Full Length Research Paper
ABSTRACT
Fungal diseases are one of the major causes of losses on wheat yield in the world. Recent studies on plant defense mechanisms have highlighted the role of amino acids and total polyphenols in disease tolerance. Thus, with the objective of identifying on the basis of morphological and physiological variables the high-performance wheat accessions in high and low altitude, we characterized sixteen wheat accessions via quantification of polyphenols (TPP) and amino acids (AA) and identified fungal diseases affecting wheat in Centre Cameroon. Using the set of the three hexaploid wheat cultivars where the 11 microsatellite markers data was available, a total of 29 alleles were detected among cultivars and the number of alleles per locus ranged from 2 to 3 with an average of 2.64. Gene diversity ranged from 0.44 to 0.67 with an average of 0.59, increasing with the number of alleles. Microsatellites markers used had an average Polymorphic Information Content (PIC) value of 0.50, indicating that these markers are useful and will make a contribution to the studies in hexaploid wheat. The assessment of wheat plant to disease tolerance permitted to identify Septoria, Fusarium wilt; tanned spot and powdery mildew in high and low altitude. Analyzes of TPP and AA have made it possible to discriminate accessions that are tolerant to diseases. Hence, accession Atilla 1 was highly tolerant and had high levels of TPP (3.5 ± 0.36 μg/mg) and AA (40.1 ± 1.21 μg/mg) while the accession Sup 152 highly susceptible to disease had low levels (2.3 ± 0.1 and 2.1 ± 0.36 μg/mg, respectively). This study will contribute to the extension of wheat in areas where conditions similar to the study sites will be localized.
Key words: Amino acids, bread wheat, high and low altitude, fungal diseases, polyphenols.
INTRODUCTION
Wheat (Triticum aestivum L.) is one of the most valuable crops with a rate of more than 45% in world trade (Mohsen et al., 2014). Cereal of the Poaceae family, its global production increased from 655 million tons in 2014 to 734.9 million tons in 2017 with 26 million tons coming from Africa. In Cameroon wheat is still the most consumed cereal. A highly strategic commodity, with a view to food security and sovereignty, wheat cultivation in Cameroon is limited to small areas despite the country's potential. (Boutraid (1982). With its 66 tons, Cameroon, then unable to meet the needs of its population which amounts to nearly 598,000 tons allocates about 144 billion CFA francs each year for the import of wheat estimated at nearly 750,000 ton. Cameroon is thus very dependent on wheat import and therefore, in order to be able to withstand the financial consequences of price fluctuations, it must imperatively reduce this dependence and work to reach a minimum threshold of self-sufficiency for this speculation. Before the closure of the company Development of Wheat Cultivation in Cameroon (Sodéblé) in 1982, Kouébo and Monté had worked on fungal diseases attacking wheat and had identified Solanika considered today as a highly productive local accession and resistant to fungal diseases. However, for intensive and extensive cultivation, it is urgent to find new accessions that can guarantee competitive yields. It then appears necessary to introduce and popularize accessions with satisfactory ecological and economic solutions. However, the extension of wheat cultivation depends on the resolution of the main constraint related to its production, which is on one hand the problem of altitudes, which seems to be one of the main factors limiting production to nearly 75% (Jabal et al., 2017). Known as Africa in miniature, Cameroon has a high level of topographic variation. However, the majority of cultivable areas are located in low-lying areas (<800 m), since bread wheat usually grows at high altitude, hence it will be important to find accessions adapted to local situations in order to increase the area under cultivation. On the other hand, the problems of fungal infestations such as rust, septoria and fusariosis account for nearly 20 to 100% of the losses on production (Tesfay and Araya, 2015). Several authors have shown that the narrowness of crop genetic diversity could lead to increased susceptibility to diseases and pests, as well as the inability of plants to respond to different environmental constraints (Bendif, 1994; Gorji and Zolnoori, 2011). So, evaluating the genetic diversity is a prerequisite for studying the adaptation of populations to new environmental conditions and hence for the selection of new varieties. In this context, the use of Simple Sequence Repeats (SSRs) markers combines many desirable marker properties such as abundance, high levels of polymorphism (unlike RFLP), very good reproducibility (compared to RAPD), and co-dominance (contrary to the AFLP for which codominance is not exploitable), but also an even coverage of the genome and the specificity of amplification (Tekeu et al., 2017).
