ABSTRACT
Indole-3-acetic acid (IAA) is an important plant growth regulator. As the very first endeavor, the study is aimed at extracting and quantifying IAA from seedlings of Bambusa tulda and evaluating its bioactivity. The extraction of IAA was performed in organic solvent followed by sample evaporation and TLC with a mobile phase composed of chloroform, methanol and formic acid (77:22:1 v/v) in isocratic mode. The extract obtained from preparatory TLC was subjected to HPLC with acetic acid and methanol (75:25 v/v) as the mobile phase in isocratic mode at a flow rate of 0.8 ml/min and operation pressure of 54 MPa at 30°C, and detection was monitored at 280 nm. Bioassay of the extracted IAA was carried out in Abelmoschus esculentus seedlings. The similar Rf value (0.412) of the extract during TLC analysis and similar peak of HPLC chromatogram with retention time 28.71 min to that of standard IAA indicated the presence of IAA in B. tulda seedling extract. The extracted IAA was quantified to be 10.28 µg/ml. In bioassay experiment, the extracted IAA significantly enhanced root length, root fresh weight, root dry weight, shoot length, shoot fresh weight, shoot dry weight and significant increase of total chlorophyll and protein in A. esculentus leaves. Therefore, B. tulda seedlings could be a potential source of IAA, and it can be utilized for production of bio-fertilizer at a commercial scale.
Key words: Abelmoschus esculentus, Bamboo, High performance liquid chromatography, indole-3 acetic acid.
The demand for agricultural yield has increased tremendously with an increase in the world’s population and thereby leading to a large scale production of chemical fertilizers. Since the use of chemical fertilizers and pesticides in the agricultural fields has caused degradation of soil quality and fertility, thus the expansion of agricultural land with fertile soil is nearly impossible. Hence researchers and scientists have shifted their attention for a safer and productive means of agricultural practices (Gouda et al., 2018). Auxin is a critical phytohormone for plant growth and orchestrates many developmental processes in plants. Complex and redundant regulation of IAA abundance, transport, and response allow an intricate system of auxin utilization that achieves a variety of purpose in plant growth and development (Woodward and Bartel, 2005). It is a low molecular weight compound highly involved in the control of plant growth and development (Porfírio et al., 2016). Although there are several naturally occurring compounds comprising auxin activity, IAA is the most abundant auxin (Simon and Petrasek, 2011), only a minor part of which resides in plant in free form, the majority being conjugated to amino acids or sugars (Ludwig et al., 2009).
IAA is considered to be the main biologically active plant hormone of the auxin class and is a product of L-tryptophan (L-Trp) metabolism. It stimulates cell elongation by modifying certain conditions, like increase in cell osmotic contents, increase in water permeability into the cell, decrease in wall pressure, increase in cell wall synthesis, and inducing specific RNA and protein biosynthesis. It promotes antioxidant activity, inhibits or delays abscission of leaves, induces flowering and fruiting (Zhao, 2010). It is a mobile signaling molecule that can be transported among cells to form auxin gradients and auxin maxima that are essential for plant development (Petrasek and Friml, 2009). The widespread use of auxin in plant propagation protocols and physiological studies (Strader et al., 2010) has led to many efforts towards the development of analytical methods for the quantification of the very low auxins levels in plants. Simultaneous quantitative profiling of IAA provides a basis for defining additive, synergistic or antagonistic hormone activities and identifying hormone networks regulating plant functions. For quantitative measurement of endogenous plant hormones in crude plant extracts, HPLC–MS/MS provides high sensitivity, specificity, accuracy and reproducibility (Gomez et al., 2002).
