ABSTRACT
The aim of this work was to evaluate the effect of changing the shake flask for a stirred-tank bench bioreactor on the performance of Thevetia peruviana cultures. Maximum cell growth of 18.59 and 8.46 g DW/L for shake flask and bench bioreactor at day 14 was obtained, respectively. The end of the exponential growth phase in shake flask was correlated with total sugar consumption, while this behavior was not observed in bench bioreactor. Preferential uptake of glucose over fructose was observed in both systems. Cell morphology was similar for both systems during culture time, exhibiting individual cells with cylindrical and elongated shape at day 0 and some aggregates with rounded cells at day 14. Polyphenol production was not affected by the system configuration and scale. Intra and extracellular antioxidant activity were directly related with phenol production in both systems. Guaiacol peroxidase activity (GPX) went from 1.87 to 7.97 µM/min/mg protein in shake flask and it correlated with low reactive oxygen species (ROS) production. This is an indication of a positive enzymatic cell response for culture in shake flask mediated by GPX. In contrast, the conditions in the bench bioreactor generated a higher stress environment, increasing ROS production without activating enzymatic or non-enzymatic responses led by guaiacol and phenolic compounds, respectively. In conclusion, bench stirred-tank bioreactor scale affects biomass production and sugars uptake negatively.
Key words: Thevetia peruviana, plant cell suspension culture, stirred-tank bioreactor, phenolic compounds, antioxidant activity.
Abbreviation:
ABTS, 2,2'-Azino-bis(3-ethylbenzothiazoline-6-sulphonic acid); GAE, gallic acid equivalent; DCFDA, 2’,7’ dichlorofluorescein diacetate; ROS, reactive oxygen species; GPX, guaiacol peroxidase activity; PVP, polyvinylpyrrolidone; TG, tetraguaiacol.
In vitro plant cell cultures have been considered over the last 50 years as an alternative of bioactive compounds production. Glycosides, alkaloids, terpenoids, phenolic acids and flavonoids are some of the bioactive metabolites that can be used as food and pharmaceutical additives (Dias et al., 2016), and could be produced in plant cell cultures. However, the establishment of in vitro plant cell cultures has technical difficulties, such as productivity loss related to scale-up from shake flask to stirred bioreactors (Ruffoni et al., 2010), among others. Up to date, commercial processes involving plant cell cultures are limited to just a handful valuable secondary metabolites such as shikonin, ginseng, digoxin, sanguinarine and placlitaxel (Paek et al., 2014). Thevetia peruviana is a shrub that belongs to the Apocynaceae family and it has been considered as an important source of biologically active compounds (Duke et al., 2002). The establishment of in vitro cultures of T. peruviana had been reported previously.
Phenolic acids with significant antioxidant effects have been reported in callus cultures of T. peruviana at shake flask scale under regular (Rincón et al., 2016) and different light conditions (Arias et al., 2016), as well as in vivo cultures (Dixit et al., 2015). These studies show the growing importance of T. peruviana as a plant species with great potential in the production of secondary metabolites with biological activity. However, it is necessary to carry out further studies that allow to maintain or increase the productivity of such metabolites on a large commercial scale. This work aims to evaluate the effect of scale change from shake flask to bench stirred-tank bioreactor on biomass, phenol production and antioxidant activity of suspension cultures of T. peruviana in order to establish a strong foundation for a successful scale-up.
Establishment of plant and cell suspension cultures
The protocol for establishment of in vitro cultures of T. peruviana from fruit pulp has already been described in detail (Arias et al., 2009). Briefly, fruits were initially disinfected using ethanol (70% v/v), sodium hypochlorite (10% w/v), and sterile distilled water. Next, fresh explants were extracted aseptically and sowed in solid SH (Schenk and Hildebrandt, 1972) medium supplemented with 30 g/L sucrose, 2 mg/L of 2,4-D and 0.5 mg/L of kinetin. Friable callus (4 g) were transferred to 250 mL Erlenmeyer flask with SH liquid medium. Cell suspension cultures were maintained at 25°C constant agitation (110 rpm) and darkness condition. All cell suspension cultures used in the experiments were in exponential growth phase (6 days from the last subculture). Experiments started with an initial cell concentration of approximately 6 g DW/L.
