Full Length Research Paper
ABSTRACT
Hepatotoxicity is reported frequently as an adverse reaction during tuberculosis (TB) and HIV treatment. This study aimed to investigate the incidence of antiretroviral and anti-tuberculosis drug-induced liver enzymes activities variation in TB and TB-HIV co-infected patients at Jamot Hospital in Yaoundé-Cameroon. From April 2018 to May 2019, 336 treatment-naïve TB patients were enrolled. Liver enzymes (AST, ALT, ALP) and total bilirubin were evaluated at baseline and 12 weeks after treatment initiation. Blood was spotted on filter paper for DNA extraction by the chelex method. Standard nested PCR followed by restriction enzyme analysis with KpnI, TaqI, and BamHI to detect NAT2 polymorphisms was performed. TB-HIV co-infection prevalence was 29.46%. There was a significant rise of transaminases (p < 0.05) at baseline in TB-HIV co-infected patients. At 12 weeks, there was a substantial rise of transaminases in TB patients, and total bilirubin in TB-HIV co-infected patients (p < 0.05). The prevalence of slow and fast acetylators was 85.71 and 14.29%, respectively. NAT2*5/5 and NAT2*5/6 genotypes were most represented. Slow acetylating NAT2 phenotype was significantly associated with drug hepatotoxicity (p < 0.05). The prevalence of TB-HIV co-infections remains high, and the rise in transaminases is linked to the slow acetylating NAT2 phenotype.
Key words: TB/HIV co-infection, hepatotoxicity, NAT2, Jamot Hospital, Cameroon.
INTRODUCTION
Tuberculosis is an infectious and contagious disease caused by the mycobacteria of the Mycobacterium tuberculosis complex and the leading cause of morbidity and mortality in sub-Saharan Africa and worldwide, in people with HIV/AIDS. In 2018, 10 million people contracted the disease, and 1.2 million died. In addition, 2.48 million new cases were recorded in Africa, with 397,000 deaths (WHO, 2019). Tuberculosis also represents one of Cameroon's major threats to public health and a significant cause of preventable mortality in the adult population (NTCP, 2019). In 2018, 47,000 cases of tuberculosis were registered, with 7,700 cases of death (WHO, 2019). In 2019, 82% of tuberculosis patients were tested for HIV, with a prevalence of TB-HIV co-infection of 27% (NTCP, 2019).
Anti-tuberculosis therapy has always been an inducing toxicity factor due to the combination of Isoniazid, Rifampicin, and Pyrazinamide (NTCP, 2012). Similarly, a 14-fold increased risk of anti-TB hepatotoxicity has been reported in patients co-infected with HIV (NTCP, 2012). That implies HIV infection increases the risk of antiretroviral (ARV)-induced hepatotoxicity (Younossian et al., 2005; Sadaphal et al., 2001; Pan et al., 2005). Efavirenz is the first antiretroviral drug usually given in combination with rifampicin-based anti-TB treatment therapy in case of TB/HIV co infection (WHO, 2012). Though effective, this antiretroviral has been associated with liver injury (Abrescia et al., 2002) and is metabolized in the liver by many liver enzymes (Yimer et al., 2011). Rifampicin, proved as a potent inducer of these enzymes, is known to reduce plasma efavirenz concentrations. Therefore overlapping toxicities can complicate multidrug therapy and cause treatment failure, relapse or drug-resistance (Tostmann et al., 2008; Saukkonen et al., 2006; Wares et al., 2003). Although rifampicin and efavirenz are the principal molecules used in the case of concomitant TB and HIV therapy in low- income countries, data are still limited in high endemic HIV/AIDS and TB areas (Desta et al., 2007).
Moreover, N-acetyltransferase2 enzyme (NAT2) initiates the metabolism of isoniazid in the liver by biochemical acetylation, with variable rates depending on the genetic component of each individual (Mc Donagh et al., 2014). Despite considerable evidence demonstrating that the relationship between hepatotoxicity induction and NAT2 gene distribution differs according to ethnicity, there are few studies involving TB/HIV co-infected individuals in sub-Saharan Africa countries, as well as in Cameroon (Lee et al., 2010; An et al., 2012; Ben Mahmoud et al., 2012). This suggests that careful monitoring should be carried out in all patients after initiation of antiretroviral therapy to identify risk factors for better management of drug-induced hepatotoxicity and design prevention measures. Hence this study was undertaken to study seroprevalence of TB-HIV co-infection and hepatotoxicity during anti-TB treatment in people living with HIV/AIDS at Jamot Hospital in Yaoundé-Cameroon.
MATERIALS AND METHODS
Study design and population
This prospective study with descriptive aims was carried out at Jamot Hospital in Yaoundé from April 2018 to March 2019. The study population consisted of patients in consultation and observation in the Pneumatology Unit. A total of 336 pulmonary tuberculosis patients were enrolled in this study. The serum, obtained from the collected blood, was directly analyzed or stored at -20°C.