Being a regulating agent in numerous plant growth phenomena, in an attempt to assess its role in plant growth and development, its quantification is crucial a step. The ability to rapidly quantify IAA in small amounts of tissue has great value in the study of auxin biology. As an essential signaling molecule, quantitative information about IAA levels has been a valuable aspect. Guney et al. (2016a) assessed the effect of hormonal applications on seed germination and seedling morphological traits in Lilium artvinense. The application of IAA and other phytohormons was recorded to be influential in germination percentage of Lilium martagon seeds (Guney et al., 2016b). Similarly, auxin has been found to enhance rooting effect in Ficus benjamina L. (Topacoglu et al., 2016) and Schefflera arboricola (Sevik et al., 2015). Therefore, IAA has been quantified from tobacco flowers (Liu et al., 2002), aerial parts of Arabidopsis thaliana L. (ecotype Columbia) plants, leaves of Triticum aestivum L., cv. Jara and Nicotiana tabacum L., cv. Bright Yellow 2, N. tabacum L., cv. Wisconsin 38 (Dobrev et al., 2005), Acer mono, A. negundo and Zea mays (Zhang et al., 2008) and A. thaliana (Barkawi et al., 2010), and leaves and crown of T. aestivum cultivars, winter cv. Samanta and spring cv. Sandra (Kosova et al., 2012). There is scanty of research on quantification of IAA from bamboo seedlings. In this research, the IAA from seedlings of B. tulda was quantified through HPLC method and its bioassay in Abelmoschus esculentus was performed with the hypothesis that bamboo seedlings could be potent source of IAA.
Sample preparation
Samples were prepared according to Barkawi et al. (2010), Pan et al. (2010), Liu et al. (2012) and Porfírio et al. (2016) with slight modifications. Seeds of B. tulda were obtained from the Department of Forest Resources, Janakpur, Nepal. The seeds were surface sterilized in 70% ethanol with 0.1% Tween 20 (polyoxyethylene sorbitan monolaurate) working in a laminar flow hood (Barkawi et al., 2010). The samples were vortexed intermittently for 5 min, and the solution was removed using a sterile Pasteur pipet, taking care to remove as much liquid as possible and seeds were dried overnight in laminar flow hood. About 25 to 35 germinating seeds were placed in sterile filter paper in petri-plates. Experiments were repeated five times and replicated three times making a total of 15 petri plates and they were randomly kept in growth chamber at 28±2°C adjusted to 16 h light and eight hours dark period for 18 days (Figure 1). Autoclaved distilled water was applied to the experiment in the morning (11:00 am-12:00 noon) to avoid water stress. After 18 days, the seedlings were harvested using sterilized forceps; blotted on a laboratory tissue paper and the separated upper portion of the seedlings were stored at -80°C in liquid nitrogen until the time of further analysis. 250 mg stored sample was ground in liquid nitrogen, then it was mixed with methanol (HPLC grade) (4 ml per gram of fresh weight) followed by centrifugation at 4°C for 15 min at 10,000 rpm. The pallet was sonicated for five minutes and re-extracted with methanol. The pH of the solution was acidified to pH 2.5 with 1 M HCL and extracted twice with methanol. The upper organic phase was transferred to a beaker, filtered by syringe filter (SFPS25X, 0.45 µm × 25 mm) and evaporated in rotatory evaporator at 35°C. The extract was dissolved in 100 ml methanol and stored at -20°C. The reference (control) solution was obtained by dissolving 5 mg standard IAA (HiMedia) in 5 ml of methanol.
Detection of IAA by thin layer chromatography (TLC)
A thin mark was made 1 cm above the bottom of the TLC plate (60GF254, 20 × 20 cm, Merck) to apply the sample and control (standard IAA) spots (Abubakar et al., 2016). Methanol fraction of 25 µl of test sample and 15 µl of control solution were spotted equidistantly (1 cm) on the TLC plate using capillary tubes and developed in the mixture of chloroform, ethyl acetate and formic acid (77:22:1 v/v) as mobile phase. The mobile phase was poured into the TLC chamber to a leveled few centimeters above the chamber bottom. The solvent was allowed to saturate the container. The plate was then immersed in such way that the sample spots were well above the level of mobile phase, but not immersed in the solvent for development. After migration of the principal components, detection was performed in UV light (254nm) by spraying with the mixture of 2 ml of 0.5 M FeCl3 in 98 ml of 35% HClO4 (Rahman et al., 2010), and the spots with retardation factor (Rf) value identical to standard IAA (HiMedia) was calculated.