Erlenmeyer flask cultures
Cell suspension cultures were inoculated in 100 mL of SH medium in 250 mL Erlenmeyer flasks. Cultures were incubated as described earlier and samples were taken every 2 to 3 days.
Bench bioreactor cultures
Cell suspension cultures were carried out in a 7 L stirred-tank bioreactor (Applikon, NL) with three baffle plates and a six-bladed disc turbine impeller. DO was maintained at 30% with air using a sterilizable DO probe (ColeParmer, USA), a Controller Alpha-DO2000PPG (Eutech Instruments, NL), and a stainless steel sparger (seven orifices) located below the turbine. pH was maintained at 5.8 using a sterilizable pH probe (ColeParmer, USA) and a Controller Alpha-pH 800 (Eutech Instruments, NL). Cells were inoculated in 5 L (working volume) of SH medium. Cultures were incubated at 25°C and rotational speed was set at 300 rpm. Samples were taken every 2 to 3 days.
Cell growth determination
Cell growth was determined by dry weight technique. Cell culture aliquots (5 mL) were subjected to vacuum filtration through a pre-dried filter paper Whatman 595. Next, the retained biomass was washed 3 times with distilled water and dried in a convection oven until it reached constant weight after 24 h at 60°C.
Viability
Cell membrane integrity was measured by the blue dye exclusion test, according to the protocol described by Gaff and Okong’o-ogola (1971) with some modifications. Briefly, 75 μL of cell suspension were incubated with 25 μL of Evan’s blue (10 mg/mL) for 5 min and microscopically analyzed. The percent viability of samples was determined based on the number of non-stained cells (viable) respect to the number of total cells.
Morphology
Photographic recording of the cells at 10 and 40X was carried out using a Leica Microscope every 2 to 3 days. Circularity measurements (width/length) and cell aggregate size, if they were present, were made using the ImageJ software (https://imagej.nih.gov/ij/).
Sugar quantification
Extracellular sucrose, fructose and glucose concentrations were analyzed using a Shimadzu Prominence HPLC system with a pumping system LC-20 CE, coupled to a refractive index detector RID-10A and the Solution LC software was used. Separation and quantification of sugars were carried out using a reverse phase amino column RP-NH2 NUCLEODUR 100-5 and mobile phase acetonitrile/water (79:21 v/v ratio), with a 2 mL/min flow and a sample size of 10 μL at 35°C.
Metabolite extraction and analysis
Phenol production was determined every 2 to 3 days during 19 days of culture. Extracellular samples (phenolic content and antioxidant activity) were prepared by microfiltration (0.2 µm) of cells free medium. Intracellular phenolic content and antioxidant activity samples were obtained from 200 mg of fresh biomass collected by filtration and resuspended in 1 mL of phosphate buffer (pH 4). Cell extracts (extra and intracellular) were stored at -20°C until use.
Phenolic content
The phenolic content was determined by the adapted Folin-Ciocalteu method (Singleton and Rossi, 1965). The extract (25 µL) was mixed with 70 µL of Folin-Ciocalteu solution and 200 µL of sodium carbonate solution (7.1% w/v). The resulting product was brought to 500 µL with distilled water. This solution was stirred and stored in darkness at 25°C for 30 min, and then the absorbance was measured at 760 nm. The absorbance values were compared with the standard (aqueous solutions of gallic acid) and the results were expressed as mg GAE/g DW and mg GAE/L for intra and extracellular samples, respectively.
Antioxidant activity
The antioxidant activity was evaluated by the 2,2'-Azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) method, according to the protocol described by Re et al. (1999). This technique determines the capacity of a sample to trap the radical cation ABTS and cause its discoloration. The extract (20 μL) was added to 1980 μL of ABTS solution and the absorbance was measured after 30 min at 732 nm. The absorbance values were compared to the reference curve prepared with Trolox as a primary standard. The results were expressed as μmol Trolox Equivalent/g DW.
Production of reactive oxygen species (ROS)
Generation of ROS during the cell growth was evaluated using 2’,7’ dichlorofluorescein diacetate (DCFDA), a ROS-sensitive dye. The protocol employed was proposed by Martín-Romero et al. (2008). Aliquots of 30 µL of the sample were warmed to 37°C before addition of 270 µL of DCFDA (40 µmol/L in phosphate buffer). The kinetics of fluorescence emission at 530 nm was measured by excitation at 490 nm for 3000 s. The oxidation rate of DCFDA is equal to the slope of the kinetic curve. DCFDA oxidation rate can be assumed as an index of ROS production, that is, high oxidation rate indicates high ROS production.
