ABSTRACT
Nanofertilizers may be a possible replacement for organic fertilizers because they can get to targeted sites in a plant and either enhance or thwart its growth depending on the toxicity level of the used nanoparticles. Zinc oxide nanoparticles are said to be a good source of nutrients for crop production and using these nanoparticles as bio fertilizers, could help to reduce the chemical residue in the grown vegetables which may sometimes be consumed raw or at an immature stage. Zinc oxide nanoparticles used in this work were biosynthesized using Ocimum gratissimum and Vernonia amygdalina leaf extracts. They were characterized using optical spectroscopy and electron microscopy and found to be of sizes ranging from 11 to 99 nm with some agglomeration. Colloidal solutions of these nanoparticles of concentrations 10, 100 and 500 mgl-1 were used as nanofertilizer for growing black-seeded and pale-seeded Amaranthus cruentus. Percent emergence was best at 69% for the pale seeded variety to which 100 mgl-1 Og-ZnO (pH 8) nanoparticles was applied and worst at 29% for the black seeded variety treated with 10 mgl-1 Og-ZnO (pH 12) nanoparticles. Best emergence index value of 2.28 was observed for the black seeded variety on the application of pH 8, 100 mgl-1 Og-ZnO nanoparticles. The pale seeded variety to which pH 12, 10 mgl-1 of Va-ZnO nanoparticles was applied gave the best emergence rate index results. The study showed that pH and concentration of nanoparticles can affect the seedling characteristics of A. cruentus seeds.
Key words: Nanofertilizer, percent emergence, emergence index, emergence rate index, seedling characteristics, electron microscopy, optical spectroscopy.
Nanoparticles have sizes in the nanometer range and exhibit novel properties which are dependent on their morphology (Vidya et al., 2013). They can be synthesized using chemical, physical and biological methods but of these three methods, biosynthesis is preferred because of its environmental friendliness (Singh et al., 2011). The use of nanoparticles spans through a number of disciplines ranging from household items to the field of electronics as sensors. While in medicine they aid drug delivery to specific targets, most recently in agriculture they have been said to play active roles in plant growth (Sabir et al., 2014). Some nanoparticles are reported to have excellent antibacterial and antimicrobial properties and others are said to exhibit considerable phototoxicity on growing plants (Lin and Xing, 2007).
Recent researches have shown that nanoparticles can be used as nanofertilizer for growing plants but though some researchers say nanoparticles thwarts the growth of some plants, others reported growth enhancement of some other plants (Aslani et al., 2014; Sabir et al., 2014). The effect of nanoparticles on plants varies with the kind of nanoparticles used, the plant species under study, the form of the nanoparticles in use and the concentration of its colloidal solution (Ma et al., 2010).
Zinc oxide nanoparticles biosynthesized using Ocimum gratissimum (Scent leaf) and Vernonia amygdalina (bitter leaf) leaf extracts were used as nanofertilizer for this study. O. gratissimum and V. amygdalina are both antioxidants with valuable food and medicinal values which contain bio molecules with reductive abilities. While O. gratissimum can be used for treating headaches, inflammation, pneumonia, haemorrhoids , common colds and infant convulsions (Akinyemi et al., 2005; Prabhu et al., 2009), V. amygdalina serves as a laxative and is used by herbalists for treating malaria probably due its Quinone content. It is also said to be effective for assisting diabetic control of sugar level (Akinjogunla et al., 2011; Katewa et al., 2004; Udochukwu et al., 2015; Ugwoke et al., 2010).
Zinc oxide nanoparticles have some antibacterial properties and a wide range of applications. They can be used to fabricate some functional device structures such as relative humidity sensors due to their resistance change upon exposure to different relative humidity conditions (Erol et al., 2010; Vaseem et al., 2010). Their ability to produce bright UV and visible fluorescence has been used for optical applications such as light emitting devices (LEDs) as well as in the field of bio-medical imaging (Vaseem et al., 2010). Organic fertilizers for growing plants are in high demand and even when available are not enough to meet the growing demand for it, nor do they completely get absorbed by the plants or get to designated sites in the plants due to hydrolysis, leaching etc (Nair et al., 2010).
