Full Length Research Paper
ABSTRACT
Tripsacum laxum Nash (Guatemala grass) is widely used globally as a forage crop. Its strong roots and long period of growth and utilization allow it to be harvested for many years, and given its high yield, high nutritional value, and good taste. Here the peduncles of T. laxum Nash were used as explants to induce shoots and then efficient shoot proliferation and regeneration system were established for the first time. Multiple shoots were proliferated on Murashige and Skoog (MS) medium to establish, for the first time, an efficient shoot proliferation and plant regeneration systems. Optimal shoot proliferation medium was MS with 3.0 mg/L 6-benzyladenine (BA) and 0.2 mg/L α-naphthaleneacetic acid (NAA), resulting in a shoot proliferation coefficient of 11.0 within 45 days. Optimal rooting medium was MS with 0.1 mg/L NAA and/or 0.1 mg/L indole-3-butyric acid (IBA), inducing 100% root formation from shoots within 30 days. The in vitro young roots, leaf sheaths and shoot bases were also used as explants, to induced embryogenic callus. The results showed that MS medium with 1.0 mg/L thidiazuron (TDZ) and 0.2 mg/L BA induced most shoots, with the least callus. Shoot bases induced beige-white callus and shoots directly on MS medium with 1.0 mg/L TDZ and 0.2 mg/L 2,4-dichlorophenoxyacetic acid (2,4-D), while leaf sheaths induced beige-white callus and shoots directly on MS medium with 1.0 mg/L TDZ and 0.2 mg/L BA. The rooted plantlets showed 99.3% survival when transplanted into a substrate of vermiculite: peat soil (1:3, v/v).
Key words: Tripsacum laxum, axillary shoots, callus, adventitious shoots, rooting, regeneration.
INTRODUCTION
The genus Tripsacum (Maydeae tribe, Panicoideae, Gramineae) includes 16 species that grow in many ecologically distinct niches and habitats that are typically distributed in tropical and subtropical regions (Gray, 1974; Wet et al., 1985). Since Tripsacum has a common ancestor with maize and teosinte, it may be important to better understand the origin and evolution of maize. Tripsacum is a perennial warm-season C4 type of grass that is often used to produce high-quality forage and biomass energy, and control soil erosion (Zhao et al., 2020). Tripsacum laxum Nash (Guatemala grass) is widely used globally as a forage crop (Munyasi et al., 2015; Maleko et al., 2018; Klapwijk et al., 2020). Its strong roots and long period of growth and utilization allow it to be harvested for many years, and given its high yield, high nutritional value, and good taste, it is suitable for cutting green feed or process into silage material, and can thus be used as feed for cattle, ducks, geese and pigs in the limestone soils and a seasonally dry habitat (Wilkes, 1972; Boonman, 1993; Boschini-Figueroa and Vargas-Rodríguez, 2018). The roots of T. laxum develop well, and when tilled into soil and used as organic matter, this improves the physical and chemical structure of the soil, so it is often used as a multi-year cover crop (Shem et al., 1995). After T. laxum was introduced to China, it is now a major source of forage feed (Jiang et al., 2002; Zhong et al., 2011). Recently, it has been completed in chloroplast genome and evolutionary relationship analysis showed that it is more closely related to Tripsacum dactyloides (Luo et al., 2021).
The chromosome number of T. laxum is 2n = 72 (Dodds and Simmonds, 1946; Zhong et al., 2011). Although most chromosomes are bivalents, there are multiple chromosomal irregularities, ultimately resulting in male sterility (Dodds and Simmonds, 1946). T. laxum is rarely propagated by stem cuttings or rhizomes (Guyadeen, 1951; Wilkes, 1972). However, the stems tend to shrink and are prone to bacterial infections (Tuley, 1961; Schieber, 1975; Asudi et al., 2015). To resolve limitations associated with proliferation and to overcome disease-related problems, the establishment of an in vitro regeneration system would allow this plant to be mass propagated and to create a platform that would allow for its genetic improvement through transgenic strategies. To our knowledge, there are no studies on the tissue culture or related biotechnologies of T. laxum. In this study, for the first time, the young peduncles of T. laxum was employed as explants to induce shoots that were then proliferated to establish an efficient in vitro regeneration system.