Similarly, preparative TLC was carried out to obtain the purity of sample for HPLC. The analysis was performed in triplicates.
Quantification of IAA by HPLC
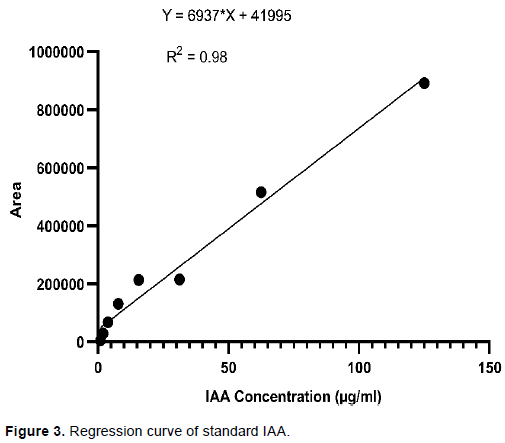
HPLC was performed to quantify the IAA in reverse-phase (RP) HPLC using an RP-C18 column (5 µm, 250 × 4 mm) with UV absorbance at 280 nm. 15 µl extract of bamboo seedlings and standard IAA was injected on RP-C18 column (Shimadzu Lab solutions). The mobile phase was composed of acetic acid: Methanol (75:25 v/v) at pH 3.8 of HPLC grade at isocratic phase. The flow rate was 0.8 ml/min and the operation pressure of 54 MPa at 30ºC. Quantity of standard IAA and test sample was individually adapted in order to estimate the quantity of IAA in bamboo seedlings. Standard calibration curves were obtained by plotting the peak areas of standard concentration of IAA (0.975-500 µg/ml) using a serial dilution and equation was generated to quantify IAA from bamboo seedlings. Experiments were repeated three times with three replication.
In-vitro bioactivity of extracted IAA on ladies’ finger (Abelmoschus esculentus) seeds
Bioactivity of extracted IAA from bamboo seedlings was demonstrated by inoculating seeds of A. esculentus in in-vitro under auxenic condition. A. esculentus was selected for this experiment because it is one of the most preferred vegetables in context of Nepal. A. esculentus seeds were collected from local agricultural cooperative, Kathmandu, Nepal. The seeds were then surface sterilized with 1% sodium hypochlorite for one minute and washed three times with autoclaved distilled water. Then the seeds were immersed in 1 mg/ml stock solution of extracted IAA, standard IAA (HiMedia) as positive control and distilled water as negative control. Seeds were dried overnight in laminar hood and 10 germinating seeds were kept in sterilized filter paper in petri plates. Each treatment was repeated three times with three replicates thus making a total of 27 petri plates which were placed in growth chamber at 30±2°C for 14 days. Autoclaved distilled water was applied to the experiment in the morning (10:00 am -11:00 am) in the interval of 48 h. After 14 days, the growth parameters like root length, shoot length, root fresh weight, root dry weight, shoot fresh weight, shoot dry weight, and total chlorophyll and protein content of A. esculentus seedlings were recorded for all the treatments. Total chlorophyll and protein content of the seedlings under in-vitro assay were estimated by reading optical density at 645, 652 and 663 nm on spectrophotometer (Shimadzu UV-1800). The amount of total chlorophyll pigments was determined by the equation of Arnon (1949) and protein by Folin reaction (Lowry et al., 1951).
Statistical analysis
Analysis of variance (ANOVA) was performed to compare growth characters of A. esculentus using IBM Statistical Package for the Social Science (SPSS). Mean values of growth characters of A. esculentus were analysed by Tukey’s Honestly significant difference (HSD) at the significance level of P < 0.05.