In contrast, a small quantity of nano fertilizer when applied to plants is said to actually get to the targeted sites in the plants (Sabir et al., 2014) and nano-encapsulated slow release of fertilizers conserve fertilizers and reduce environmental pollution (Nair et al., 2010). Nanoparticles are beneficial to agriculture and can serve as an alternative to organic fertilizer. This, according to Siddiqui et al. (2015), is because nano-particles with high surface areas are highly reactive and can get to targeted sites in a plant in order to enhance its metabolism. Furthermore, surface area and surface properties of nanoparticles (Ma et al., 2010) rather than their concentrations influence phytotoxicity of seedlings.
Ramesh et al. (2014) compared the effect of bulk and nano Zinc oxide (ZnO) and Titanium dioxide (TiO2) on seed germination, mitosis, seeds, shoot and root growth of wheat (Triticum aestivum Linn) and reported that bulk metal oxides had no significant effect on any of those rather nano ZnO raised Chlorophyll and protein levels of treated seeds. A related research (Stampoulis et al., 2009) which studied the effect of bulk and nano forms of five nanomaterials namely silver, copper, zinc oxide, silicon and multiwalled CNTs (MWCNTs on Field pumpkin (Cucurbita pepo), observed that seedling germination percentage was higher for seeds treated with nano ZnO than those treated with bulk ZnO powder and that percent emergence of the seeds was dependent on the sizes of the nanoparticles used for treatment of seeds.
Nanoscale metals such as zinc or aluminum or metal oxides like ZnO or TiO2 have been applied to selected plants and their toxicology effects on the seeds, roots or growth have been studied. While TiO2 is said to shield plant Chlorophyll from destruction due to long exposure to light, it has also been mixed with SiO2 and applied to soybean to boost its antioxidant effect and intake of water and other essential nutrients (Lu et al., 2001).
While studying the toxicity effects of five nanoparticles (MWCNT, Al2O3, ZnO, Al, and Zn) with concentrations ranging from 10 mgl-1 to as high as 2000 mgl-1 on six plants (rape, radish, rye grass, lettuce, corn and cucumber), the researchers (Lin and Xing, 2007) reported that none of the applied nanoparticles showed inhibition to growth except for nano zinc, which affected rye grass and nano zinc oxide which affected corn.
While these researches show the extent of phytotoxicity experienced by the studied plants due to the applied nanoparticles, none of them addressed the interactive effect of nanoparticles of various pH values and their concentration on the seedling characteristics of their chosen species of plants.
This work will not discuss the biological process behind the assimilation of the zinc oxide nanoparticles into the plant cells but will dwell on the physical characteristics of the nanoparticles and how the concentration of the ZnO nanofertilizer and various seed treatments make observable changes in the seedling characteristics of the Amaranthus cruentus seeds. It will also show how these changes depend on the pH of the synthesized zinc oxide nanoparticles and the concentration of its colloidal solution.
The ZnO nanoparticles were biosynthesized using leaf extracts of O. gratissimum and V. amygdalina and then characterized using UV-vis, scanning electron microscope (SEM), Transmission electron microscope (TEM), XRD, Fourier transform infrared spectroscopy (FTIR) and PL studies. Colloidal solutions of these nanoparticles of different concentrations were prepared and used as fertilizer for growing black-seeded and pale - seeded A. cruentus. The interactive effect of pH of the nanoparticles of different concentrations on the seedling characteristics of these seeds namely percent emergence (%E). Emergence Index (EI) and Emergence rate index (ERI) of these two varieties of A. cruentus were investigated.
A. cruentus which belongs to a family of plants called Amaranthaceae is used in place of spinach in some countries. It is said to have grains which are rich in protein and leaves which are rich in vitamins. It is cheap, common, and grows generally well in the tropics.