Inclusion and exclusion criteria
According to the defined inclusion criteria, participants in this study were aged 15 and more, regardless of gender ethnicity. In addition, only volunteers who agreed to sign an informed consent form after being informed on the nature, study procedure, potential benefits, and foreseeable risks were recruited, excluding patients with forms of tuberculosis other than pulmonary TB.
Data collection procedure and laboratory analyses
Peripheral venous blood samples (10 mL) were obtained from all studied patients at baseline and after 12 weeks of anti-TB and antiretroviral treatment. For confidentiality management, an anonymous identification code was assigned to each patient for laboratory analyses, data entry, and data analyses. Sputum samples were collected successively over three days after microscopic confirmation of positive cases. In parallel, blood samples were collected in dry tubes and EDTA tubes, in strict compliance with the conditions of asepsis. The serum was separated by centrifugation at 3000 (g) for 5 min, aliquots done, and stored at -20°C for subsequent analyses. Anti-HIV antibodies were determined using the whole blood by immuno-chromatography using ALERE Determine, and all positive cases were confirmed using Oraquick. An aliquot of the serum was then used to determine the serum enzymes activities of Alanine Aminotransferase (ALT), Aspartate Aminotransferase (AST), Alkaline Phosphatase (ALP) by kinetic method, and the total bilirubin using colorimetric method. The kinetic method for determining ALT and AST was based on the recommended International Federation of Clinical Chemistry (IFCC). Hepatotoxicity was defined as elevated aminotransferase levels and identified as three times higher than normal before initiating TB treatment, with associated symptoms considered jaundice, nausea, vomiting, dyspepsia, and asthenia. The reference values adopted were AST 37 UI/mL and ALT 40 UI/mL, ALP 92 UI/mL and total bilirubin 1
mg/dL) according to the manufacturer’s instructions. The biological variables potentially related to hepatotoxicity were analyzed, and the use of antiretroviral drugs at the time of initiating TB treatment was confirmed through medical records. The results of all the 336 tests for HIV antibodies conducted on the 173 PLWHA patients included in this study were positive. All patients who had initiated TB treatment due to clinical diagnosis or after a bacteriological test confirmation were said to have TB infection.
NAT2 polymorphism evaluation
The most common alleles in the African population were investigated. They contained the following mutations: C481T (rs1799929, silent mutation, amino acid change L161L), G590A (rs1799930, amino acid change R197Q), A803G (rs1208, amino acid change K268R) and G857A (rs1799931, amino acid change G286E). The primers used to amplify the gene was: NAT2 (+) 5'-GCCTCAGGTGCCTTGCATTT-3' and NAT2 (-) 5'-CGTGAGGGTAGAGAGGATAT-3'. The PCR amplification was carried out using a T3 thermal cycler (Biometra, UK), and performed in a total volume of 25 μl containing: nuclease-free water, 10× thermopol buffer, 10 mM dNTPs (200 μM of each deoxyribonucleotide), 20 pmol primers, 5 U/μL Taq polymerase, and 3 ng of gDNA. After initial denaturation at 95°C for the 5 min, 30 cycles of amplification was carried out with denaturation at 95°C for the 50 s, annealing at 55°C for the 50 s and extension at 72°C for the 50 s, followed by a final extension at 72°C for the 5 min. To confirm the presence of NAT2 alleles, PCR products were electrophoresed on a 2% agarose gel and polymorphisms determined by restriction endonuclease digestion of amplified gene fragments as previously described (Chen et al., 2007). The amplicons were digested under conditions stipulated for the restriction enzymes KpnI and BamHI (New England Biolabs, USA), followed by inactivation at 80°C for 20 min, a 2% agarose gel electrophoresis with Ethidium Bromide, and the analysis of the migration by UV trans-illumination.
Acetylator genotype classification
NAT2 acetylator genotypes were produced according to previously published data (Yokogawa et al., 2001). In this procedure, homozygotes (NAT2*4/NAT2*4) or heterozygotes (NAT2*4/NAT2*5, NAT2*4/NAT2*6, and NAT2*4/NAT2*7 combinations) for the dominant NAT2*4 wild type allele were classified as fast acetylator genotypes, while homozygotes of the mutant alleles (NAT2*5, NAT2*6 ,and NAT2*7) were classified as slow acetylator genotypes.
Data preparation and analysis
The sample size was calculated by the formula
n = z2×p(1-p)/m2
With p = 0.5, z = 1.96, m = margin of error (5%).
