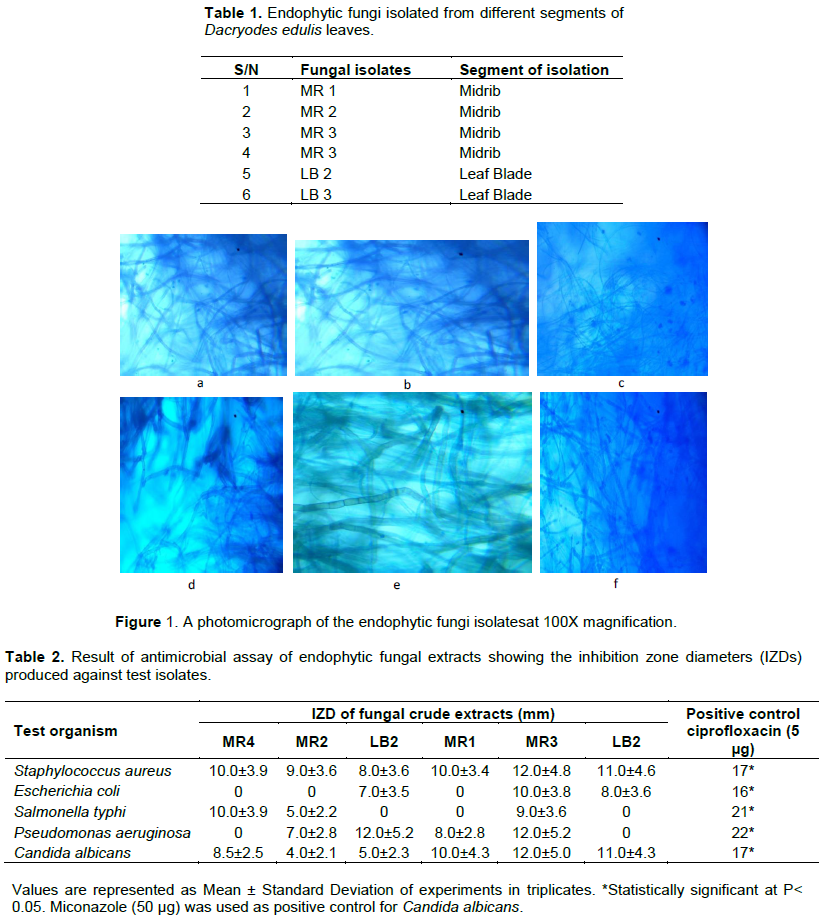
D. edulis has been constantly used in African traditional medicine, and the leaf extracts have been reported to exhibit antimicrobial and antioxidant activities (Okunomo and Egho, 2010). In this study, six (6) endophytic fungi were isolated. Four of the endophytic fungi were isolated from the plant leaf midrib (MR 1-4), while two where isolated from the leaf blade (LB 1 and 2) (Table 1). Thus, based on our results, fungal community appeared to be higher in the leaf midrib than in the leaf blade. The results of the morphological characterization are as shown in Figure 1.
In this study, the idiolites of the endophytic fungi from D. edulis exhibited antimicrobial activity at varying degrees at a concentration of 1 mg/ml. Table 2 represents the result of the antibacterial activities of the tested fungal extracts. Extract MR3 exhibited the best antibacterial activity against 100% of the test microorganisms employed in this study. The endophytic fungal extract MR3 was not only observed to be active against all tested bacteria strains, but also displayed the highest inhibition zone diameter values of 12.0±5.2 and 12.0±5.2 against S. aureus and P. aeruginosa, respectively. However, there is a significant difference (P < 0.05) when compared with the values of the positive control (Table 2). The extract MR4 demonstrated the best activity against Salmonella typhi (IZD = 10.0±3.9 mm), but was totally resisted by E. coli and P. aeruginosa. This is not surprising as the findings of Kibret and Abera (2011) stated that the antimicrobial resistance in E. coli has been reported worldwide. The increasing rates of resistance among E. coli are a growing concern both in the developed and developing countries. Several previous studies of Marilyn et al. (2012), Nwobodo et al. (2020b), and Egbujor et al. (2020) reported that resistance development in P. aeruginosa is multifactorial, with mutations in several genes contributing for resistance to β-lactams, carbapenems, aminoglycosides, fluoro-quinolones and sulphonamides. S. aureus was sensitive to all tested extracts, while E. coli was sensitive to only 50% of the tested extracts (Table 2). There was a complete resistance by P. aeruginosa to two (MR4 and LB2) of the six extracts tested. Similarly, in a recent study, Mordi et al. (2019) reported the resistance of P. aeruginosa to the seed oil extract of D. edulis. The mean values of the zones of inhibition obtained for the fungal extracts are statistically not significant as p > 0.05. However, when compared with the positive control, p < 0.001.