MATERIALS AND METHODS
Establishment of in vitro tissue culture
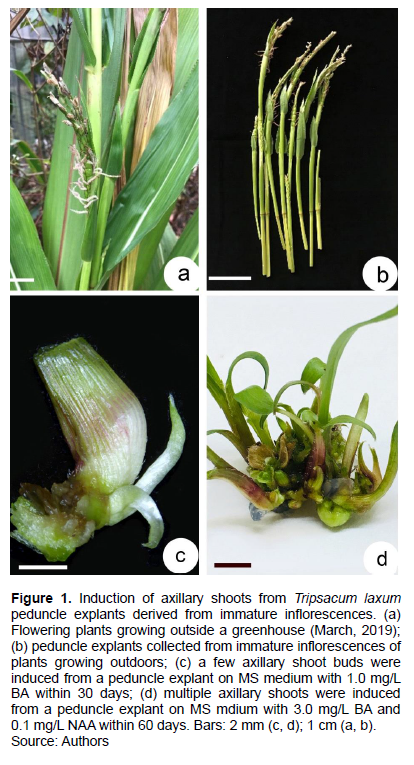
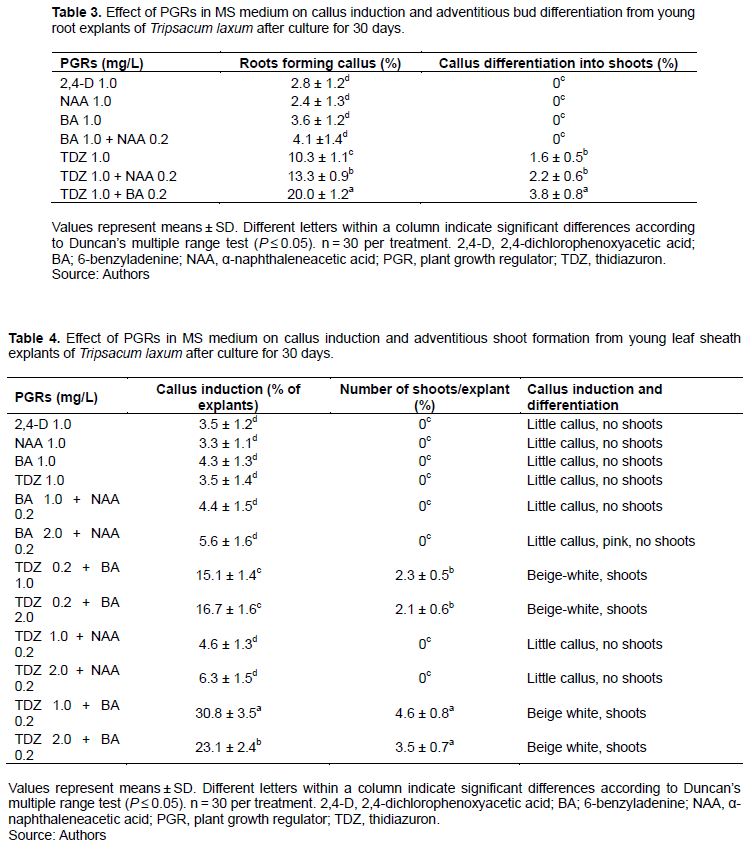
T. laxum plants growing on a farm in Guigang city, Guangxi province were brought back to Guangzhou in 2010. All the studies comply with relevant institutional, national, and international guidelines and legislation. It has been specified under the appropriate permissions and licenses for the collection of plant specimens. Plants were propagated by cutting and grown in a test field of South China Botanical Garden, Guangzhou, Guangdong Province. The plants flowered every year but no seed were produced (Figure 1a). Stems were cut into 30 cm long cuttings, planted in a field and allowed to grow naturally. Plants were identified by Dr. Qing Liu, a botanist in South China Botanical Garden. When the plants began to flower, between March and April of 2016, young inflorescences of T. laxum were removed with a surgical knife (Figure 1b). The peduncles segments (5 cm long) were the first surface disinfected with 75% ethanol using cotton balls, dipped into 0.1% (w/v) mercuric chloride solution (HgCl2) for 10 min, then washed three times with sterile distilled water. Surface-disinfected explants (2-3 cm long peduncles) were inoculated into Murashige and Skoog (MS) basal medium (Murashige and Skoog, 1962) containing 1.0 mg/L 6-benzyladenine (BA) and 30 g/L sucrose. Medium pH was adjusted to 6.0 before being solidified with 0.7% (w/v) agar (Sigma-Aldrich, St. Louis, MO, USA), then autoclaved at 121°C for 20 min. Culture jars (height = 10 cm; diameter = 8 cm) were placed in an air-conditioned culture room at 25 ± 2°C with a 12-h photoperiod and 100 μM m−2 s−1 fluorescent light (Philips, Tianjin, China). Tissue culture conditions were identical to those used for another grass Lepturus repens (Xiong et al., 2021). After 15 days in culture, some axillary shoots buds (Figure 1c) were induced from peduncle internodes. Axillary shoots were subcultured on the same medium every 45 days. When sufficient stock was proliferated, experiments were initiated.