The data obtained were entered, cleaned, and analyzed using the statistical software for social sciences (SPSS) version 22.1. The means, frequencies, and percentages were used to summarize the descriptive statistics of the data. The chi-square test (c2) was used to assess the relationships between the qualitative variables, namely sex, marital status, level of education, occupation. Values ??of P ≤ 0.05 were considered to be statistically significant.
Ethical considerations
Ethical approval for this study was obtained from the Regional Ethics Committee of the Centre Region, Cameroon N°: 2018/01/970/CE/SP. An authorization from the “Jamot Hospital in Yaoundé” collection site (N°:00001478/L/MINSANTE/SG/DHJY) was also obtained. All participants were fully informed about the objectives, procedures, potential risks, benefits, and purpose of the study. The included study population gave their signed informed consent form (provided in French and English languages).
RESULTS
Socio-demographic characteristics of the subjects of study
A total of 336 newly diagnosed pulmonary tuberculosis patients were recruited prospectively in this study, and they were followed up to 12 weeks. Baseline demographic and clinical data, plus laboratory results of the 336 patients at 12 weeks, were used for analyses. ALT, AST, PAL, and bilirubin levels were evaluated at baseline and 12 weeks. Of the total TB patients, 215 (63.98%) were men, while 121 (36.01%) were women, with a male to female ratio of 1.77:1. The average age of the patients was (35.16 ± 14.04 years), the minimum was 15 years, and the maximum was 94 years. Regarding marital status and level of education, 222 (66.07%) were single, 94 (27.97%) were married, 14 (4.16%) were widowed, and 6 (1.78%) were divorced, while 17 (5.05%) were out of school, 77 (22.91%) had a primary level, 194 (57.73%) had a secondary level, and 48 (14.28%) had a university level. Regarding the HIV status of the study patients, 99 (29.46%) tested positive for HIV. Table 1 presents the various socio-demographic characteristics in a grouped manner.
Biochemical parameters
Table 2 presents the values ??of liver enzymes activity in TB and TB-HIV patients. It appears that in TB-HIV co-infected patients, there is a significant rise in Alanine Aminotransferase (ALT: 28.97±2.98) (p = 0.01) and Aspartate Aminotransferase (AST: 67.98±5.75) (p= 0.008).
To assess the variation in liver enzymes and the impact of anti-tuberculosis treatment on hepatotoxicity, two samples were taken on Day 0 before the anti-TB treatment and three months after initiation. The results of Table 3 show that in TB mono-infected patients, there is a significant rise in ALT (32.2 ± 3.56) (p=0.01) and AST (64.67 ± 5.23) (p=0.008), three months after treatment initiation, compared to the value on Day 0. While in TB-HIV co-infected patients, there is a significant rise in Alkaline Phosphatase (ALP) (143.8±8.75; p= 0.001) and total bilirubin (1.44±0.27; p=0.018), but an insignificant increase in ALT.
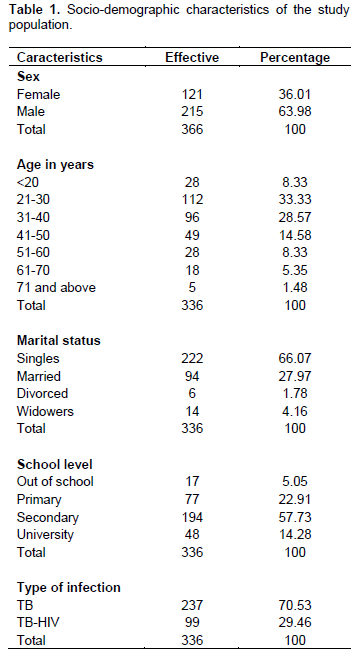
Genetic polymorphism of NAT2 and association with hepatotoxicity
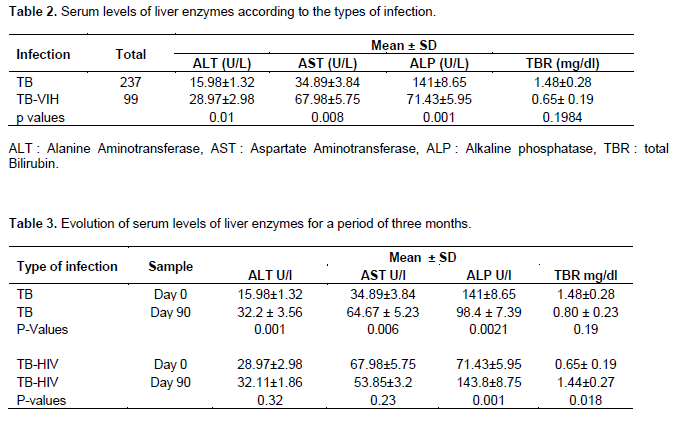
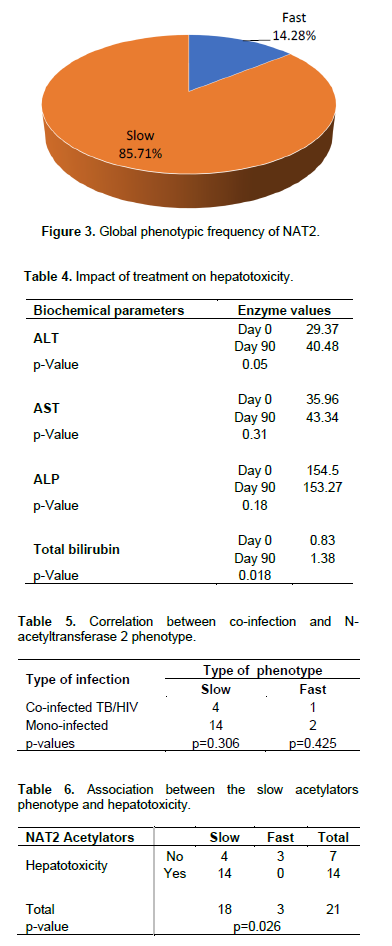