Nanoparticles synthesis and colloidal solution preparation
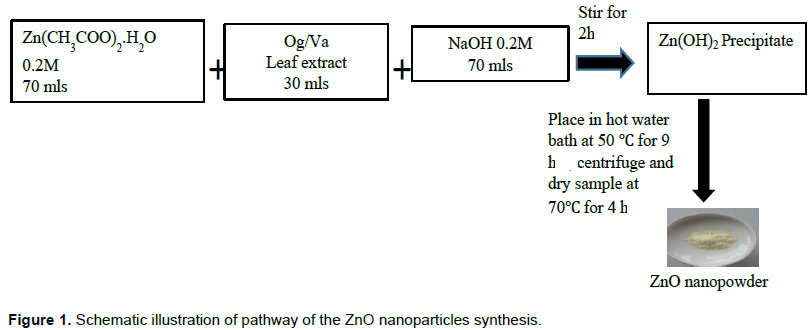
Two samples of zinc oxide nanoparticles were biosynthesized using 15% leaf extracts of O. gratissimum (Og) and V. amygdalina (Va) with zinc acetate dihydrate and NaOH as precursor materials (Sidra et al., 2014) and the resulting precipitate was kept in a hot water bath at 50°C for 9 h after which it was centrifuged and dried in an oven for 7 h (Figure 1).
Three samples each of Og-ZnO and Va ZnO nanoparticles of pH values P1= pH 8, P2= pH 10 and P3=pH 12 were synthesized and characterized using optical spectroscopy and electron microscopy. Colloidal solutions of the zinc oxide nanoparticles of concentrations N1=10, N2=100, and N3=500 mgl-1 were prepared for each sample.
Seeds and their planting
Two varieties of A. cruentus, namely, the black-seeded and the pale-seeded varieties were used for the study. These seeds were given some extra treatments before planting: (i) soaked in nanoparticles solution for 10 min (ii) soaked in water for 10 min and (iii) not soaked. The soil used for the planting was analyzed for its constituent elements before planting and after harvesting the plants.
The soil was of pH 6.0 and consisted of 79.5% of sand, 10.5% of clay and 10.0% of silt. Organic carbon was 1.50%, and its zinc content was 0.24 mg kg-1. The pots used for the seed planting each contained soil of volume 8×10-3 m3 and the seeds were planted in pots at a depth of 0.7 cm. Each treatment had three replications and the blank control pots had soil with no nanoparticles treatment. A screen house was used for shielding the pots, used for the seed planting from pests to ensure that only the nanoparticles played a role in seed germination. The International Seed Testing Association (ISTA) method in which 100 seeds are planted in each pot was used and after emergence the plants were thinned to eight with four of those actually tagged for all the measurements that were done. The study of the seedling germination was done over a period of one week and the parameters of interest were the percent emergence % E the emergence index EI, and the emergence rate index ERI of the planted seeds. Four factors were introduced in the experiment namely (i) pH of synthesized nanoparticles (ii) seed varieties (iii) concentration of colloidal solutions of the zinc oxide nanoparticles (iv) seed soaking.
Preparation of zinc oxide nanofertilizer
The different concentrations of the colloidal solutions of the zinc oxide nanoparticles were prepared and labelled accordingly with pH 8, 10 and 12 referred to as P1, P2 and P3, respectively. The concentrations of solutions prepared were 10, 100 and 500 mgl-1 referred to in this report as N1, N2 and N3, respectively. The zinc oxide nanoparticles solution is used as nanofertilizer in this experiment. The soil in the pots in which the seeds were to be planted was treated with the nanofertilizer before planting and was repeated a week after seed emergence. The blank control experiments pots were designated as performance of the blank control (P0N0) and had soil with no nanoparticles treatments.
Statistical analysis
Data of the interactive effect of nanoparticles pH and concentration on the seedling characteristics was subjected to the analysis of variance (ANOVA) and tests were done at 5% probability using the Genstat release 10.3 DE. The effects of soaking the seeds before planting were however ignored because it was not significant.