Guaiacol peroxidase activity
Intracellular guaiacol peroxidase activity was measured according to the method described by Villegas et al. (2017) with some modifications. Briefly, 0.2 g of biomass, previously macerated with liquid nitrogen, were mixed with 1 mL of phosphate buffer 10 mM, pH 7.0 with 4% (w/v) of PVP. The mixture was centrifuged at 14000 g for 20 min at 4°C; the supernatant was used as crude extract. 60 μL of crude extract were mixed with 2340 μL of phosphate buffer 10 mM, pH 7.0, 600 μL of guaiacol (1% w/v) and 1.5 μL of H2O2. The absorbance was measured at 470 nm. Specific guaiacol peroxidase activity (GPX) activity was expressed as μM of TG/min/mg protein, where total intracellular proteins were measured by the Bradford (1976) method using the crude extract.
Statistical analysis
All data was reported as the mean values ± standard deviation (SD). All experiments were performed in duplicate (n=2). The difference between treatments was established via two-way analysis of variance (ANOVA) followed by Tukey’s test (p < 0.05) for multiple group comparisons, using the Statgraphics Centurion XV version 15.2.05 software.
Cell growth and substrate consumption
A maximum cell growth (Figure 1) of 18.59 and 8.46 g DW/L for shake flask and bench reactor were obtained at day 14, respectively. The end of the exponential growth phase in shake flask was correlated with the total sugar consumption, especially sucrose and glucose. This behavior was not observed in bench bioreactor, which did not show a clear exponential phase and accumulated fructose in the culture medium over the time. The lower production of biomass in bench reactor corresponded to a lower degree of sucrose hydrolysis and total consumption of fructose; whereas in the shake flask on day 14, sucrose hydrolysis and glucose consumption were complete, while fructose consumption was close to completion. These behavioral differences in the dynamics of cell growth, sucrose hydrolysis, and sugar consumption between the two systems may be due to different mixing regimes and hydrodynamic stress.
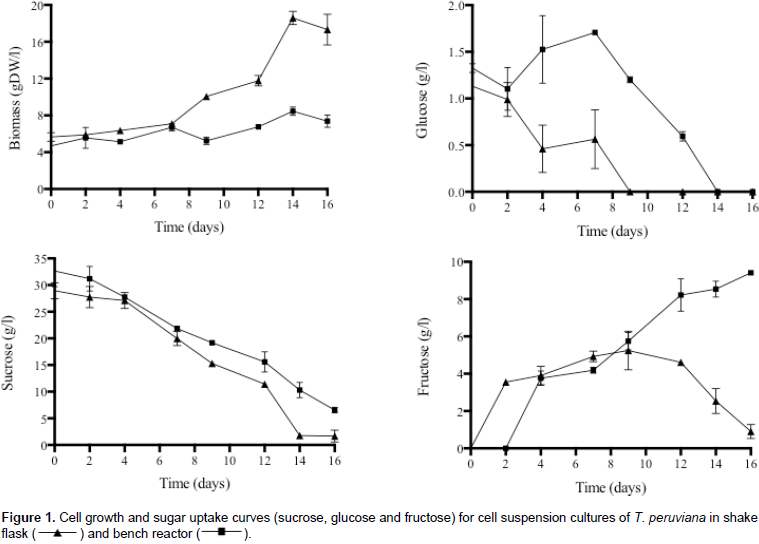
The type of agitation has been related to hydrodynamic stress especially in mechanically stirred-tank reactor (Kieran et al., 2000), which increases the shear stress in the cultures. Differences between maximum values ​​of biomass in shake flask and reactor have been reported in other plant cell species. Cell cultures of Rubia tinctorum showed a 29% decrease in biomass concentration at reactor scale compared to shake flask (Busto et al., 2008). However, Villegas et al. (2017) observed a decrease in biomass production from shake flask (15.69 g DW/L) to reactor scale (10.53 g DW/L at 400 rpm) in cell suspension cultures of Azadirachta indica. In our experiments, both systems showed preferential glucose uptake in contrast to fructose. This last one remained in higher concentration during the time of culture. Preferential glucose consumption has been reported for other plant species at shake flask (e.g., Daucus carota (Krook et al., 2000), Taxus chinensis (Li et al., 2003), and Centella asiatica (Omar et al., 2006), and bioreactor scale (e.g., Elaeis guineensis (Gorret et al., 2004)).