Qualitative analysis of IAA by TLC
IAA was extracted from bamboo seedlings using methanol solvent. TLC results showed that this method may be used efficiently to detect target compounds. The methanol extract of bamboo seedlings affirmed the presence of IAA with Rf value similar to the standard IAA (0.412) indicating that IAA was present in B. tulda seedling extract (Figure 2A and B). IAA has been implicated in almost every aspect of plant growth and development from embryogenesis to senescence (Perry et al., 2005).
Plant growth regulators are difficult to analyze because they occur in very low amounts in plant extracts which are rich in interfering substances, especially secondary metabolites (Dobrev et al., 2005). Biochemical experiments regarding quantification of IAA as we have performed from bamboo seedlings are the very first attempt in context of Nepal. Similar research in relation to isolation and quantification of plant growth regulators like IAA in bamboo seedlings as well as in other plants were unavailable to compare our research in context of Nepal.
Sporadic efforts performed to analyze IAA in other plants most commonly in abroad have been gathered to discuss with our results.
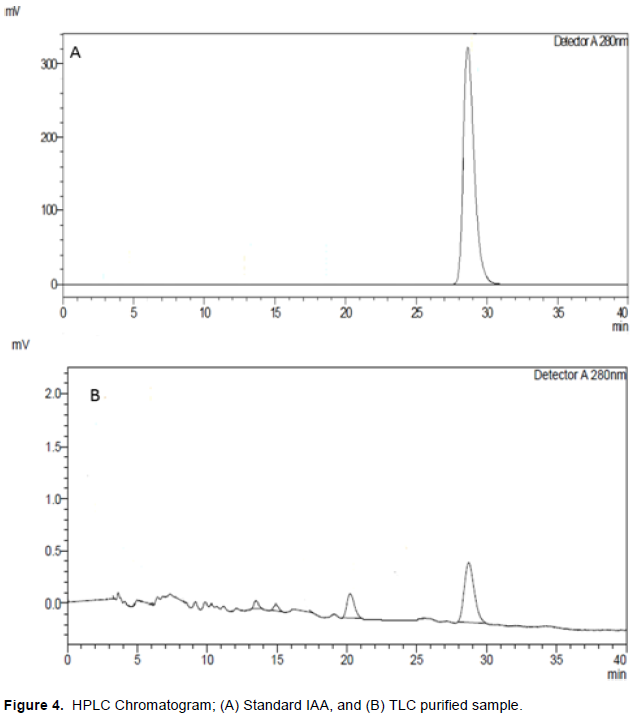
We used TLC followed by HPLC for quantification of our target bioactive compounds (IAA) from B. tulda seedlings. Although a wide variety of methods are available for auxin isolation and analysis, chromatographic methods are still the predominant and established technique within the laboratory (Porfírio et al., 2016). The IAA on a reversed phase HPLC column was quantified under isocratic program. Extracted IAA from B. tulda seedlings showed similar peak to that of standard IAA (HiMedia) in HPLC analysis. The retention time of extracted IAA was found to be 28.71 min (Figure 4A and B). There was a linear relationship between the concentration of standard IAA and area of HPLC peak and an equation Y= 6937x + 41995 (R2= 0.98) (Figure 3) was obtained. Amount of IAA production by B. tulda seedlings was quantified to be 10.28 µg/ml. Sharma et al. (2014) mentioned that the goal of HPLC is to promote the measurable IAA and reduce the amount of unexpected substances in sample. Junior et al. (2011) mentioned that HPLC detected IAA levels which are undetectable in colorimetric analysis. The amount of IAA extracted from B. tulda seedlings by HPLC was 10.28±0.1µg/gm in this experiment. Kim et al. (2006) reported the 20.4±6.1 and 16.2±11 nmol/gm from Zea mays between 1 and 2 cm of primary root tip. Similarly, Nakurte et al. (2012) reported 14.03±1.84 ng/gm IAA from shoot extract of Hordeum vulgare while Hussain and Hasnain (2011) reported 293.33 ng/gm IAA from seedling extract of Triticum aestivum var. Uqab 2000 during ultra-performance liquid chromatography coupled to a tandem mass spectrometer through electrospray interface. Higher level of extracted IAA in this experiment may be attributed to rapid growth nature of bamboo during in-vitro growth condition. However, the growth conditions of Hordeum vulgare (Nakurte et al., 2012) at 22°C for 14 days and that of Triticum aestivum var. Uqab 2000 (Hussain and Hasnain, 2011) incubated under autoclaved calcinated sand for 14 days were quite different than was set in this experiment. The amount of IAA may vary depending on the types of plants and growth condition in in-vitro experiments.