Also, the extract MR3 displayed the best antifungal activity against C. albicans (12.0±5.0), followed by the extract of LB2 (11.0±4.3). There was no significant difference in values obtained in the antifungal assay, across all extracts and organisms (P>0.05).
The ability of the fungal extracts to inhibit both Gram positive and Gram negative organisms, denotes the broad spectrum potentials of the fungal metabolic extracts. These findings indicate the potentials of idolites of endophytic fungi isolated from D. edulis as potential value as starting point in the development of new broad spectrum antibacterial and antifungal agents. To the best of the authors’ knowledge, this is the first study on the endophytic fungi isolated from D. edulis, hence, there are limited information on this area. However, several authors have isolated endophytic fungi from plants of this same botanical family (Burseraceae). A high diversity of fungal species was also found in leaves and stems of Boswellia sacra (Fierro-Cruz et al., 2017). Similarly, Guerrero and Dalisay (2018) isolated 15 fungal endophytes from Canarium ovatum fruit.
In this study, the endophytic fungal extracts of D. edulis were found to possess potential broad-spectrum antimicrobial activities against clinical human pathogens. It could be of much relevance to the development of new antimicrobials or chemotherapeutic drugs to address the ever increasing problems of drug resistance, which is fast encroaching, even to the last line antimicrobials.
The authors have not declared any conflict of interests.
REFERENCES
|
Akpotu MO, Eze PM, Abba CC, Nwachukwu CU, Okoye FBC, Esimone CO (2017). Metabolites of endophytic fungi isolated from Euphorbia hirta growing in Southern Nigeria. Chemical Science Review Letters 21:12-19.
|
|
|
|
Bergey DH, Holt JG (2000). Bergey's manual of determinative bacteriology. 9th ed. Philadelphia: Lippincott Wiliams and Wilkins. Little, Christopher R, Carol M, Stiles LMC (2014). Introduction to Fungi.
|
|
|
|
|
Chirlei G, Fabiana T, Josiane GF, Daiani S, Vania AV, Beatriz HLN, Sales M, Yvelise MP (2012). Antimicrobial activity of endophytes from Brazilian medicinal plants. INTECH 239-254.
|
|
|
|
|
Debbab A, Aly AH, Chaidir C (2011). Bioactive secondary metabolites from endophytes and associated marine derived fungi. Fungal Diversity 49(1):1-12.
Crossref
|
|
|
|
|
Egbujor MC, Nwobodo DC, Egwuatu PI, Abu IP, Ezeagu CU (2020). Sulphonamidedrugs and Pseudomonas aeruginosa resistance: A review. International Journal of Modern Pharmaceutical Research 4(1):78-83.
|
|
|
|
|
Eze PM, Ojimba NK,Abonyi DO, Chukwunwejim CR, Abba CC, Okoye FBC, Esimone CO (2018). Antimicrobial activity of metabolites of an endophytic fungus isolated from the leaves of Citrus jambhiri (Rutaceae). Tropical Journal of Natural Product Research 2(3):145-149.
Crossref
|
|
|
|
|
Fierro-Cruz JE, Jiménez P, Coy-Barrera E (2017). Fungal endophytes isolated from Protium heptaphyllum and Trattinnickia rhoifolia as antagonists of Fusariumoxysporum. Revista Argentina de Microbiología 49(3):255-263.
Crossref
|
|
|
|
|
Guerrero JJG, Dalisay TU (2018). Fungal endophytes across tissue layers of Canarium ovatum (Burseraceae) fruit. Australian Journal of Mycology 27:11-21.
|
|
|
|
|
Hassan-Olajokun RE, Deji-Agboola AM, Olasunkanmi OO, Banjo TA, Olaniran O (2020). Antimicrobial activity of fractioned components from Dacryodesedulis: Invitro Study. European Journal of Medicinal Plants 31(9):71-82.
Crossref
|
|
|
|
|
Khare E, Mishra J, Arora NK (2018). Multifaceted interactions between endophytes and plant: Developments and prospects. Frontiers Microbiology 15:1-17.
Crossref
|
|
|
|
|
Kibret M, Abera B (2011). Antimicrobial susceptibility of E.coli from clinical sources in northeast Ethiopia. African Health Sciences 11(1):40-45.