This preferential glucose uptake could partially be explained by a higher affinity of the hexose carrier in the plasma membrane for glucose over fructose (Krook et al., 2000). Cell viability (Figure 2) remained constant for shake flask cultures (85.78%±1.48). Regarding the bench reactor, a slight decrease in cell viability was observed in days 4 and 12; however, the value remains around 83.52%±3.60 during culture. These results suggest that the change in hydrodynamic conditions due to culture system does not affect cell integrity significantly, indicating that T. peruviana cells could be shear tolerant. Same behavior was observed in suspension cultures of Beta vulgaris (Rodríguez and Galindo, 1999) and Uncaria tomentosa (Trejo et al., 2005), where biomass production was affected at bench stirrer bioreactor scale while cell viability remains equal. In contrast, suspension cultures of R. tinctorum (Busto et al., 2008) and Centaurea calcitrapa (Raposo and LimaCosta, 2006) presented a 6-fold reduction and 34% decrease on cell viability respectively, compared to shake flask.
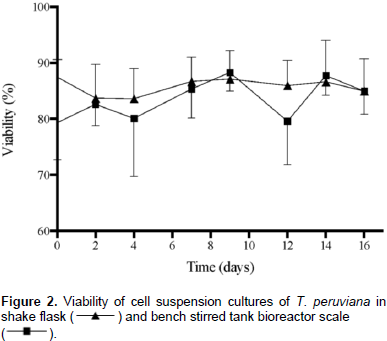
Morphology
Shake flask cells presented a continuous increase of cell circularity from 0.44±0.19 at day 0 to 0.82±0.15 at day 16. Bench stirred tank bioreactor cells showed a slightly decrease from 0.38±0.09 at day 0 to 0.25±0.06 at day 4, and finally an increase to 0.78±0.15 at day 16 (Figure 3). Both systems tend to increase cell circularity throughout the culture. However, the circularity is greater in the shake flask than in the bench bioreactor. This behavior is most likely associated with the higher hydrodynamic stress present in the mechanically stirred bench reactor. At the beginning of the cultures, cells show a cylindrical and elongated shape in both systems (Image 1A and C); this led to the formation of structures similar to fungal hyphae. Through the culture, cells are more rounded and tend to form some small agglomerates. Elongated cells have been reported for other plant cell species, e.g., Nicotiana tabacum (Curtis and Emery, 1993; Mcdonald et al., 2001) and Solanum chrysotrichum (Trejo et al., 2001).
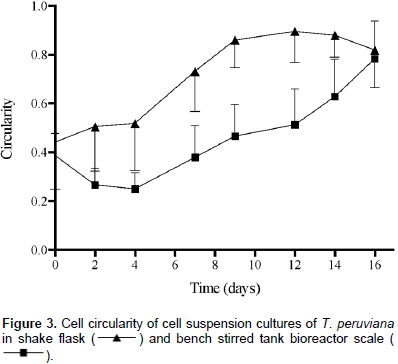
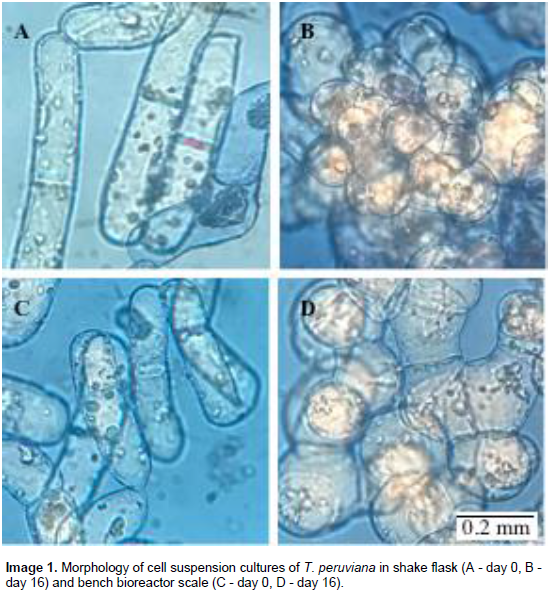
Similarly, rounded shape has been reported for U. tomentosa (Trejo et al., 2005) and Trichosanthes kirilowii (Mcdonald et al., 2001). The initial decrease in circularity and subsequent lower values for stirred tank bioreactor, compared to shake flask during culture time, could be a cell response to the change in hydrodynamic conditions. These results agree with other plant species report found in the literature. Suspension cultures of S. chrysotrichum at bench reactor scale showed a clear tendency to change from an elongated to a round form (Trejo et al., 2001). In contrast cell shape in U. tomentosa cultures changed from round to elongate in reactor culture (Trejo et al., 2005), indicating that this response is specific by species.