On the other hand, phytosanitary constraints being a function of environmental conditions (Siou, 2013), knowledge of the potential of accessions through agronomic and biochemical descriptors remains to be done to choose and make an informed decision on the best accession to introduce (Nda et al., 2014). Several methods are then available for such work, namely the identification of local situations that meet the biological requirements of each accession, and the detection of the elements involved in the defense mechanism against diseases. Indeed, Bushnell (1984); Lattanzio et al., (2006) and Patil et al. (2011) have shown that wheat genotypes in response to pathogens synthesize substances such as polyphenols and total amino acids that, in addition to their roles in growth metabolism, also protect the plant. Our study has been investigated to identify high performance wheat accessions in high and low altitude zone in Cameroon.
MATERIALS AND METHODS
Plant material and morphological characterization
A total of 16 winter and spring wheat varieties were obtained from the International Maize and Wheat Improvement Center (CIMMYT) and the International Center for Agricultural Research in the Dry Areas (ICARDA) (Table 1). Field trials were conducted in two areas of different altitudes of the bimodal humid forest zone of Cameroon (Mount Mbankolo: high-altitude area of 1057 m above the sea level; and Nkolbisson: low altitude area of 650 m above the sea level). At each trial site, an incomplete alpha-lattice design with two replications was used. Each accession was planted in a single 3-m row with 25-cm spacing between the rows.
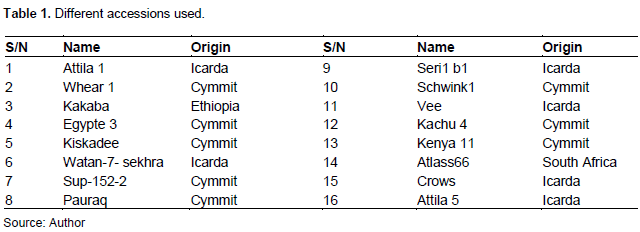
For each wheat accession, plant height (measured using a graduated ruler) and systemic identification of pathogens were assessed, based on observation of symptoms on infected plants and appearance of colonies on the plants via culture medium. Thus, seeding was done under a laminar flow host near a Bunsen burner flame. After visual examination of organs affected by pathogens, isolations were performed according to the protocol of Mahfoud and Lasbuhani (2015). From organ fragments with characteristic symptoms of diseases, including leaves, neck, roots or seeds, small portions were cut (leaves and stems) and disinfected with 2% sodium hypochlorite (1 minute). These fragments were then rinsed three times with sterile distilled water (1 min each time). Using a scalpel previously flamed and immersed in an alcoholic solution, the dried fragments on blotting paper were seeded in Petri dishes containing Potato Dextrose Agar (PDA) on which was indicated the name of the sample, as well as the date of seeding. Then, the seeded Petri dishes were incubated for 7 days until a good fungal development appeared. After good development, colonies were sub-cultured into new Petri dishes containing PDA. The goal was to purify the mushrooms by minimizing the risk of contamination. Finally, these boxes were also incubated for 3 to 4 days. The microscopic identification consisted in observing after putting on the slide a few drops of methyl blue, the appearance of the mycelium and spores if sporulation existed thanks to a photonic microscope with a magnification of 400 x after which the different species observed were determined by criteria established by (Leslie and Summerell (2006).
Genomic DNA extraction
An adjusted Doyle and Doyle (1990) protocol was used to extract genomic DNA (gDNA) from seedlings at the two to three leaf stage. Approximately, 100 mg of plant tissue were cut up and placed into a micro centrifuge tube. 500 µl of 2% (m/v) CTAB extraction buffer [1.4 M NaCl, 20 mM Na2EDTA (pH 8), 100 mM Tris-HCl (pH 8)] and tow sterilized steel bearings were added to each sample. A Qiagen®TissueLyser (Qiagen (Pty) Ltd; local distributer: Southern Cross Biotechnology, Claremont, RSA) was used to grind the samples 2 times for 2 min (min) at 30 Hz. This mixture was then incubated in a water bath for 20 min at 60°C. 200 µl and 50 µl of chloroform: isoamyl-alcohol (C:I::24:1, v/v) were added and the solution was centrifuged for 5 min at 12000 rpm. The supernatant was transferred to a clean centrifuge tube and another 250 µl of chloroform: isoamyl-alcohol was added, followed by centrifugation for 5 min at 12000 rpm. The supernatant was once again transferred to a clean centrifuge tube. 50 µl of 3 M Sodium acetate (pH 5.0) was added, followed by 500 µl of ice cold 100% ethanol. The tubes were carefully inverted and the gDNA was precipitated. After incubation, the pellet underwent two wash steps with 500 µl of 70% ethanol. Then, the tubes were centrifuged for 5 min at 12000 rpm. The supernatant was discarded and the pellet was left to air dry. The pellet was finally re-suspended in 30 μl of DNase/RNase- free water and incubated at 60°C for 2 min. The extracted gDNA was quantified using a Nanodrop® ND-1000 spectrophotometer. The DNA was diluted with DNase/RNase-free water to a concentration of 100 ng/μl and stored at the fridge (-20ºC).