Characterisation of zinc oxide nanoparticles
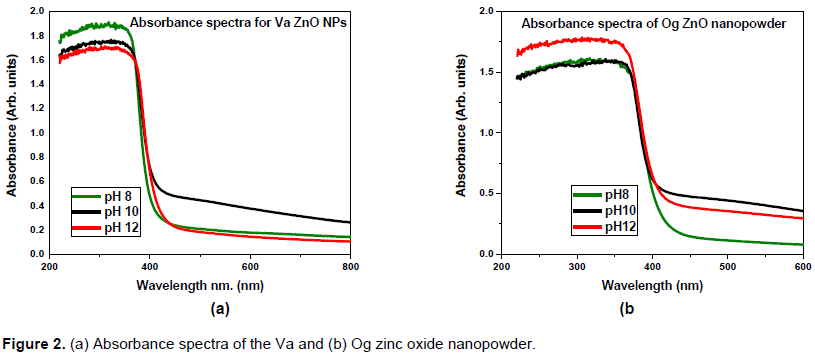
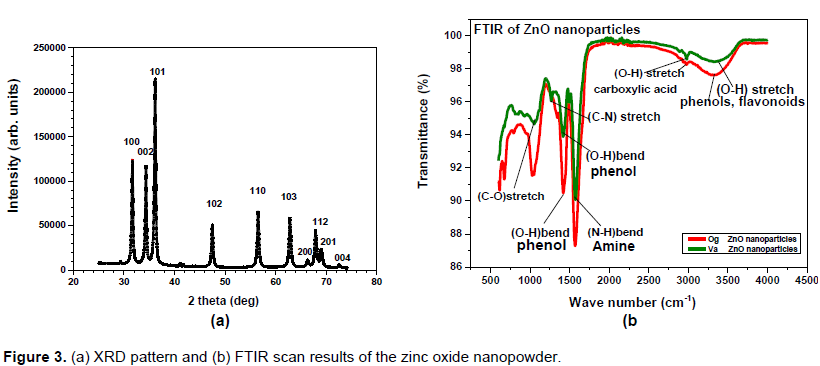
The absorbance spectrum for zinc oxide nanopowder (Figure 2) shows that the Va sample interacts better with light than the Og sample, with a higher peak absorbance at wavelength 355 nm as against 360 nm for Og sample. The absorbance increased with pH for Og zinc oxide nanoparticles but the reverse is true for Va zinc oxide nanoparticles. While the XRD pattern of the ZnO nanopowder (Figure 3a) shows that it has the hexagonal wurtzite structure, the FTIR spectra of zinc oxide nanoparticles (Figure 3b) revealed the functional groups on the nanoparticles surface, which are present in flavonoids, phenols, amides and polysaccharides. These biomolecules may have been involved in the reduction of the zinc ions to nanoparticles.
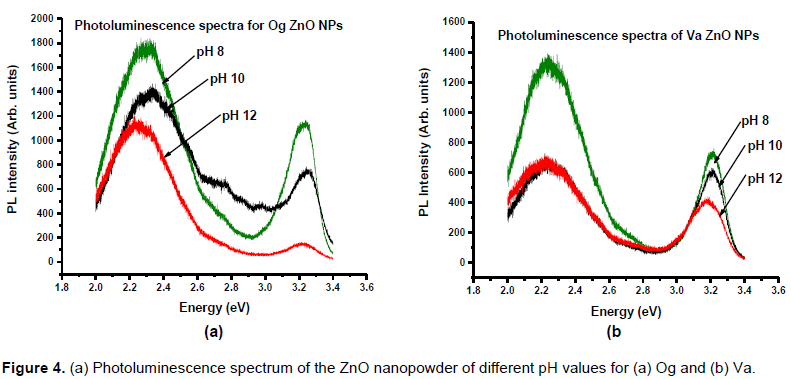
The near-ultraviolet (NUV) Photoluminescence spectra of the zinc oxide nanoparticles (Figure 4) show two
emission peaks. Lower wavelength peaks attributed to the near bandgap excitonic emission and higher wavelength peaks due to singly ionized oxygen vacancies (Sangeetha et al., 2011). These have values ranging from 381 to 384 nm for pH 8 to 10 Og - zinc oxide nanoparticles and 532 to 551 nm while for the Va - zinc oxide nanoparticles, the values were in the range 386 to 390 nm and 553 to 556 nm, respectively. The PL intensities were found to decrease for higher pH samples. The SEM images (Figure 5a) show the particles to be spherical with aggregation and with low pH particles larger in size than higher pH nanoparticles.