In this study, a total of 7 genotypes were obtained with variable proportions NAT2*5/5 (47.61%), NAT2*5/6 (19.04%), NAT2*5/7 (14.28%), NAT2*4/6 (4.76%), NAT2*4/7 (4.76%), NAT2*4/5 (4.76%), NAT2*6/7 (4.71%), with a predominance of four alleles: NAT2*5 (66.66%), NAT2*6 (14.28%), NAT2*7 (11.98%), and NAT2*4 (7.14%). And the results of phenotypes, genotypes and alleles are represented by Figures 1 to 3: NAT2*5/5 fast phenotype frequency 14.28%, and slow phenotype frequency 85.71%.
Impact of anti-tuberculosis treatment in tuberculosis patients and co-infected TB-HIV and variation in liver enzymes
In Table 4, liver enzyme values after three months of initiation of anti-tuberculosis treatment show variations in
ALT (from 29.37 to 40.48 UI), AST (from 35.96 to 43.34 UI), and total bilirubin (from 0.83 to 1.38).
Association between hepatotoxicity and NAT2 polymorphism
In this study, 21 patients were genotyped. Among these, 5 were co-infection TB/HIV cases and 16 cases of mono-infection TB. Unfortunately, no statistically significant difference was found between acetylation phenotype and co-infection, as shown in Table 5.
The results of Table 6 have shown a significant association (p=.026) between the slow acetylators phenotype and the hepatotoxicity in mono-infected TB patients and TB/HIV co-infected patients.
DISCUSSION
Hepatotoxicity is reported frequently as an adverse reaction during the treatment of tuberculosis. This study aimed to study the seroprevalence of TB-HIV co-infection and hepatotoxicity during anti-TB treatment in people living with HIV/AIDS at Jamot Hospital in Yaoundé- Cameroon.
This study showed that TB/HIV co-infection prevalence was high (29.46%) (Table 1). This prevalence is higher than those reported by Assam-Assam et al. (2011) and Sidze et al. (2014) in Cameroon, which was 20.77 and 28.5%, respectively, but remains lower than that reported by Sama et al. (2017), which was 32.8%. This high prevalence was linked to Cameroon's being classified as a ??highly endemic area for TB, with the national prevalence of TB-HIV co-infection of 27% (NTCP, 2019). Previous studies have shown that HIV+ patients have an increased risk of TB infection (Havlir and Barnes, 1999; Colebunders and Lambert, 2002). The cellular immunosuppression induced by HIV infection prepares the entry of Mycobacterium tuberculosis and is a factor of TB recrudescence (Gollub et al., 1997). TB-HIV co- infected subjects are 20 to 30 times more likely to develop active TB than HIV-negative subjects (WHO, 2018).
Also, the obtained prevalence value of TB-HIV co-infection was slightly high among men (15.18%) compared to women (14.28%), more common in the 21 to 49 age group, representing 82.82% of co-infected cases, and also more prevalent among singles and married people as well, with 88.88% of co-infected cases. Similar results have been reported by other authors (Sama et al., 2017) and can be explained that TB is the main opportunistic disease in HIV-positive people. Moreover, in the Demographic and Health Survey reports and Multiple Indicators, Cameroon's most sexually active age group is between 15 and 49 years (Demographic and Health Survey and Multiple Indicators, 2011).