Effects of plant growth regulators on axillary shoot proliferation
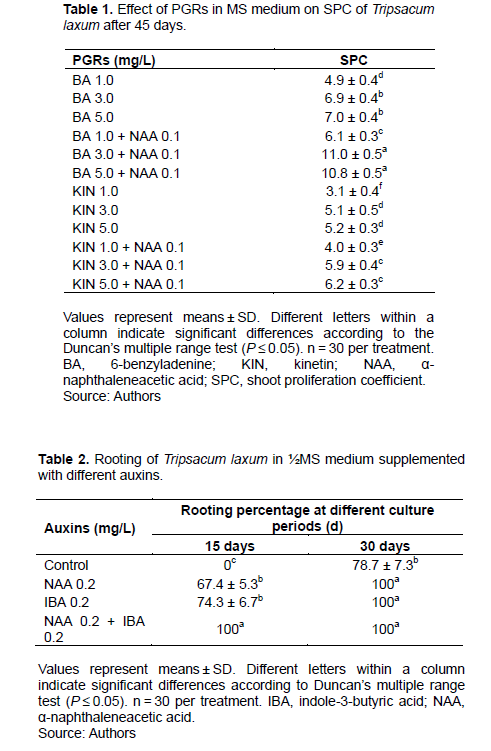
Using a similar technique as was employed for Scaevola sericea (Liang et al., 2020), axillary shoot clusters were cut into smaller clusters, each with three shoots. These were “inoculated onto MS medium containing different combinations and concentrations of plant growth regulators (PGRs) for axillary shoot proliferation (Table 1). For each treatment, 10 jars were used. Each jar contained three shoot clusters. After culture for 45 days, axillary shoot proliferation coefficient (SPC) was assessed as: number of axillary shoots after proliferation for 45 days/number of axillary shoots before proliferation” (Liang et al., 2020).
Adventitious root formation
Adventitious root formation used a similar method as was employed for S. sericea (Liang et al., 2020). Axillary shoots were separated and cultured on rooting medium (½MS) “supplemented with different concentrations and combinations of indole-3-butyric acid (IBA) and α-naphthaleneacetic acid (NAA) (Table 2). In each treatment, 10 jars were inoculated and each jar contained three shoots. PGR-free ½MS medium was used as the control. After 15 and 30 days of culture, rooting percentage was observed and assessed, as follows (Liang et al., 2020):
(number of buds that rooted after 30 days/number of inoculated buds) × 100%.
Effects of plant growth regulators on callus induction from three explant types
Young roots, young leaf sheaths and shoot bases were used as explants. Roots were derived from 15 day-old plantlets that had been rooted in ½MS medium with 0.1 mg/L NAA. Roots were cut into 1.0 cm long explants. The young leaf sheaths and shoot bases were derived from shoots that had been proliferated on MS medium with 1.0 mg/L BA for 45 days. These tissues were cut into explants 0.5 cm2 in size and inoculated onto MS-based media with different plant growth regulators (PGRs) to induce callus and observe differentiation after 30 days (Tables 3 to 5).