Microsatellite markers and PCR amplification
Eleven wheat microsatellite markers for 11 loci located in the chromosomes 1A, 2A, 2D, 3A, 3B, 4D, 5D, 6B and 7D, were used for genetic diversity analysis. Xgwm and Xwmc markers were obtained respectively from Röder et al. (1998) and Somers and Isaac (2004).
PCR reactions were carried out in 14 µl reaction mixture of KAPA2GTM Fast Multiplex PCR Mix, 6.25 µM of each forward and reverse primer (Stellenbosch University, Stellenbosch, South Africa), 1 µl gDNA and dH2O. The PCR cycling conditions was set at 94ºC for 3 min of denaturation, followed by 45 cycles of 1 min at 94°C, 1 min at the annealing temperature (Ta), 2 min at 72°C and then 72°C for 10 min for extension. The PCR products were electrophoresed on 6% denaturing polyacrylamide gels containing 1xTBE (Tris Borate EDTA). The amplified band sizes for each SSR locus were determined on the basis of their migration relative to the 50 bp marker.
Assessment of the incidence and prevalence of fungal diseases
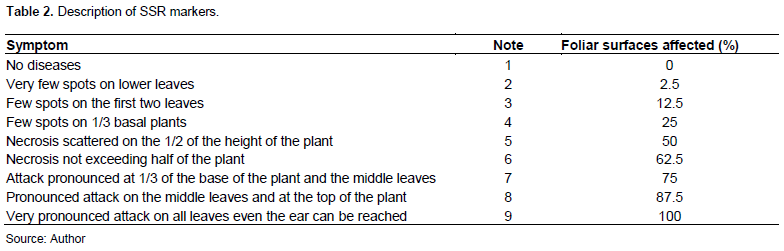
The incidence was calculated using the formula of Dajoz, (1985): I = Nm / NT × 100, where Nm: number of diseased plants per accession, NT: total number of plants per accession, and I: incidence (%). The prevalence was expressed through the formula of Zahri et al. (2014): P = n / N × 100, where n: number of sick accessions for each disease, N: total accession number, and P: prevalence (%). To estimate the ability of plant to fight against the pathogen, the severity was expressed as a percentage of sick leaf area on a log scale (Table 3) described by Notteghem et al. (1980), and is calculated using the formula preconized by Chumakov (1990)., where ? (a x b): sum of sick plants (a) having the degree of infection (b); N: Total number of diseased plants; S: Severity (% of SFM).
Biochemical analyzes
Extraction and dosage of total polyphenols
The extraction of the total polypheanols was carried out following the modified method of Boizot (2006). Briefly, 1 g of leaves was mixed with 2 mL of 80% methanol and incubated at room temperature. The mixture was centrifuged (Labofuge 400R) at 4500 rpm for 15 min. However, the method used to assay polyphenols is that of Marigo (1973). 50 μL of each extract was added 3 mL of distilled water and 0.5 mL of Folin-ciocalteu reagent. The mixture was incubated for 2 min in dark and room temperature. Then, 2 mL of Na2CO3 (75 g/L) were added and the whole was stirred on a vortex and then incubated for 15 min at 50°C in a water bath in the dark at 760 nm and was immediately measured (Jenway 6305, spectrophotometer) against a blank in which the extract is replaced by 80% methanol. Results were expressed in μg/mg gallic acid equivalents (GAE).