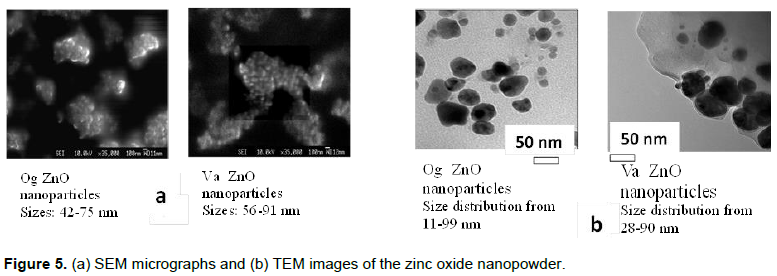
The Og pH 8 zinc oxide nanoparticles had sizes ranging from 42 to 75 nm, giving an average size of 63 nm while the pH 10 nanoparticles had sizes ranging from 36 to 55 nm and an average size of 43 nm. The Va zinc oxide nanoparticles of pH 8 had sizes ranging from 56 to 91 nm and an average size of 71 nm while the pH 10 nanoparticles had sizes in the range of 50 to 78 nm and an average size of 54 nm. The TEM images (Figure 5b) show the nanoparticles to be spherical in shape with some aggregation, with sizes ranging from 11 to 99 nm for Og and Va samples.
Seedling characteristics
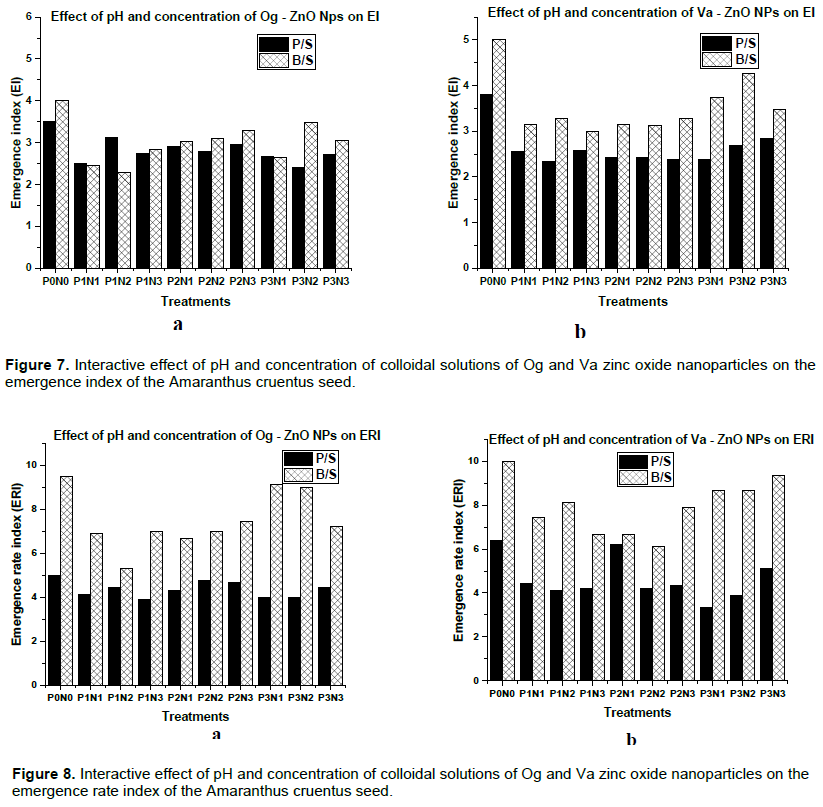
The number of days to emergence of the planted seeds was two and it took a little above a week for all the viable seeds to germinate. The interactive effects of pH and concentration of the Og and Va zinc oxide nanoparticles, resulting from the analysis of variance (ANOVA) of the collected data was done. The blank control P0N0 was compared with those of the treated seeds and the result of the seedling characteristics are as shown in the plotted bar charts (Figures 6 to 8).