Acclimatization and transplantation
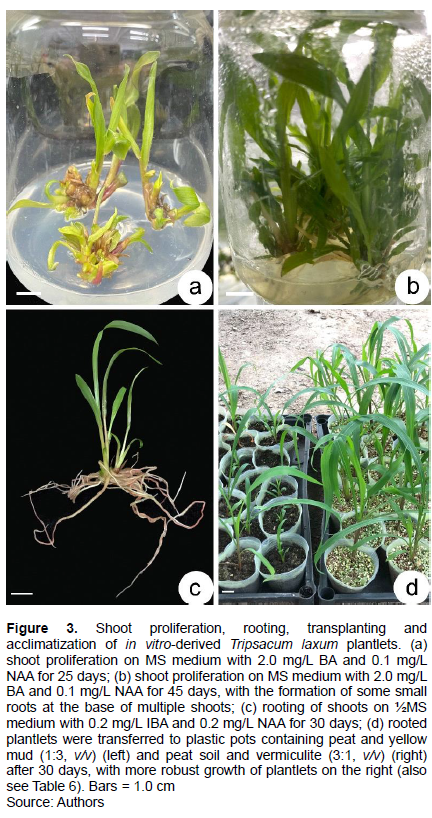
An acclimatization protocol, similar to that which was used for S. sericea (Liang et al., 2020), was employed. Culture jars with shoots that were rooted in ½MS medium with 1.0 mg/L IBA for 30 days were transferred to natural light for 7 days. Using tap water, agar was gently rinsed off roots. Rooted plantlets were transplanted into plastic pots (height and diameter = 10 cm) containing yellow mud and peat soil (1:1, v/v), or peat and vermiculite (3:1, v/v). A single plantlet was planted in each plastic pot, and each treatment had 30 plantlets. Plants were watered every morning with tap water. After 30 days, plantlet height was determined. Survival percentage of transplanted plantlets was assessed as (Liang et al., 2020):
(number of living plantlets before transplanting / number of living plantlets after transplanting for 30 d) × 100%.
Statistical analyses
All experiments were repeated three times within one week. Data are reported as mean ± standard deviation (SD). Means were statistically analyzed by one-way analysis of variance (ANOVA). Treatment means were considered to be significantly different from controls after applying Duncan’s multiple range test (P ≤ 0.05) using SPSS v. 19.0 (IBM, New York, NY, USA).
RESULTS
Shoot proliferation on different media
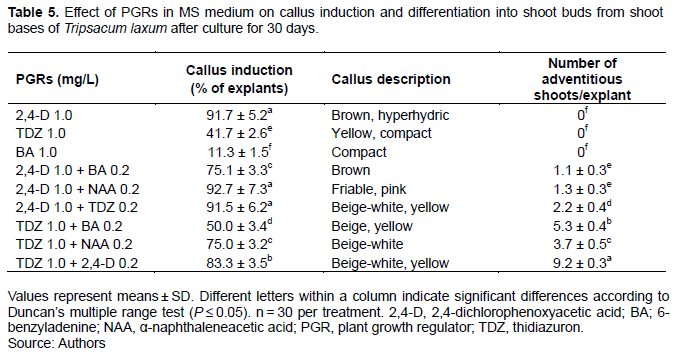
BA induced shoots more effectively than KIN, as assessed by SPC, but not when its concentration exceeded 3.0 mg/L (Table 1). When 6-benzyladenine (BA) was supplemented with 0.2 mg/L NAA, axillary shoot number increased significantly (Table 1 and Figure 1d), with 3.0 mg/L BA and 0.2 mg/L NAA assessed as the optimal medium for shoot proliferation (Figure 3a). When culture period was extended to 45 day, some shoots formed roots at their base (Figure 3b), suggesting that rooting was easy.
Root formation
After 15 days, 67 to 75% of shoots induced roots when medium contained 0.1 mg/L NAA or IBA, or 100% if 0.1 mg/L of both these auxins were employed (Figure 3c and Table 2). Control (no auxins) shoots did not induce roots within 15 days. However, after 30 days, 100% of shoots on any medium with an auxin formed roots (85% in the control) (Table 2).