Analysis of the assay data showed a significant rise in serum transaminase activity (ALT/AST, p = 0.005) in TB-HIV coinfected patients. These results agree with the work of Sama et al. (2017) conducted in a TB-HIV coinfected population in the West region of Cameroon and show a rise in the levels of the transaminases. The high level of ALT and AST transaminases obtained in the present study could be explained by the percentage of HIV-positive patients in the study population (29.46%), already under ARV treatment for most of them at the start of the study. Indeed, HIV has been shown to directly attack liver cells (Oluwafemi et al., 2003) and cause cell death. The release in the environment of cell contents made of 20% of enzymes (Wild-Up et al., 1990) can be responsible for the significant increase in liver enzymes in infected patients confirmed by several studies in ARV-naive patients. In addition, tuberculosis, which accelerates morbidity and mortality in HIV-positive people, can lead to the degeneration of the liver connective tissue (Margulis et al., 1986) and result from hepatobiliary obstruction occurring in these patients. This high level of transaminase might also be due to the difficulties in accessing treatment in developing countries where most of the population barely visits hospitals for treatment and use drugs from unknown origin, which can lead to severe hepatotoxicity and even cirrhosis.
The results of the second sampling from patients infected by tuberculosis and co-infected with TB-HIV after three months showed a significant increase in ALP (143.8±8.75UI) and total bilirubin (1.44±0.27 mg/dl) in TB-HIV co-infected patients (Table 4). This high rise in ALP and total bilirubin could be due to cumulative hepatotoxicity arising from the combined anti-tuberculosis (RHEZ) and antiretroviral therapies the patients took during the three-month treatment period. In addition, cotrimoxazole (Yang et al., 2014) and Isoniazid (Raghu and Karthikeyan, 2016) have been shown to induce liver damages resulting in raised levels of these liver enzymes. On the other hand, in TB patients, a significant rise of ALT (32.2 ± 3.56 UI) and AST (67 ± 5.23 UI) transaminases was noted after three months of the start of Anti-TB treatment, confirmed in other published studies (Van Hest et al., 2004; Schechter et al., 2006). In addition, anti-tuberculosis therapy in treating tuberculosis has the problem of hepatotoxicity resulting from the combination of these anti-tuberculosis drugs (Isoniazid, Rifampicin, Pyrazinamide) (Schechter et al., 2006).
Indeed, the information gathered on the distribution of the genetic polymorphism of genes that encode N- acetyltransferase2 (NAT2) in populations is essential for understanding the inter-individual differences in drug metabolism and the risk of developing side effects. In this study, seven genotypes were identified, and among them, NAT2*5/5 (47.61%), NAT2*5/6 (19.04%), and NAT2*5/7 (14.28%) were predominant. These results do not corroborate those published by Kengne et al. (2016) in a study in children under ten years of age, having malaria and receiving antimalarial treatment, in the North-West and South-West regions of Cameroon. In this previous study, the following genotypes were described: fast acetylators: NAT2*4/4; slow acetylators: NAT2*5/5, NAT2*6/6, NAT2*5/6, NAT2*5/7, NAT2*6/7, and intermediate acetylators: NAT2*4/5, NAT2*4/6, NAT2*4/7. The difference observed with the results of our study could be linked to the disease (malaria instead of TB) and the small size of the study population. Our findings indicated 85.71% of slow metabolizers and 14.28% of fast acetylators, as mentioned in the previous study (Kegne et al., 2016; Achonduh et al., 2013). Similarly, previous work done in Africa and around the world on tuberculosis patients or TB/HIV co-infected individuals has also reported high frequencies of slow metabolizers (Soukaina et al., 2014, 2016; Mariz et al., 2020). Indeed, the main side effects of anti-tuberculosis drugs are ocular toxicity, hypersensitivity reactions, and liver toxicity. These are the most severe side effect of the adverse effects of anti-TB medications due to isoniazid in particular (Tostmann et al., 2008). Factors that initiated this hepatotoxicity include metabolizer phenotype, anti-tuberculosis drug doses, patient's nutritional status, and disease severity. The relationship between the genetic factors and hepatotoxicity induced by anti-TB drugs has been published already (Soukaina et al., 2016; Hyun et al., 2007; Sun et al., 2008). The TB/HIV co-infected patients on anti-TB treatment and characterized by a slow acetylation phenotype were significantly more likely to develop hepatotoxicity (p <0.05) in a previous study (Mariz et al., 2020). One of the reasons could be their capacity to slowly remove drugs from the body that can lead to drug persistence and liver toxicity. Likewise, some authors suggested the role of translational or post-translational defects in the NAT2 enzyme degradation acceleration (Blum et al., 1991), inducing an accumulation of toxic metabolites and free radicals generation. Our results showed that slow acetylators are the most frequent phenotype among TB patients who have developed high liver toxicity (p= 0.026). It provides an existing correlation between slow acetylation and anti-TB drugs induced hepatotoxicity among the study participants. These results agree with other researches carried out by several authors (Khalili et al., 2011; Leiro-Fernandez et al., 2011; Ben-Fredji et al., 2016). However, it should be noted that this study has some limitations on the size of the samples used for this NAT2 genetic polymorphism evaluation that should be extended in the future. In addition, assessing the CYP450 enzymes involved in the biotransformation of drugs might be essential to draw more valuable conclusions.