Percent emergence (% E)
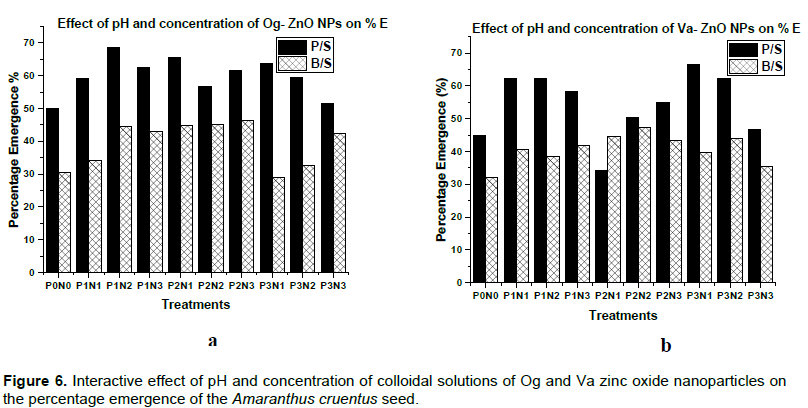
Percent emergence values give an indication of the number of seeds out of the 100 planted which germinated. High values are considered to be good. For black seeded variety and for all three pH levels, increasing Og-ZnO nanoparticles concentration increased the percent emergence.
For the pale seeded variety however, increasing the pH 8 nanoparticles concentration increased the percent emergence but increasing the pH 10 and 12 nanoparticles concentration, decreased the percent emergence
(Figure 6a). Increasing the pH 8 Va-ZnO nanoparticles concentration raised the percent emergence of the black seeded variety but lowered that of the pale seeded variety.
Similarly high concentrations of the pH 10 nanoparticles reduced the black seeded variety percent emergence but increased that of the pale seeded variety. High concentrations of the pH 12 nanooparticles reduced the percent emergence of both the pale and black seeded variety (Figure 6b).
Emergence Index (EI)
The emergence index shows the uniformity in growth of the planted seeds. Low EI values are considered to be better than higher ones. The results for the emergence index (EI) as shown in Figure 7 seem to be equally distributed between the two seed varieties with Og zinc oxide nanofertilizer pH 8, 100 mgl-1 giving the best value of 2.28 for the black seeded variety (Figure 7a), and the worst value of 4.26 was recorded for same seed variety treated with pH 12, 100 mgl-1Va zinc oxide nanofertilizer (Figure 7b).
Increased Og ZnO nanoparticles concentration of all three pH levels increased EI values for both the pale and black seeded varieties. Raising the concentration of pH 8 Va ZnO nanoparticles increased the EI values of the pale seeded variety but reduced that of the black seeded variety. High concentrations of pH 10 Va ZnO nanoparticles gave low EI values for pale seeded variety but high EI values for black seeded variety. Furthermore, high concentrations of pH 12 Va ZnO nanoparticles gave better EI values for black seeded variety but poor EI values for pale seeded variety.
Emergence Rate Index (ERI)
Emergence rate index values show on a scale of zero to ten, how long it actually took all the planted seeds to emerge. Low ERI values are preferred to higher ones. Results of the emergence rate index (Figure 8) showed that, the pale seeded variety did better than the black seeded variety when treated with either Og or Va zinc oxide nano fertilizer. However the best result of 3.35 was observed for pH 12, 10 mgl-1 Va zinc oxide nanofertilizer (Figure 8b) and the worst result was for the black-seeded variety with ERI 9.36 due to pH 12, 500 mgl-1 Va zinc oxide nano fertilizer (Figure 8b).
Increasing the concentration of pH 8 Og ZnO nanofertilizer increased ERI values for the black seeded variety but lowered the ERI values for the pale seeded variety. For both the pale seeded and black seeded variety, increasing the concentration of pH 10 nanoparticles raised their ERI values however, high concentrations of the pH 12 nanoparticles lowered the ERI values of the black seeded variety but increased that of the pale seeded variety, implying that better growth uniformity could be achieved using lower concentrations of nanoparticles of all pH levels.