In-vitro bioactivity of extracted IAA on ladies’ finger (Abelmoschus esculentus) seeds
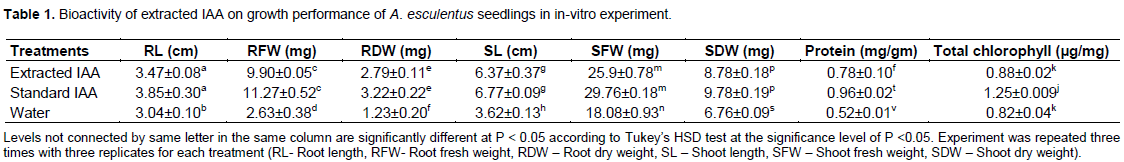
Analysis of growth characters such as shoot length (6.37±0.37), shoot fresh weight (25.93±0.78), shoot dry weight (8.78±0.18), root length (3.4789±0.08), root fresh weight (9.90±0.05) and root dry weight (2.79±0.11) of A. esculentus seedlings inoculated with extracted IAA were found significant as compared to negative controls (P < 0.05) (Table 1). However, these growth parameters of A. esculentus treated with standard IAA (HiMedia) were not significantly different (P< 0.05) to the seedlings inoculated with extracted IAA. Similarly, extracted IAA also contributed increased biosynthesis of protein content (0.78±0.1 mg /gm) and total chlorophyll (0.88±0.02) in A. esculentus leaves. K.C. et al. (2020) reported marked influence of IAA on production of total chlorophyll in B. tulda seedlings. The results revealed an increase of growth performance of A. esculentus seedlings inoculated with extracted and standard IAA than the negative control. Enhanced chlorophyll content may be an indication of interaction that stimulates the chlorophyll related enzymes for increased biosynthesis of chlorophyll (Kang et al., 2014). The explicit role of IAA has been witnessed in almost every aspect of plant growth and development from embryogenesis to senescence (Perry et al., 2005).
The study quantified and highlighted the importance of IAA for growth and development of plants. Bioassay of extracted IAA from B. tulda seelings in A. esculentus were found to be effective in growth of shoot length, shoot biomass, root length, root biomass, and biosynthesis of total chlorophyll and protein in leaves. This IAA deserves the potential of being used as exogenous source of phytohormone although it requires further purification. An assessment of growth response of this extracted IAA in agronomically important crops under greenhouse and field conditions are warranted. Bamboo seedlings are an important but less explored source of plant growth regulators. Therefore, similar types of follow up research in seeds of other bamboo species as well as juvenile culms are essential.
The authors declared that there is no conflict of interests.
The author would like to acknowledge the President Chure Tarai Madhesh Conservation and Development Board (KU/CHURE/PROJECT/01) for financial support, and Organic Farming and Natural Product Research Centre (ONRC), Kathmandu University for laboratory facilities. Authors are thankful to Sharmila Chimouriya, Ashesh Acharya and Nabin Bogati for their assistance in laboratory analysis.
REFERENCES
|
Abubakar N, Shehu K, Yahaya MM, Tafinta IY, Imonikhe MA (2016). Phytochemical Screening and Thin Layer Chromatographic Studies of Guiera senegalensis GF Gmel (Egyptian mimosa). Annals of Biological Sciences 4(1):26-30.
|
|
|
|
Arnon DI (1949). Copper enzymes in isolated chloroplasts. Polyphenoloxidase in beta vulgaris. Plant Physiology 24(1):1-15.