Callus induction and adventitious shoot induced from root explants
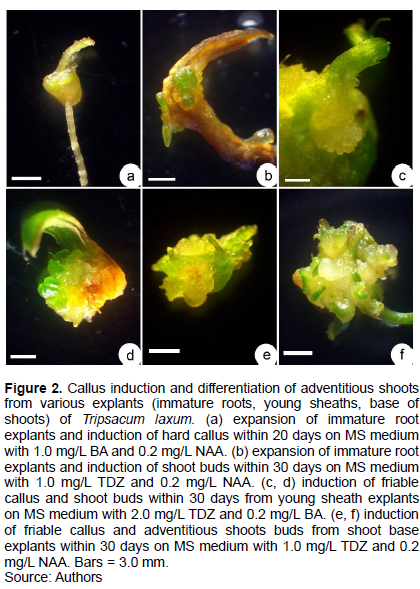
When BA, 2,4-D and NAA were used alone, almost no callus was induced from root explants, and only TDZ induced some expansion of the root explant and the induction of some callus. In all cases, adventitious shoot buds developed (Figure 2a). When thidiazuron (TDZ) and NAA were combined, the percentage of explants inducing callus increased to 13.3%, ultimately forming 2.2 adventitious shoot buds per explant after 30 days. Callus induction percentage (20% of explants) and number of adventitious shoot buds/explant (3.8) were largest on MS medium with 1.0 mg/L TDZ and 0.2 mg/L BA (Figure 2b and Table 3).
Callus and adventitious shoot buds induced from young sheath explants
PGRs, when used alone, or a combination of BA/TDZ with NAA, could not induce callus from young sheath explants while TDZ with BA induced a low frequency (3.3-4.3%) of callus after 30 days. This callus was granular and beige-white (Figure 2c). Adventitious shoot buds were visible after 30 days. Optimal medium contained 1.0 mg/L TDZ and 0.2 mg/L BA, resulting in highest callus induction frequency (30.8%) most adventitious shoot buds/explant (4.6) (Figure 2d and Table 4).
Callus inducing from shoot basal meristem explants
When TDZ or 2,4-D were used alone, some hyperhydric pink callus was induced, but it was unable to differentiate, and eventually turned brown and died. BA did not induced callus, instead inducing adventitious shoots from callus. When 2,4-D was combined with BA and TDZ, they induced a low frequency of callus in 1 to 2% of explants after 30 days (Table 5). Milky white or yellow granular callus possessed a strong ability to develop adventitious shoot buds directly, especially the combination of 1.0 mg/L TDZ and 0.2 mg/L 2,4-D (9.2 adventitious shoot buds/explant) (Figure 2e and f), followed by 1.0 mg/L TDZ and 0.2 mg/L BA (5.3 adventitious shoot buds/explant) (Table 5).
Acclimatization and transplanting
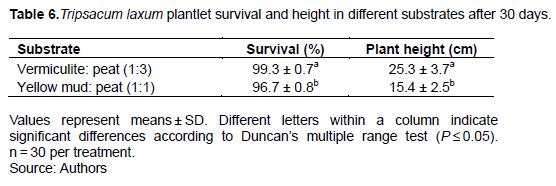
Both treatments resulted in a high survival percentage, 99.3% in vermiculite: peat (1:3, v/v), and 96.7% in yellow mud and peat (1:1, v/v) (Table 6 and Figure 3d).
DISCUSSION
The tissue culture of several species of the Gramineae employed various explants. For example, callus was induced from leaves in sugarcane on MS with 1.0 mg/L 2,4-D (Garcia et al., 2007), callus were induced from meristem tips in MS with 4.0 µM BA and 40.0 µM NAA (Lakshmanan et al., 2006; Tang et al., 2011), and callus were induced from sorghum immature embryos in MS with 2.0 mg/L 2,4-D (Assem et al., 2014). In the present experiment, peduncles were selected as explants because seed are not produced in nature (Dodds and Simmonds, 1946; Zhong et al., 2011). Since explants derived from field-grown plants are easy to become contaminated in vitro after inoculation on medium, despite surface disinfection, peduncles were selected as explants, reducing contamination-associated problems to about 3% in our initial trial.
Axillary shoot proliferation (that is, SPC) was enhanced in the presence of a cytokinin and NAA (Table 1), similar to the tissue culture of L. repens, another Gramineae plant (Xiong et al., 2021). In T. dactyloides, mature zygotic embryos were used to induce embryogenic callus cultures on MS medium with dicamba (10 or 20 μM) and sucrose (3 or 6%), while plantlets were regenerated on PGR-free MS medium containing 2% sucrose (Furini and Jewell, 1991). In our study on T. laxum, only TDZ was able to induce callus from root explants, while the further addition of BA also stimulated shoot formation (Table 3). In dicotyledonous plants, the use of TDZ or BA are popular PGRs to induce shoot buds (Zhang et al., 2017; Liang et al., 2020), although TDZ might also induce somaclonal variation (Dewir et al., 2018). In monocotyledonous plants, 2,4-D has been used to induce callus and shoots from roots in sorghum (Mishra and Khurana, 2003), rice (Guo et al., 2018) and maize (Wang et al., 2021).