Extraction and determination of total amino acids
To extract the total amino acids from wheat leaf, 1 g of plant material was crushed and then diluted in 2 ml of citrate buffer (0.2 M, pH 4.5). The mixture obtained was homogenized using a vortex and centrifuged at 4500 rpm for 15 min at room temperature (25 ± 2°C). The supernatants were recovered and placed in the 2 ml tubes. Further extractions were repeated with 1 ml of solution and the two supernatants were mixed into a single extract according to the method of Singh et al. (1999). The total amino acids were then determined according to the ninhydrin modified method of Yemm and Cocking (1955). Briefly in tubes, 5.1 ml of the reaction medium, 0.5 ml of 80% ethanol, 25 μl of alcohol extract, 0.5 ml of citrate buffer (0.2 M, pH 5) and 2 ml of reagent Potassium Cyanide- Ninhydrin acetone (1 % ninhydrin and 0.06 % KCN in acetone). The mixture was heated in a boiling water bath for 15 min. A blue-purple complex was formed whose intensity of the coloration was proportional to the concentration of the amino acid in the solution. After cooling at room temperature, 8 ml of distilled water was added to the mixture and finally the absorbance was read at 570 nm with the spectrophotometer. For each extract, three readings were made and the amino acid content was evaluated by reference to the standard curve made with pure glycine.
Data analysis
The molecular diversity within all accessions was estimated for each SSR locus, using the PowerMarker 3.25 software (Liu and Muse, 2005). To measure the informative character of the SSR markers, the Polymorphism Information Contents (PIC) for each marker was calculated using the formula of Nei (1973): PIC = 1-?i=1k P2, where k is the total number of alleles detected per locus and Pi the frequency of the allele i in all 17 accessions.
Genetic similarity (GS; Dice, 1945) was calculated as: GS = 2Nij/(Ni + Nj) where, Nij is the number of fragment common to individual i and j, and (Ni + Nj) is the total number of fragment in both individuals.
Genetic distance (GD) among group pairs was calculated following Nei and Li (1979); (GDxy) = 1-(2Nxy/Nx + Ny). The dendrogram was constructed using the method based on the genetic distance (SAHN method, UPGMA algorithm) of the 17 accessions using the software Statistica 12. To calculate allele frequency (Axy) from one of variation to another in each locus, the formula of Khlestkina et al. (2004) was used: Axy = ?IPxi – PyiINxy, where Pxi and Pyi are the frequencies of the ith allele in regions X and Y, respectively, and Nxy is the total number of alleles for the two groups X and Y. The allelic frequency variation was calculated separately for each of the 11 loci and then for all of them as an average. All fragments were used to generate a GS matrix for Principal Component Analysis (Sneath and Sokal, 1973).
The statistical analyzes as well as the classification of the accessions in the dendrogram were carried out using the SPSS 20.0 software. The data of different variables were subjected to an analysis of variances (ANOVA), the averages compared to the Tukey test at the threshold of 0.05 and the correlations carried out using the Pearson test.
RESULTS
Diversity of wheat microsatellite markers
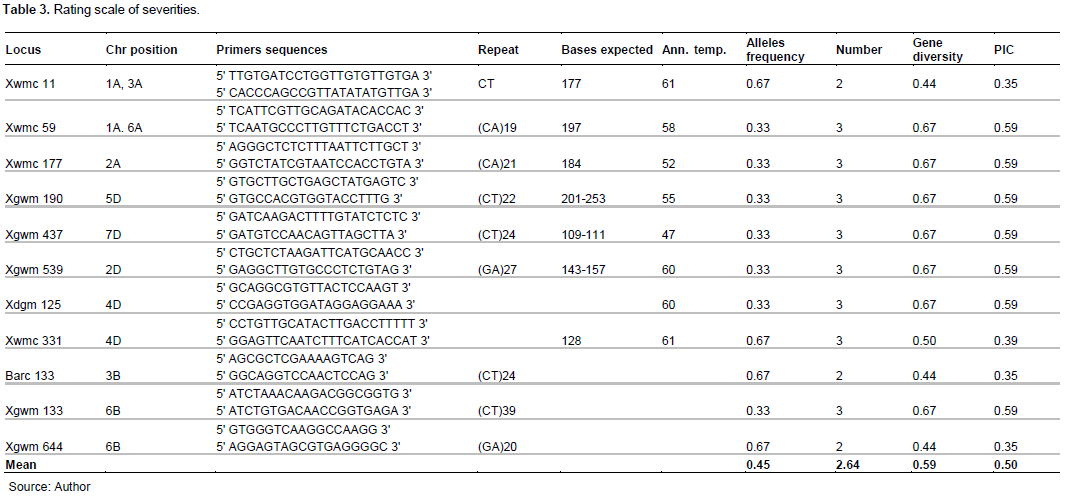
All pairs of primers specific for SSR locus used gave amplifications with allelic variations in size on all DNA wheat accessions. A total of 29 microsatellite alleles were detected. The number of alleles per locus varied from 2 to 3, with an average of 2.64 alleles per locus. The polymorphism information Content (PIC) varied from 0.35 to 0.59, with an average of 0.50 (Table 2). The results indicated a significant correlation over 11 loci (R2=1, P < 0.01) between gene diversity and number of alleles across wheat accessions (Table 3).