Zinc oxide nanoparticles of three pH levels were biosynthesized using O. gratisimum and V. amygdalina x biosynthesized using O. gratisimum and V. amygdalina and characterized using optical spectroscopy and electron microscopy. They were found to be spherical in shape with sizes which were found to be pH dependent. Higher pH zinc oxide nanoparticles were smaller in size than the lower pH zinc oxide nanoparticles for both the Og and Va zinc oxide nanoparticles. The Va zinc oxide nanoparticles were more sensitive to light giving a higher UV-Vis absorbance peak than the Og zinc oxide nanoparticles, with a lower UV-Vis absorbance peak.
The TEM images showed the nanoparticles to be spherical in shape and of a range of sizes while the SEM micrographs present them as aggregated entities with sizes which were pH dependent. The FTIR scans of the zinc oxide nanoparticles showed functional groups present in the nanoparticles and point to the presence of biomolecules such as flavonoids, phenols and polysaccharides which probably aided the reduction of the zinc ions to zinc nanoparticles.
Colloidal solutions of zinc oxide nanoparticles of three different pH values were used as fertilizer for growing pale and black seeded A. cruentus. A significant positive effect of the prepared nanofertilizer on the seedling characteristics was observed as the blank control seeds gave poorer results when compared with the treated seeds. The treatments which resulted in best and worst results are as shown in Table 1. While high concentrations of the Og ZnO nanoparticles of all three pH levels raised the percent emergence of the black seeded variety, high concentrations of the pH 12 Va ZnO nanoparticles and lowered the percent emergence of both seed varieties.
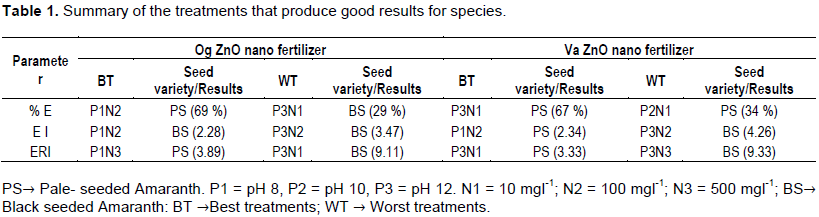
Increased concentrations of Og ZnO nanoparticles of all pH levels gave high EI values and hence poor growth uniformity of the two seed varieties. Lower concentrations of Og ZnO nanoparticles of all pH levels gave the pale seeded variety low ERI values and showed that all seeds of that variety could germinate in four days as against the black seeded variety, which needed as much as ten days for the germination of all its viable seeds. This work showed that pH and concentration of the colloidal solution of the zinc oxide nanoparticles used as nano fertilizer affected the seedling characteristics of the cultivated seeds and corroborated the results of similar researches (Lu et al., 2001; Stampoulis et al., 2009) on other crops, which attest to the effectiveness of nano zinc oxide in seedling growth.
Since none of the zinc oxide nanoparticles treatments choked the planted seeds, the biosynthesized ZnO nanoparticles could be explored for possible use as an active ingredient of a nanofertilizer and could supplement the role of organic fertilizer, whose current supply does not seem to meet demand. Furthermore, this work can chart a new course for the two leaves O. gratisimum and V. amygdalina used rather than their present use only for food and medicinal values
The authors have not declared any conflict of interests.
REFERENCES
|
Akinjogunla O, Ekoi O, Odeyemi A, Odeyemy A (2011). Phytochemical screening and in vitro antibacterial assessment of aqueous leaf extracts of Vernonia amygdalina (Asteraceae) and Ocimum gratissimum (Lamiaceae) on moxifloxacin resistant Escherichia coli isolated from clinical and environmental samples. Nat. Sci. 9(7):42-52.
|
|
|
|
Akinyemi KO, Oladapo O, Okwara CE, Ibe CC, Fasure KA (2005). Screening of crude extracts of six medicinal plants used in South-West Nigerian unorthodox medicine for anti-methicillin resistant Staphylococcus aureus activity. BMC Complement. Altern. Med. 5(1):1-7.
Crossref
|
|
|
|
|
Aslani F, Bagheri S, Julkapli MN, Juraimi AS, Hashemi FSG, Baghdadi A (2014). Effects of engineered nanomaterials on plants growth: an overview. Sci. World J.