The base of leaf sheaths were used as explants to induce callus, although only TDZ combined with BA successfully induced callus, which differentiated into adventitious buds (Table 4). In sugarcane, in light, shoot induction from leaf explants was possible within 3 weeks on MS medium with 10 to 60 µM NAA and 4 to 8 µM BA (Lakshmanan et al., 2006), whereas in dark, callus was induced from stem segments or immature leaves when exposed to 4.5 μM 2,4-D (Garcia et al., 2007).
CONCLUSION
A protocol was developed for the regeneration of shoots from T. laxum peduncles via a direct shoot induction and shoot proliferation route and an indirect (callus-induced regeneration) route. The development of a protocol that will allow for the mass propagation of this species, will allow the resource allocation needs of this forage crop to be met, and allow for additional research such as genetic engineering to fortify abiotic stress tolerance.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
ABBREVIATIONS
2,4-D, 2,4-Dichlorophenoxyacetic acid; BA, 6-benzyladenine; IBA, indole-3-butyric acid; KIN, kinetin; MS medium, Murashige and Skoog (1962) medium; NAA, α-naphthaleneacetic acid; PGR, plant growth regulator; SPC, shoot proliferation coefficient; TDZ, thidiazuron.
ACKNOWLEDGEMENTS
This work was supported by the National Key Research Plan of China (2021YFC3100400) and conservation and utilizaiton of the important strategic wild plant resource in Guangdong Province (Grant No. 2022B1111040003).
REFERENCES
|
Assem SK, Zamzam MM, Hussein BA (2014). Evaluation of somatic embryogenesis and plant regeneration in tissue culture of ten sorghum (Sorghum bicolor L.) genotypes. African Journal of Biotechnology 13(36):3672-3368. |
|
|
Asudi GO, Johnnie V, Midega C, Pittchar J, Pickett JA, Khan ZR (2015). Napier grass stunt disease in east africa: farmers' perspectives on disease management. Crop Protection 71:116-124. |
|
|
Boschini-Figueroa CC, Vargas-Rodríguez F (2018). Nutritional composition of Tripsacum laxum fertilized with nitrogen, phosphorous and potassium. Agronomía Mesoamericana 29(1):150-162. |
|
|
Dewir YH, Nurman S, Naidoo Y, Teixeira da Silva JA (2018). Thidiazuron-induced abnormalities in plant tissue cultures. Plant Cell Reports 37(11):1451-1470. |
|
|
Dodds KS, Simmonds NW (1946). A Cytological basis of sterility in Tripsacum laxum. Annals of Botany 10(38):109-116. |
|
|
Furini A, Jewell DC (1991). Somatic embryogenesis and plant regeneration of Tripsacum dactyloides L. Euphytica 55(2):111-115. |
|
|
Garcia R, Cidade D, Castellar A, Lips A, Magioli C, Callado C, Mansur E (2007). In vitro morphogenesis patterns from shoot apices of sugarcane are determined by light and type of growth regulator. Plant cell, Tissue and Organ Culture 90(2):181-190. |
|
|
Gray JR (1974). The genus Tripsacum L. (Gramineae): Taxonomy and chemosystematics, PhD thesis, University of Illinois at Urbana-Champaign, IL, USA. |
|
|
Guo F, Zhang H, Liu W, Hu X, Han N, Qian Q, Xu L, Bian H (2018). Callus initiation from root explants employs different strategies in rice and Arabidopsis. Plant and Cell Physiology 59(9):1782-1789. |
|
|
Guyadeen KD (1951). A promising fodder - Guatemala grass. Tropical Agriculture, Trinidad and Tobago 28:76-79. |
|
|
Jiang CS, Liu GD, Zou DM, Wei JS, Zhao JS (2002). Regional experimental study of several tropical herb. Grassland and Turf 3:12-14. |
|
|
Klapwijk CJ, Schut M, Asten P, Vanlauwe B, Descheemaeker K (2020). Micro-livestock in smallholder farming systems: the role, challenges and opportunities for cavies in South Kivu, eastern DR congo. Tropical Animal Health and Production 52(3):1167-1177. |