Phenolic content and antioxidant capacity
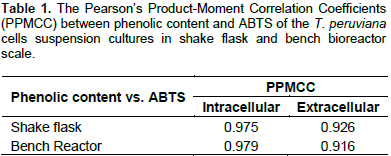
The intra and extracellular phenolic contents and ABTS values of T. peruviana cell suspension cultures in shake flask and bench reactor are as shown in Figure 4. Results show that the production of polyphenols was not affected by the system configuration and scale. Only day 2 showed a significant difference. Kinetic behavior in phenols production indicates an increase until day 7, followed by a decrease. The highest intracellular phenol content was 13.86 and 12.24 mg GAE/g DW at day 7 in bench reactor and shake flask, respectively. The highest extracellular phenol content was 39.05 mg GAE/L at day 7 for bench reactor and 38.19 mg GAE/L at day 9 for shake flask. Production of phenolic compounds in this study is higher than that obtained for other T. peruviana cultures, callus (2.34 mg GAE/g DW) and leaves (2.34 mg GAE/g DW) (Zibbu and Batra, 2012). Therefore, plant cell suspension cultures of T. peruviana are a feasible biotechnological platform for phenol production with important use in food industry.
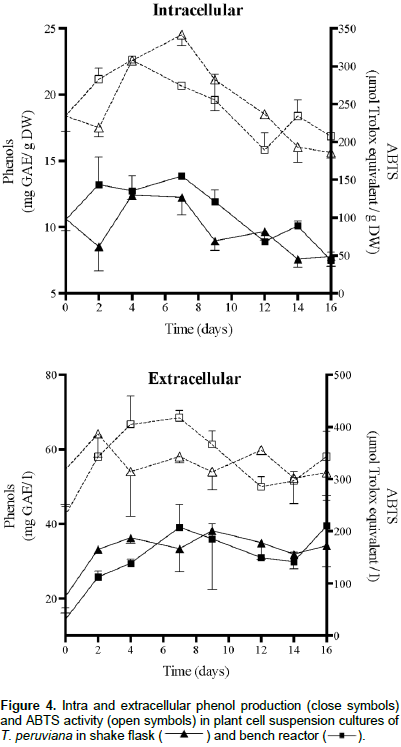
Intra and extracellular ABTS values were directly related with phenol production in both systems. The correlation between phenolics and ABTS was calculated by the Pearson’s Product-Moment Correlation Coefficient (PPMCC). PPMCC values were closed to one (Table 1) indicating positive and perfect correlation between the two variables. This behavior has been reported in previous studies (Arias et al., 2016), showing the consistency of the relationship between the production of phenolic compounds and the antioxidant capacity in plant cell suspension cultures of T. peruviana. These results suggest that phenol production does not constitute an indicator of cell exposure to higher shear environment. To the best of our knowledge there is only one study showing a correlation between phenol accumulation in cell suspensions of C. calcitrapa, in stirred tank reactor, and different agitation rates. Therefore, suggesting that shear-sensitivity of these cells is responsible for an increased phenol production (Raposo and LimaCosta, 2006).
Other authors have reported shear-sensitivity in plant cell suspension cultures and changes in metabolite production as a response to shear rates. Suspension cultures of R. tinctorum showed an increase of 233% in anthraquinones content in bioreactor compared to Erlenmeyer flasks (Busto et al., 2008). Chen and Huang (2000) observed a 64% increase in L-3,4-dihydroxyphenylalanine (L-DOPA) production in Stizolobium hassjoo cells related to a rotational speed of 400 rpm. Shi et al. (2003) studied the shear stress applied in short term on cell suspension cultures of T. chinensis obtaining inhibition on phenylalanine ammonia-lyase (PAL) activity at low shear rate and high PAL activity at higher shear rate.