CONCLUSION
Finally, the present study has demonstrated that providing more significant support for TB patients living with HIV/AIDS undergoing anti-TB treatment and antiretrovirals may well contribute to more excellent management and control of the drugs used, especially those TB patients co-infected or not, with genotypes NAT2*5/5 (47.61%), NAT2*5/6 (19.04%) and NAT2*5/7 (14.28 %). Thus, we can say that NAT2*5/5 (47.61%) and NAT2*5/6 (19.04%) variant alleles are the main risk factors for developing hepatotoxicity. TB patients having these genotypes present a higher chance of developing liver toxicity during anti-TB treatment. These findings demonstrate that the NAT2 phenotype may also be a good indicator for assessing adverse events due to anti-TBs drugs or in combination with antiretrovirals. Further studies with different populations and larger sample sizes are required to verify the validity of these findings. Values from control individuals not having TB or HIV might bring interesting complementary information for a better understanding and use of liver enzymes activities variation as indicators of toxicity prevention in these populations.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
ACKNOWLEDGEMENTS
The authors greatly thank EDCTP (European and Developing Clinical Trials Partnership) for having financially supported the realization of this work through CANTAM (Central Africa Network for Tuberculosis, AIDS/HIV and Malaria) Network and its Coordinator Prof Francine Ntoumi. The authors also thank Prof. Assam Assam Jean-Paul, Associate Professor in Microbiology and research scientist at the LTR Laboratory (BTC/UYI), for all the scientific and technical support and fieldwork supervision; Dr. Cedric Tchinda Fossi (LTR, BTC, UYI) and Mrs. Augusta Tsasse for their technical assistance in the laboratory investigations. In addition, all the study participants, counselors, and health staff from the Jamot Hospital and Day Care of the Central Hospital in Yaoundé are appreciated for their technical support in carrying out this research work. Finally, sincere thanks go to the National Tuberculosis Control Program (NTCP).
REFERENCES
|
Abrescia N, D'Abbraccio M, Figoni M, Busto A, Butrico E, Marco MD, Viglietti R (2002). Fulminant hepatic failure after the start of an efavirenz-based HAART regimen in a treatment-naive female AIDS patient without hepatitis virus co-infection. Journal Antimicrobial Chemotherapy 50(5):763-765. |
|
|
Achonduh OA, AtoghoTB, Mbulli IA, Chedjou JP, Achu M, Nji AM, Fokou E, Kamgue E, Veyee V, Karana O, Sahfe D, Mbacham WF (2013). Adverse events clustering with NAT2 slow metabolisers following deparasitization in children in Bangolan, NWR Cameroon. Journal of Life Science 7(7):742-748. |
|
|
An HR, Wu XQ, Wang ZY, Zhang JX, Liang Y (2012). NAT2 and CYP2E1 polymorphisms associated with antituberculosis drug-induced hepatotoxicity in Chinese patients. Clinical and Experimental Pharmacology and Physiology 39(6):535-543. |
|
|
Assam-Assam JP, Beng VP, Cho-Ngwa F, Tedom JC, Anyangwe AI, Titanji VPK (2011). Mycobacterium tuberculosis complex drug resistance pattern and identification of species causing tuberculosis in the West and Center regions of Cameroon. BMC Infectious Diseases 11(1):1-6. |
|
|
Ben Mahmoud L, Ghozzi H, Kamoun A, Hakim A, Hachicha H, Hammami S, Sahoung Z, Zalila N, Makmih H, Zeghal K (2012). Polymorphism of the N-acetyltransferase 2 gene as a susceptibility risk factor for antituberculosis drug-induced hepatotoxicity 364 in Tunisian patients with tuberculosis. Pathologie Biologie 60(5):324-330. |
|
|
Ben-Fredj N, Gam R, Kerkni E, Chaabane A, Chadly Z, Boughattas N, Aouam K (2016). Risk factors of isoniazid-induced hepatotoxicity in Tunisian tuberculosis patients. Pharmacogenomics Journal 17(4):372-377. |
|
|
Blum M, Demierre A, Grant DM, Heim M, Meyer UA (1991). Molecular mechanism of slow acetylation of drugs and carcinogens in humans. Proceedings of the National Academy of Sciences of the United States of America 88(12):5237-5241. |