|
|
Lakshmanan PRJG, Geijskes RJ, Wang LF, Elliott A, Grof CPL, Berding N, Smith GR (2006). Developmental and hormonal regulation of direct shoot organogenesis and somatic embryogenesis in sugarcane (Saccharum spp. interspecific hybrids) leaf culture. Plant Cell Reports 25(10):1007-1015. |
|
|
Liang HZ, Xiong YP, Guo BY, Yan HF, Jian SG, Ren H, Zhang XH, Li Y, Zeng SJ, Wu KL, Zheng F, Teixeira da Silva JA, Xiong YH, Ma GH (2020). Shoot organogenesis and somatic embryogenesis from leaf and root explants of Scaevola sericea. Scientific Reports 10(1):1-11. |
|
|
Luo L, Lin H, Luo HL, Li QQ, Lin DM, Lu GD (2021). The complete chloroplast genome of Tripsacum laxum (Gramineae). Mitochondrial DNA Part B 6(11):3207-3208. |
|
|
Maleko D, Msalya WT, Mwilawa G (2018). Seasonal variations in the availability of fodder resources and practices of dairy cattle feeding among the smallholder farmers in western Usambara highlands, Tanzania. Tropical Animal Health and Production 50(7):1653-1664. |
|
|
Munyasi JW, Auma EO, Ngode L, Muyekho FN (2015). Evaluation of biomass yield of selected fodder species in relation to frequency of harvest alongside defoliation heights in western Kenya. Journal of Agricultural Extension and Rural Development 7(8):257-262. |
|
|
Mishra A, Khurana P (2003). Genotype dependent somatic embryogenesis and regeneration from leaf base cultures of Sorghum bicolor. Journal of Plant Biochemistry and Biotechnology 12(1):53-56. |
|
|
Murashige T, Skoog F (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum 15(3):473-497. |
|
|
Schieber E (1975). Puccinia polysora rust found on Tripsacum laxum in jungle of Chiapas, Mexico. Plant Disease Reporter 59:625-626. |
|
|
Shem MN, Ørskov ER, Kimambo AE (1995). Prediction of voluntary dry-matter intake, digestible dry-matter intake and growth rate of cattle from the degradation characteristics of tropical foods. Animal Science 60(1):65-74. |
|
|
Tuley P (1961). Puccinia polysora on Tripsacum laxum and Zea mays. Nature 190(4772):284-284. |
|
|
Wang C, Ma H, Zhu W, Zhang J, Zhao, X, Li X (2021). Seedling-derived leaf and root tip as alternative explants for callus induction and plant regeneration in maize. Physiologia Plantarum 172(3):1570-1581. |
|
|
Wet J, Cohen CE, Brink DE (1985). Seed proteins and systematics of Tripsacum. Biochemical Systematics and Ecology 13(3):231-237 |
|
|
Wilkes HG (1972). Maize and Its Wild Relatives: Teosinte and Tripsacum, wild relatives of maize, figured prominently in the origin of maize. Science 177(4054):1071-1077. |
|
|
Xiong YP, Wei ZP, Yu XC, Pang JH, Zhang T, Wu KL, Ren H, Jian SG, Teixeira da Silva JA, Ma GH (2021). Shoot proliferation, embryogenic callus induction, and plant regeneration in Lepturus repens (G. Forst.) R. Br. In Vitro Cellular & Developmental Biology-Plant 57(6):1031-1039. |
|
|
Zhao YL, Wen XD, Sun RL, Iqbal MZ, Tang QL (2020). Research and utilization of maize' s wild relative the genus Tripsacum. Journal of Maize Sciences 28(2):1-10. |
|
|
Zhang YY, Chen YL, Zhang XH, Teixeira da Silva JA, Ma GH (2017). Adventitious shoot induction from internode and root explants in a semiparasitic herb Monochasma savatieri Franch ex Maxim. Journal of Plant Growth Regulation 36(3):799-804. |
|
|
Zhong S, Huang MF, Xue SM (2011). Breeding and Applying of Tripsacum laxum Nash cv. Yingjiang. Chinese Journal of Tropical Crops 32(5):806-810. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0