Crossref
|
|
|
|
|
Erol A, Okur S, Comba B, Mermer Ö, Arıkan MC (2010). Humidity sensing properties of ZnO nanoparticles synthesized by sol–gel process. Sens. Actuators B Chem. 145(1):174-180.
Crossref
|
|
|
|
|
Katewa SS, Chaudhary BL, Jain A (2004). Folk herbal medicines from tribal area of Rajasthan, India. J. Ethnopharmacol. 92(1):41-46.
Crossref
|
|
|
|
|
Lin D, Xing B (2007). Phytotoxicity of nanoparticles: inhibition of seed germination and root growth. Environ. Pollut. 150(2):243-250.
Crossref
|
|
|
|
|
Lu C, Zhang C, Wen J, Wu G, Tao M (2001). Research of the effect of nanometer materials on germination and growth enhancement of Glycine max and its mechanism. Soybean Sci. 21(3):168-171.
|
|
|
|
|
Ma X, Geiser-Lee J, Deng Y, Kolmakov A (2010). Interactions between engineered nanoparticles (ENPs) and plants: phytotoxicity, uptake and accumulation. Sci. Total Environ. 408(16):3053-3061.
Crossref
|
|
|
|
|
Nair R, Varghese SH, Nair BG, Maekawa T, Yoshida Y, Kumar DS (2010). Nanoparticulate material delivery to plants. Plant Sci. 179(3):154-163.
Crossref
|
|
|
|
|
Prabhu KS, Lobo R, Shirwaikar AA, Shirwaikar A (2009). Ocimum gratissimum: a review of its chemical, pharmacological and ethnomedicinal properties. Open Compl Med. J. 1:1-15.
Crossref
|
|
|
|
|
Ramesh M, Palanisamy K, Babu K, Sharma NK (2014). Effects of bulk & nano-titanium dioxide and zinc oxide on physio-morphological changes in Triticum aestivum Linn. J. Glob. Biosci. 3:415-422.
|
|
|
|
|
Sabir S, Arshad M, Chaudhari SK (2014). Zinc oxide nanoparticles for revolutionizing agriculture: synthesis and applications. Sci. World J. 1-8.
Crossref
|
|
|
|
|
Sangeetha G, Rajeshwari S, Venckatesh R (2011). Green synthesis of zinc oxide nanoparticles by aloe barbadensis miller leaf extract: Structure and optical properties. Mater. Res. Bull. 46(12):2560-2566.
Crossref
|
|
|
|
|
Siddiqui MH, Al-Whaibi MH, Firoz M, Al-Khaishany MY (2015). Role of nanoparticles in plants. Nanotechnology and Plant Sciences, Springer. pp. 19-35.
Crossref
|
|
|
|
|
Singh RP, Shukla VK, Yadav RS, Sharma PK, Singh PK, Pandey AC (2011). Biological approach of zinc oxide nanoparticles formation and its characterization. Adv. Mater. Lett. 2(4):313-317.
Crossref
|
|
|
|
|
Stampoulis D, Sinha SK, White JC (2009). Assay-dependent phytotoxicity of nanoparticles to plants. Environ. Sci. Technol. 43(24):9473-9479.
Crossref
|
|
|
|
|
Udochukwu U, Omeje FI, Uloma IS, Oseiwe I (2015). Phytochemical analysis of Vernonia amygdalina and Ocimum gratissimum extracts and their antibacterial activity on some drug resistant bacteria. Am. J. Res. Commun. 3(5):225-235.
|
|
|
|
|
Ugwoke C, Nzekwe U, Ameh G (2010). Phytochemical constituents and ethnobotany of the leaf extract of bitter leaf (Vernonia amygdalina) Del. J. Pharm. Allied Sci. 7:3.
|
|
|
|
|
Vaseem M, Umar A, Hahn Y-B (2010). ZnO nanoparticles: growth, properties, and applications. Metal oxide nanostructures and their applications, 5:1-36.
|
|
|
|
|
Vidya C, Hiremath S, Chandraprabha MN, Antonyraj MAL, Gopal IV, Jain A, Bansal K (2013). Green synthesis of ZnO nanoparticles by Calotropis Gigantea. Int. J. Curr. Eng. Technol. 1:118-120.
|
|
|