Phenotypic characterization of accessions
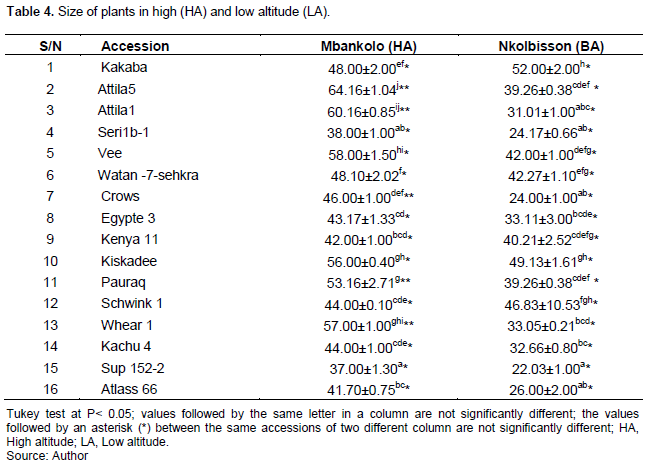
A great variability in the height of the wheat plants was observed according to the accessions but also according to the sites. Within accessions, Attila 1 (60.16 ± 0.85 cm) and Attila 5 (64.16 ± 2 cm) appeared best in Mbankolo while Kakaba (52.00 ± 2 cm) and Kiskadee (49.13 ± 1.61 cm) are the best in Nkolbisson. However, the Watan 7 accession behaves both in Mbankolo and Nkolbisson (48.10 ± 2.02 and 46.83 ± 10.53 cm respectively). Significant differences between the two sites have been observed and it appears that all accessions in general develop better in Mbankolo than in Nkolbisson. For Mount Mbankolo (high altitude), values between accessions range from 37 cm (Sup 152-2) to 64.16 cm (Attila 5) versus 22.03 cm (sup 152-2) to 52 cm (Kakaba) for the Nkolbisson site (low altitude) (Table 4).
Assessment of the status of diseases
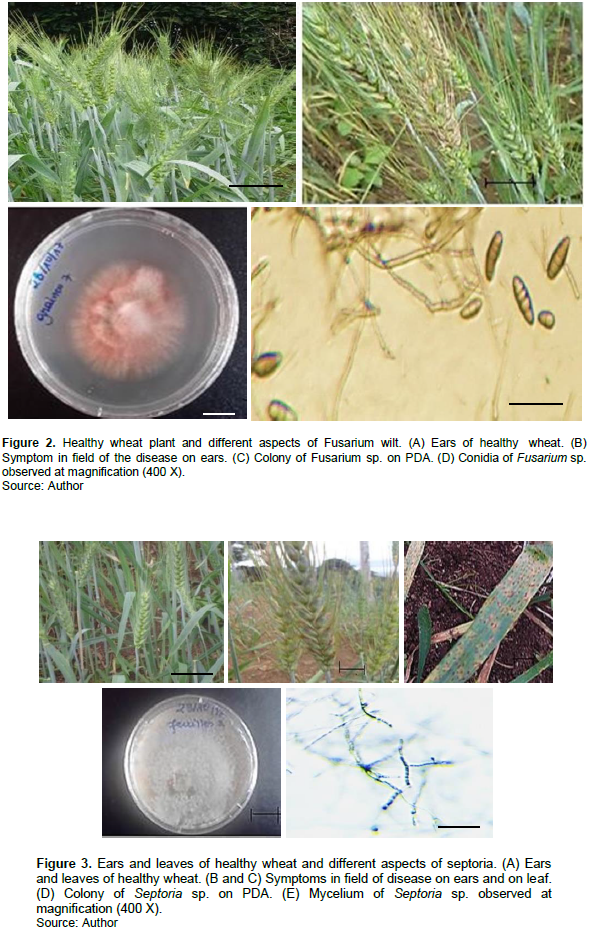
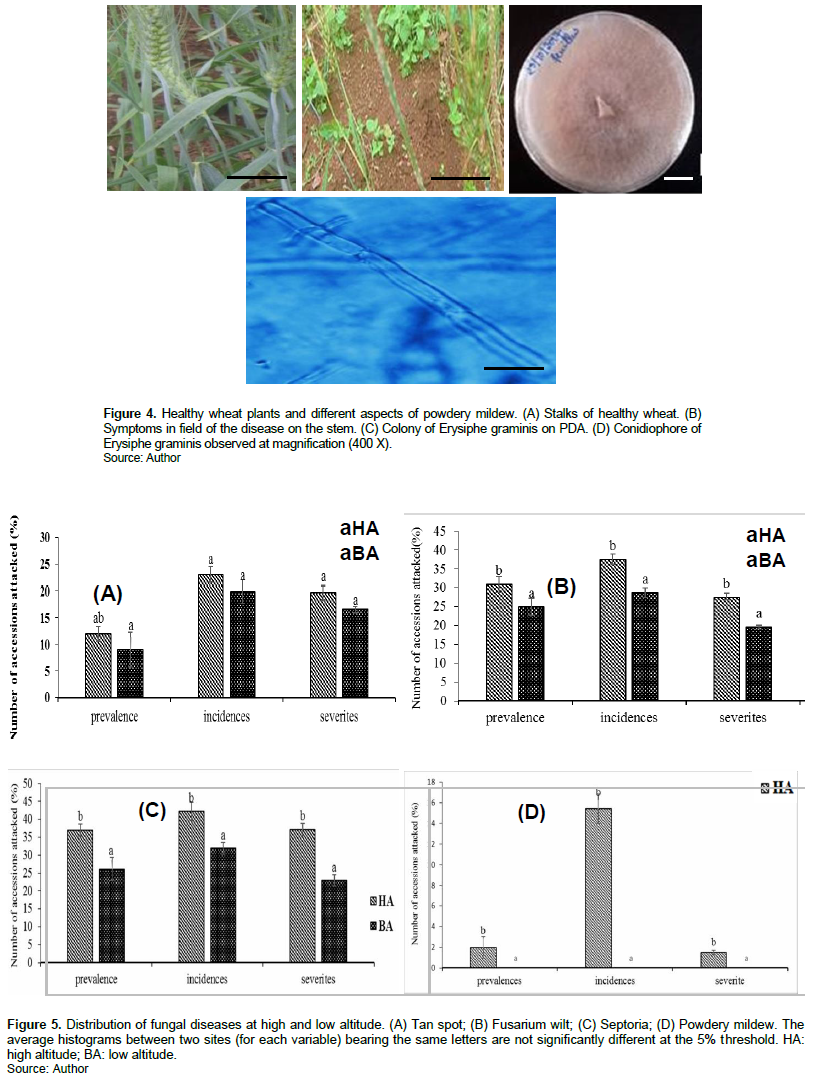
Observations of cultures isolates from fragments of infected plants on the macroscopic and microscopic level allowed the realization that isolates obtained were characteristic of the pathogens responsible for the main diseases of