GPX and ROS production
The technique used in this work for ROS quantification depicts an overall view of the oxidative state of cells as response to culture conditions. Previous studies with other plant species have focused primarily on hydrogen peroxide (H2O2) as a ROS and its effect on cellular behavior (Busto et al., 2008; Chin et al., 2014; Trejo et al., 2007). However, the probe 2,7-dichlorodihydrofluorescein may be oxidized by the presence of other ROS besides hydrogen peroxide (Gomes et al., 2005), contributing to a broader assessment of ROS production. Figure 5 describes GPX activity and intracellular ROS production at shake flask and bench reactor scale in this study. GPX activity was higher at shake flask than in bench stirred tank bioreactor scale during the entire culture time. Increase in GPX from 1.87 to 7.97 μM/min/mg protein in shake flask correlated to low ROS production (0.09 RFU/seg). These results indicate a positive enzymatic cell response to culture condition at this scale mediated by GPX.
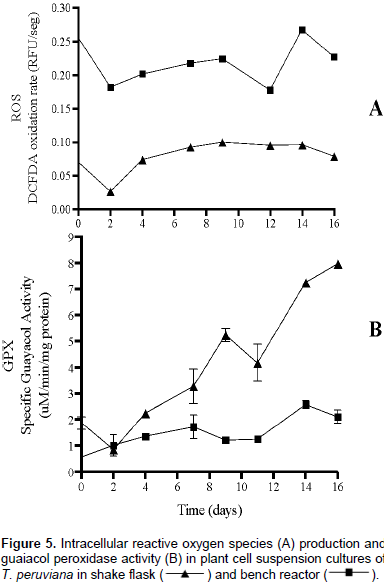
Figure 5A presents a higher intracellular ROS concentration for cell culture at bench stirred tank bioreactor scale compared to shake flask. This behavior was consistent throughout the culture time compared. Low GPX (Figure 5B) and high ROS production at bench reactor scale indicate that the antioxidative defense mechanism in T. peruviana cells is not responding or, at least, is not mainly mediated by GPX to counter the initial stressful condition; hence, leading to the observed decrease in cell growth and morphology changes without affecting cell viability. Other enzymes activities, such as superoxide dismutase (SOD), ascorbate peroxidase (APX), and catalase (CAT), as well as non-enzymatic processes, could be taking place (Åžen, 2012) and could be considered in future studies.
The results reported in our study are consistent with those reported by Villegas et al. (2017) who used the same technique for ROS quantification in suspension cultures of A. indica. In this case, the enzymatic response to high shear stress condition in reactor scale was not mediated by guaiacol enzyme. Finally, from our study it can be inferred that there was a stressful condition for the plant cells of T. peruviana cultivated at reactor scale, because their growth was considerably affected. However, the high cell viability, considerable sugar consumption, and observed morphology, in combination with the lack of enzymatic and non-enzymatic responses (by action of guaiacol peroxidase and production of phenolic compounds, respectively) suggest that the cell’s metabolism could be affected to another level not identified yet.
This is the first study related to the change of plant cell suspension cultures of T. peruviana from shake flask to bench stirred tank reactor in the production of phenolic compounds. Bench reactor scale affected biomass production and sugars uptake negatively. Preferential consumption of glucose over fructose for cell suspension cultures of T. peruviana was found, while cell morphology, viability, phenol production, and antioxidant activity were not significantly affected. Increased ROS production, as an indicator of a metabolic response to a high stress condition in bench reactor scale, did not activate enzymatic and non-enzymatic responses led by guaiacol or phenolic compounds, respectively. Experimental observations indicate that the T. peruviana cell line is not as sensitive as other plant cell species (Busto et al., 2013; Raposo and LimaCosta, 2006; Shi et al., 2003) to shear forces under hydrodynamic stress environment. However, it is necessary to carry out further experiments in order to identify if other level of the cellular metabolism may be affected by hydrodynamic stress variations.
The authors have not declared any conflict of interests.