Crossref
|
|
|
|
|
Barkawi LS, Tam YY, Tillman JA, Normanly J, Cohen JD (2010). A high-throughput method for the quantitative analysis of auxins. Nature Protocols 5(10):1609-1618.
Crossref
|
|
|
|
|
Dobrev PI, Havlicek L, Vagner M, Malbeck J, Kaminek M (2005). Purification and determination of plant hormones auxin and abscisic acid using solid phase extraction and two-dimensional high performance liquid chromatography. Journal of Chromatography A 1075(1-2):159-166.
Crossref
|
|
|
|
|
Gomezâ€Cadenas A, Pozo OJ, Garciaâ€Augustin P, Sancho JV (2002). Direct analysis of abscisic acid in crude plant extracts by liquid chromatography-electrospray/tandem mass spectrometry. Phytochemical Analysis: An International Journal of Plant Chemical and Biochemical Techniques 13(4):228-234.
Crossref
|
|
|
|
|
Gouda S, Kerry RG, Das G, Paramithiotis S, Shin HS, Patra JK (2018). Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiological Research 206:131-140.
Crossref
|
|
|
|
|
Guney K, Cetin M, Sevik H, Guney KB (2016a). Effects of some hormone applications on germination and morphological characters of endangered plant species Lilium artvinense L. Seeds, New Challenges in Seed Biology-Basic and Translational Research Driving Seed Technology, Dr. Susana Araújo. InTech 4:97-112.
Crossref
|
|
|
|
|
Guney K, Cetin M, Sevik H, Guney KB (2016b). Influence of germination percentage and morphological properties of some hormones practice on Lilium martagon L. seeds. Oxidation communications 39(1):466-474.
|
|
|
|
|
Hussain A, Hasnain S (2011). Interactions of bacterial cytokinins and IAA in the rhizosphere may alter phytostimulatory efficiency of rhizobacteria. World Journal of Microbiology and Biotechnology 27:2645-2654.
Crossref
|
|
|
|
|
Junior RFG, Pedrinho EAN, Castellane TCL, de Macedo Lemos EG (2011). Auxin-producing bacteria isolated from the roots of Cattleya walkeriana, an endangered Brazilian orchid, and their role in acclimatization. Revista Brasileira de Ciencia do Solo 35(3):729-737.
Crossref
|
|
|
|
|
K.C. BM, Gauchan DP, Khanal SN, Chimouriya S, Lamichhane J (2020). Extraction of indole-3-acetic acid from plant growth promoting rhizobacteria of bamboo rhizosphere and its effect on biosynthesis of chlorophyll in bamboo seedlings (Online published). Indian Journal of Agricultural Research Article Id: A-5578.
|
|
|
|
|
Kang SM, Radhakrishnan R, You YH, Joo GJ, Lee IJ, Lee KE, Kim JH (2014). Phosphate solubilizing Bacillus megaterium mj1212 regulates endogenous plant carbohydrates and amino acids contents to promote mustard plant growth. Indian Journal of Microbiology 54:427-433.
Crossref
|
|
|
|
|
Kim YJ, Oh YJ, Park WJ (2006). HPLC-based quantification of indole-3-acetic acid in the primary root tip of maize. Journal of Nanobiotechnology 3(1):40-45.
|
|
|
|
|
Kosova K, Prasil IT, Vítamvas P, Dobrev P, Motyka V, Flokova K, Novak O, Tureckova V, Rolcik J, Pesek B, Travnickova A (2012). Complex phytohormone responses during the cold acclimation of two wheat cultivars differing in cold tolerance, winter Samanta and spring Sandra. Journal of plant physiology 169(6):567-576.
Crossref
|
|
|
|
|
Liu BF, Zhong XH, Lu YT (2002). Analysis of plant hormones in tobacco flowers by micellar electrokinetic capillary chromatography coupled with on-line large volume sample stacking. Journal of Chromatography A 945(1-2):257-265.