|
|
Colebunders R, Lambert ML (2002). Management of co-infection with HIV and TB: improving tuberculosis control programmes and access to highly active antiretroviral treatment is crucial. British Medical Journal 342(7341):802-803. |
|
|
Demographic and Health Survey and with Multiple Indicators (2011). |
|
|
Desta Z, Saussele T, Ward B, Blievernicht J, Lang L, Klein K, Flockhart D, Zanger UM (2007). Impact of CYP2B6 polymorphism on hepatic efavirenz metabolism in vitro. Pharmacogenomics 8(6):547-558. |
|
|
Gollub EL, Trino R, Salmon M, Moore L, Dean JL, Davidson BL (1997). Co-occurrence of AIDS and tuberculosis: results of a database "match" and investigation. Journal of Acquired Immune Deficiency Syndromes 16(1):44-49. |
|
|
Havlir DV, Barnes PF (1999). Tuberculosis in Patients with Human Immunodeficiency Virus Infection. New England Journal of Medicine 340:367-373. |
|
|
Hyun JC, Won JK, Yon JR, Chang SK, Myung HN, Jong WK, Soo Y (2007). Genetic polymorphisms of NAT2 and CYP2E1 associated with antituberculosis drug-induced hepatotoxicity in Korean patients with pulmonary tuberculosis. Tuberculosis 87(6):551-556. |
|
|
Kengne CJP, Akindeh MN, Innocent MA, Achonduh OA, Ekollo MA, Mbacham WF (2016). Predominance of N-acetyl transferase 2 slow acetylator alleles in children less than ten years experiencing adverse treatment events following artemisinin-based combination therapy in North and South West Regions of Cameroon. African Journal of Biotechnology 15(25):1285-1291. |
|
|
Khalili H, Fouladdel S, Sistanizad M, Hajiabdolbaghi M, Azizi E (2011). Association of N-Acetyltransferase-2 Genotypes and Anti-Tuberculosis Induced Liver Injury; First Case-Controlled Study from Iran. Current Drug Safety 6(1):17-22. |
|
|
Lee SW, Chung LS, Huang HH, Chuang TY, Liou YH, Wu LS (2010). NAT2 and CYP 2E1 polymorphisms and susceptibility to first-line anti-tuberculosis drug-induced hepatitis. International Journal of Tuberculosis and Lung Diseases 14(5):622-626. |
|
|
Leiro-Fernandez V, Valverde DX, Vázquez-Gallardo K, Botana-Rial M, Constenla L, Agúndez JA, Fernández-Villar A (2011). N-acetyltransferase 2 polymorphisms and risk of anti-tuberculosis drug-induced hepatotoxicity in Caucasians. International Journal of Tuberculosis and Lung Diseases 15(10):1403-1408. |
|
|
Margulis SJ, Honig CL, Soave R, Govoni AF, Mouradian JA (1986). Biliary Tract Obstruction in the Acquired Immunodeficiency Syndrome. Annals of Internal Medicine 105(2):207-210. |
|
|
Mariz CA, Albuquerque MFPM, Lopes EP, Ximenes RAA, Lacerda HA, Filho DBM, Martins BBC, Pastor AFP, Santos BA (2020). Hepatotoxicity during TB treatment in people with HIV/AIDS related to NAT2 polymorphisms in Pernambuco, Northeast Brazil. Annals of Hepatology 19(2):153-160. |
|
|
Mc Donagh EM, Boukouvala S, Aklillu E, Hein DW, Altman RB, Klein TE (2014). PharmGKB summary: very important pharmacogene information for N acetyltransferase 2. Pharmacogenetics and Genomics 24(8):409-425. |
|
|
NTCP (2012). Epidemiological situation of tuberculosis in Cameroon. |
|
|
NTCP (2019). Epidemiological situation of tuberculosis in Cameroon. |
|
|
Oluwafemi O, Oguntibeju B, Olatubosan B (2003). A Study on the Activities of Liver Enzymes in HIV/ AIDS Patients. Journal of Medicine and Science 3:106-109. |
|
|
Pan L, Jia ZS, Chen L, Fu EQ, Li GY (2005). Effect of anti-tuberculosis therapy on liver function of pulmonary tuberculosis patients infected with hepatitis B virus. World Journal of Gastroenterology 11(16):2518-2521. |
|
|
Raghu R, Karthikeyan S (2016). Zidovudine and isoniazid induced liver toxicity and oxidative stress: evaluation of mitigating properties of silibinin. Environmental Toxicology and Pharmacology 46:217-226. |
|
|
Sadaphal JP, Astemborski NM, Graham, Sheely L, Bonds M, Madison A, Vlahov D, Thomas DL, Sterling TR (2001). Isoniazid preventive therapy, hepatitis C virus infection, and hepatotoxicity among injection drug users infected with Mycobacterium tuberculosis. Clinical Infectious Diseases 33(10):1687-1691. |
|
|