REFERENCES
|
Arias JP, Zapata K, Rojano B, Arias M (2016). Effect of light wavelength on cell growth, content of phenolic compounds and antioxidant activity in cell suspension cultures of Thevetia peruviana. J. Photochem. Photobiol. B Biol. 163:87-91.
Crossref
|
|
|
|
Arias M, Angarita MJ, Restrepo, JM, Caicedo LA, Perea M (2009). Elicitation with methyl-jasmonate stimulates peruvoside production in cell suspension cultures of Thevetia peruviana. In vitro Cell. Dev. Biol. Plant 46:233-238.
|
|
|
|
|
Bradford MM (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254.
Crossref
|
|
|
|
|
Busto VD, Calabró A, Rodríguez J, Giulietti AM, Merchuk JC (2013). Anthraquinones production in Rubia tinctorum cell suspension cultures: Down scale of shear effects. Biochem. Eng. J. 77:119-128.
Crossref
|
|
|
|
|
Busto VD, Rodríguez J, Giulietti AM, Merchuk JC (2008). Effect of shear stress on anthraquinones production by Rubia tinctorum suspension cultures. Biotechnol. Prog. 24:175-181.
Crossref
|
|
|
|
|
Chen SY, Huang SY (2000). Shear stress effects on cell growth and L -DOPA production by suspension culture of Stizolobium hassjoo cells in an agitated bioreactor. Bioprocess Biosyst. Eng. 22:5-12.
Crossref
|
|
|
|
|
Chin WYW, Annuar MSM, Tan BC, Khalid N (2014). Evaluation of a laboratory scale conventional shake flask and a bioreactor on cell growth and regeneration of banana cell suspension cultures. Sci. Hortic. 172:39-46.
Crossref
|
|
|
|
|
Curtis WR, Emery AH (1993) Plant cell suspension culture rheology. Biotechnol. Bioeng. 42:520-526.
Crossref
|
|
|
|
|
Dias MI, Sousa MJ, Alves RC, Ferreira ICFR (2016). Exploring plant tissue culture to improve the production of phenolic compounds: A review. Ind. Crops Prod. 82:9-22.
Crossref
|
|
|
|
|
Dixit A, Singh H, Sharma RA, Sharma A (2015). Estimation of antioxidant and antibacterial activity of crude extracts of Thevetia peruviana (Pers.) K. Schum. Int. J. Pharm. Pharm. Sci. 7:55-59.
|
|
|
|
|
Duke JA, Bogenschutz G MJ, DuCellier J, Duke PAK (2002). Handbook of Medicinal Herbs, Second. ed. CRC Press. Boca Raton, London, New York, Washington DC.
|
|
|
|
|
Gaff DF, Okong'o-ogola O (1971). The use of non-permeating pigments for testing the survival of cells. J. Exp. Bot. 22:756-758.
Crossref
|
|
|
|
|
Gomes A, Fernandes E, Lima, JLFC (2005). Fluorescence probes used for detection of reactive oxygen species. J. Biochem. Biophys. Methods 65:45-80.
Crossref
|
|
|
|
|
Gorret N, bin Rosli SK, Oppenheim SF, Willis LB, Lessard P, Rha C, Sinskey AJ (2004). Bioreactor culture of oil palm (Elaeis guineensis) and effects of nitrogen source, inoculum size, and conditioned medium on biomass production. J. Biotechnol.108:253-263.
Crossref
|
|
|
|
|
Kieran PM, Malone DM, MacLoughlin PF (2000). Effects of hydrodynamic and interfacial forces on plant cell suspension systems. Adv. Biochem. Eng. Biotechnol. 67:139-177.
Crossref
|
|
|
|
|
Krook J, Vreugdenhil D, Van Der Plas LHW (2000). Uptake and phosphorylation of glucose and fructose in Daucus carota cell suspensions are differently regulated. Plant Physiol. Biochem. 38:603-612.
Crossref
|
|
|
|
|
Li C, Yuan Y, Wu J, Hu Z (2003). A structured kinetic model for suspension cultures of Taxus chinensis var. mairei induced by an oligosaccharide from Fusarium oxysporum. Biotechnol. Lett. 25:1335-1343.