Crossref
|
|
|
|
|
Kusari S, Lambshoft M, Zuhike S, Spiteller M (2008). An endophytic fungus from Hypericumperforatum that produces hypericin. Journal of Natural Products 71(2):159-162.
Crossref
|
|
|
|
|
Marilyn PG, José VB, Santiago NC (2012). Overview of Multidrug-Resistant Pseudomonasaeruginosa and novel therapeutic approaches. Journal of Biomaterials and Nanobiotechnology 3:519-527.
Crossref
|
|
|
|
|
Mordi RC, Olasehinde GI, Okedere AP, Elegwule AN, Ayo-ajayi JI, Johnathan HO, Onibokun AE, Ajayi AA, Uchenna DO (2019). Antibacterial activity of moderately volatile components of the oil extracted from the seeds of Dacryodes edulisg. LAM. Asian Journal of Pharmaceutical and Clinical Research 12(3):246-249.
Crossref
|
|
|
|
|
Nicoletti R, Fiorentino A (2015). Plant bioactive metabolites and drugs produced by endophytic fungi of Spermatophyta.
Crossref
|
|
|
|
|
Nwobodo DC, Ihekwereme CP, Ugwu MC, Okoye FBC (2017). Screening of endophytic fungal secondary metabolites from Garcinia kola and Cola nitidafor antioxidant properties. Open Access Journal of Pharmaceutical Research 1(6):1-8.
Crossref
|
|
|
|
|
Nwobodo DC, Ihekwereme CP, Ikem CJ, Okoye FBC (2020a). The anti-pseu¬domonal potentials of metabolites from some endophytic fungi isolated from Garcinia kola leaves. Novel Research in Microbiolo¬gy Journal 4(3):845-855.
Crossref
|
|
|
|
|
Nwobodo DC, Ihekwereme CP, Okoye FBC (2020b). Screening of endophytic fungal secondary metabolites from Cola nitidaleaves for antimicrobial activities against clinical isolates of Pseudomonas aeruginosa. The EuroBiotech Journal 4(3):161-166.
Crossref
|
|
|
|
|
Okoye FBC, Lu S, Nworu CS, Esimone CO, Proksch P, Chaldi A, Debbab A (2013). Depsidone and diaryl ether derivatives from the fungus Corynesporacassiicola, an endophyte of Gongronemalatifolium. Tetrahedron Letters 54:4210-4214.
Crossref
|
|
|
|
|
Okezie UM, Eze PM, Okoye FBC, Ikegbunam MN, Ugwu MC, Esimone CO (2017). Biologically active metabolites of an endophytic fungus isolated from Vernoni aamygdalina. African Journal of Pharmaceutical Research and Development 9(1):24-26.
|
|
|
|
|
Okunomo K, Egho EO (2010). Economic importance of some underexploited tree species in Nigeria: Urgent need for separate research centers. Continental Journal of Biological Sciences 3:16-32.
|
|
|
|
|
Okwu DE, Nnamdi FU (2008). Evaluation of the chemical composition of Dacryodesedulis and Raphiahookeri Mann exudates used in herbal medicine in South Eastern Nigeria. African Journal of Traditional and Complementary Alternative Medicine 5(2):194-200.
Crossref
|
|
|
|
|
Olasunkanmi OO, Adeniyi PO (2017). Antibacterial and antioxidant activities of D.edulismethanolic leaf extract. Journal of Advances in Medical and Pharmaceutical Sciences 14(1):1-11.
Crossref
|
|
|
|
|
Qadri M, Johri S, Shah BA, Khajuria A, Sidiq A, Lattoo SK, Abdin MZ, Riyaz-Ul-Hassan S (2013). Identification and bioactive potential of endophytic fungi isolated from selected plants of the Western Himalayas. Springer Plus 2(8):1-14.
Crossref
|
|
|
|
|
Selim K, El-beih A, Abdel-rahman T, El-diwany A (2012). Biology of Endophytic Fungi. Current Research in Environmental and Applied Mycology 2(1):31-82.
Crossref
|
|
|
|
|
Verma SK, Kumar A, Lal M, Debnath M (2016). Antimicrobial activity of extract from endophytic fungus in Calotropisprocera root. Research in Environment and Life Sciences 9(2):212-216.
|
|
|
|
|
Zhang K, Su Y, Cai L (2013). An Optimized Protocol of Single Spore Isolation for Fungi. Mycologie 34(4):349-356.
Crossref
|
|