Sama LF, Nganou-Djinou OI, Wam EC, Bamou R, Ali IM, Noubom M, Tume CB (2017). Sero-prevalence of Hepatitis B and C virus and High Risk of Hepatotoxicity among TB / HIV Positive and HIV Negative Population in Western Cameroon. Global Journal of Infectious Diseases 3(1):001-008. |
|
|
Saukkonen JJ, Cohn DL, Jasmer RM, Schenker S, Jereb JA, Nolan CM, Peloquin CA, Gordin PM, Nunes D, Strader DB, Bernardo J, Venkataramanan R, Sterling TR (2006). An official ATS statement: hepatotoxicity of antituberculosis therapy. American Journal of Respiratory and Critical Care Medicine 174(8):935-952. |
|
|
Schechter M, Zajdenverg R, Falco G, Barnes GL, Faulhaber JC, Coberly JS, Moore RD, Chaisson RE (2006). Weekly rifapentine / isoniazid or daily rifampin / pyrazinamide for latent tuberculosis in household contacts. American Journal of Respiratory and Critical Care Medicine 173(8):922-926. |
|
|
Sidze LK, Mouafo TE, Kuaban C, Assam Assam JP, Tedom JC, Eyangoh S, Fouda FX, Nolna D, Ntoumi F, Frank M, Beng VNP (2014). Strong Decrease in Streptomycin-Resistance and Absence of XDR 12 Years after the Reorganization of the National Tuberculosis Control Program in the Central Region of Cameroon. Plos One 9(6):e98374. |
|
|
Soukaina G, Ilham R, Fatima ZL, Siham CE, Imane CJ, Amina B, Abdelaziz S (2014). Distribution of allelic and genotypic frequencies of NAT2and CYP2E1variants in Moroccan population. BMC Genetics 15(1):1-6. |
|
|
Soukaina G, Ilham R, Omaima El Bouazzi, Sanaa H, Amina T, Jamal EB, Rachida SB, and Abdelaziz S (2016). NAT2 Genotypes in Moroccan Patients with Hepatotoxicity Due to Antituberculosis Drugs. Genetic Testing and Molecular Biomarkers 20(11):680-684. |
|
|
Sun F, Chen Y, Xiang Y, Zhan S (2008). Drug-metabolising enzyme polymorphisms and predisposition to anti-tuberculosis drug-induced liver injury: a meta-analysis. International Journal of Tuberculosis and Lung Diseases. 12(9):994-1002. |
|
|
Tostmann A, Boeree MJ, Aarnoutse RE, de Lange WC, van der Ven AJ, Dekhuijzen R (2008). Antituberculosis drug-induced hepatotoxicity: concise up -to-date review. Journal of Gastroenterology and Hepatology 23(2):192-202. |
|
|
Van Hest R, Baars H, Kik S, van Gerven P, Trompenaars MC, Kalisvaard N, Keizer S, Borgdorff M, Mensen M, Cobelens F (2004). Hepatotoxicity of rifampin-pyrazinamide and isoniazid preventive therapy and tuberculosis treatment. Clinical Infectious Diseases 39(4):488-496. |
|
|
Wares DF, Singh S, Acharya AK, Dangi R (2003). Non-adherence to tuberculosis treatment in the eastern Tarai of Nepal. International Journal of Tuberculosis and Lung Disease 7(4):327-335. |
|
|
Wild-Up M, Fortuin F, Whittle DHC, Hall AJ, Wolf CR, Montesonai R (1990). Liver and hepatitis B virus in Gambian children. Cancer Epidemiology, Biomarkers and Preventions 2:555-561. |
|
|
World Health Organization (2012). Library Cataloguing-in-Publication Data: Treatment of tuberculosis: guidelines - 4th edition. (accessed 28 May 2012).WHO/HTM/TB/2009.420. |
|
|
World Health Organization (2018). Global tuberculosis report 2018. Geneva (Switzerland). |
|
|
World Health Organization (2019). Global tuberculosis report 2019. Geneva (Switzerland). |
|
|
Yang JJ, Huang CH, Liu CE, Tang HJ, Yang CJ, Lee YC, Lee KY, Tsai MS, Lin SW, Chen YH, Lu PL, Hung CC (2014). Multicenter study of trimethoprim/sulfamethoxazole related hepatotoxicity: incidence and associated factors among HIV-infected patients treated for Pneumocystis jirovecii pneumonia. PloS One 9(9):e106141. |
|
|
Yimer G, Ueda N, Habtewold A, Amogne W, Suda A, Kiedel KD, Burhenne J, Aderaye G, Lindquist L, Mkonnen E, Aklillu E (2011). Pharmacogenetic and Pharmacokinetic Biomarker for Efavirenz Based ARV and Rifampicin Based Anti-TB Drug Induced Liver Injury in TB-HIV Infected Patients. PloS One 6(12):e27810. |
|
|
Younossian AB, Rochat T, Ketterer JP, Wacker J, Janssens JP (2005). High hepatotoxicity of pyrazinamide and ethambutolfor treatment of latent tuberculosis. European Respiratory Journal 26(3):462-464. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0