Crossref
|
|
|
|
|
Martín-Romero FJ, Miguel-Lasobras EM, Domínguez-Arroyo JA, González-Carrera E, Álvarez IS (2008). Contribution of culture media to oxidative stress and its effect on human oocytes. Reprod. Biomed Online 17:652-661.
Crossref
|
|
|
|
|
Mcdonald KA, Jackman AP, Hurst S (2001). Characterization of plant suspension cultures using the focused beam reflectance technique. Biotechnol. Lett. 23:317-324.
Crossref
|
|
|
|
|
Omar R, Abdullah MA, Hasan MA, Rosfarizan M, Marziah M (2006) Kinects and modelling of cell growth and substrate uptake in Centella asiatica cell culture. Biotechnol. Bioprocess Eng. 11:223-229.
Crossref
|
|
|
|
|
Paek K, Murthy HN, Zhong J (2014). Production of Biomass and Bioactive Compounds Using Bioreactor Technology. Springer.
Crossref
|
|
|
|
|
Raposo S, LimaCosta ME (2006). Rheology and shear stress of Centaurea calcitrapa cell suspension cultures grown in bioreactor. Biotechnol. Lett. 28:431-438.
Crossref
|
|
|
|
|
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 26:1231-1237.
Crossref
|
|
|
|
|
Rincón J, Rodríguez L, Ruíz VM, Abud M, Luján MC, Ruiz N, González D, Gutiérrez FA (2016). Fatty acids profile, phenolic compounds and antioxidant capacity in elicited callus of Thevetia peruviana (Pers.) K. Schum. J. Oleo Sci. 318:311-318.
Crossref
|
|
|
|
|
Rodríguez M, Galindo E (1999). Broth rheology, growth and metabolite production of Beta vulgaris suspension culture: A comparative study between cultures grown in shake flasks and in a stirred tank. Enzyme Microb. Technol. 24:687-693.
Crossref
|
|
|
|
|
Ruffoni B, Pistelli L, Bertoli A, Pistelli L (2010). Plant cell cultures: bioreactors for industrial production. Adv. Exp. Med. Biol. 698:203-221.
Crossref
|
|
|
|
|
Schenk RU, Hildebrandt AC (1972). Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant cell cultures. Can. J. Bot. 50:199-204.
Crossref
|
|
|
|
|
Åžen A (2012). Oxidative Stress Studies in Plant Tissue Culture, In. Antioxidant Enzyme. pp. 59-88.
|
|
|
|
|
Shi Z, Yuan Y, Wu J, Shang G (2003). Biological responses of suspension cultures of Taxus chinensis var. mairei to shear stresses in the short term. Appl. Biochem. Biotechnol. 110:61-74.
Crossref
|
|
|
|
|
Singleton VL, Rossi JJ (1965). Colorimetric of total phenolics with phosphomolybdic-phosptungstic acid reagents. Am. J. Enol. Vitic. 16:144-158.
|
|
|
|
|
Trejo G, Cerda CM, Rodríguez M, Ramos AC (2005). Monoterpenoid oxindole alkaloid production by Uncaria tomentosa (Willd) D.C. cell suspension cultures in a stirred tank bioreactor. Biotechnol. Prog. 21:786-792.
Crossref
|
|
|
|
|
Trejo G, Jiménez A, Villarreal L, Rodríguez M (2001). Broth rheology and morphological analysis of Solanum chrysotrichum cultivated in a stirred tank. Biotechnol. Lett. 23:1943-1946.
Crossref
|
|
|
|
|
Trejo G, Sepúlveda G, Trejo JL, Cerda CM, de la Torre M, Rodríguez M, Ramos AC (2007). Hydrodynamic stress induces monoterpenoid oxindole alkaloid accumulation by Uncaria tomentosa (Willd) DC cell suspension cultures via oxidative burst. Biotechnol. Bioeng 98:230-238.
Crossref
|
|
|
|
|
Villegas S, Martínez AD, Hoyos R, Rojano B, Orozco F (2017). Hydrodynamic stress and limonoid production in Azadirachta indica cell culture. Biochem. Eng. J. 122:75-84.
Crossref
|
|
|
|
|
Zibbu G, Batra A (2012). In vitro and in vivo determination of phenolic contents and antioxidant activity of desert plants of Apocynaceae family. Asian J. Pharm. Clin. Res. 5:75-83.
|
|