Crossref
|
|
|
|
|
Liu X, Hegeman AD, Gardner G, Cohen JD (2012). Protocol: high-throughput and quantitative assays of auxin and auxin precursors from minute tissue samples. Plant Methods 8(1):31.
Crossref
|
|
|
|
|
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951). Protein measurement with the Folin phenol reagent. Journal of biological chemistry 193:265-275.
|
|
|
|
|
Ludwig-Muller J, Decker EL, Reski R (2009). Dead end for auxin conjugates in Physcomitrella? Plant Signaling and Behavior 4(2):116-118.
Crossref
|
|
|
|
|
Nakurte I, Keisa A, Rostoks N (2012). Development and validation of a reversed-phase liquid chromatography method for the simultaneous determination of indole-3-acetic acid, indole-3-pyruvic acid, and abscisic acid in barley (Hordeum vulgare L.). Journal of analytical methods in chemistry 2012:1-6.
Crossref
|
|
|
|
|
Pan X, Welti R, Wang X (2010). Quantitative analysis of major plant hormones in crude plant extracts by high-performance liquid chromatography-mass spectrometry. Nature protocols 5(6):986-992.
Crossref
|
|
|
|
|
Perry J, Dai X, Zhao Y (2005). A mutation in the anticodon of a single tRNAala is sufficient to confer auxin resistance in Arabidopsis. Plant physiology 139(3):1284-1290.
Crossref
|
|
|
|
|
Petrasek J, Friml J (2009). Auxin transport routes in plant development. Development 136(16):2675-2688.
Crossref
|
|
|
|
|
Porfírio S, da Silva MDG, Peixe A, Cabrita MJ, Azadi P (2016). Current analytical methods for plant auxin quantification-A review. Analytica Chimica Acta 902:8-21.
Crossref
|
|
|
|
|
Rahman A, Sitepu IR, Tang SY, Hashidoko Y (2010). Salkowski's reagent test as a primary screening index for functionalities of rhizobacteria isolated from wild dipterocarp saplings growing naturally on medium-strongly acidic tropical peat soil. Bioscience, Biotechnology and Biochemistry 74:2202-2208.
Crossref
|
|
|
|
|
Sevik H, Guney K, Topaçoglu O, Unal C (2015). The influences of rooting media and hormone applications on rooting percentage and some root characters in Schefflera arboricola. International Journal of Pharmaceutical Science Invention 4(2):25-29.
|
|
|
|
|
Sharma S, Verma PP, Kaur M (2014). Isolation, Purification and Estimation of IAA from Pseudomonas sp. using High-performance liquid Chromatography. Journal of Pure and Applied Microbiology 8:3203-3208.
|
|
|
|
|
Simon S, Petrasek J (2011). Why plants need more than one type of auxin. Plant Science 180(3):454-460.
Crossref
|
|
|
|
|
Strader LC, Culler AH, Cohen JD, Bartel B (2010). Conversion of endogenous indole-3-butyric acid to indole-3-acetic acid drives cell expansion in Arabidopsis seedlings. Plant Physiology 153(4):1577-1586.
Crossref
|
|
|
|
|
Topacoglu O, Sevik H, Guney K, Unal C, Akkuzu E, Sivacioglu A (2016). Effect of rooting hormones on the rooting capability of Ficus benjamina L. cuttings. Sumarski list 140(1-2):39-44.
Crossref
|
|
|
|
|
Woodward AW, Bartel B (2005). Auxin: regulation, action, and interaction. Annals of Botany 95(5):707-735.
Crossref
|
|
|
|
|
Zhang FJ, Jin YJ, Xu XY, Lu RC, Chen HJ (2008). Study on the extraction, purification and quantification of jasmonic acid, abscisic acid and indoleâ€3â€acetic acid in plants. Phytochemical Analysis 19(6):560-567.
Crossref
|
|
|
|
|
Zhao Y (2010). Auxin biosynthesis and its role in plant development. Annual Review of Plant Biology 61:49-64.
Crossref
|
|