wheat such as: Tan spot (Pyrenophara tritici- repentis), Fusarium wilt (Fusarium sp.), Septoria leaf blotch (Septoria sp.), and powdery mildew (Erysiphe graminis). On PDA media, showed from colonies obtained from Pyrenophora-tritici-repentis, a streak and low green mycelium that turned gray on aging. This was observed in Attila 1 (3.5 ± 0.36 μg EAG/mg), whereas accession Sup-152-2 had lower accumulation (2.3 ± 0.1 μg EAG/mg) in condition of sickness. The sample from healthy accessions had accumulated lower total polyphenol content than the sample from sick accessions with values that ranged between 0.8 and 2.2 μg EAG/mg (Figure 9A). Accumulation of Amino acid was very important in Attila 1 accession with 40.1 ± 1.21 μg conidia observed were cylindrical and slightly tapered (Figure 1). The isolation on PDA medium from Fusarium sp. showed colonies with whitish to pinkish mycelium. Microscopic observation showed spores in short and thinned form at both ends with 2 to 4 septa (Figure 2). Colonies obtained on PDA Septoria sp. were whitish in the form of cream. The pycnidiospores observed under the microscope, after 10 days of incubation, were hyaline, narrow, curved at the end and filiform divided by 3 to 7 septa (Figure 3).
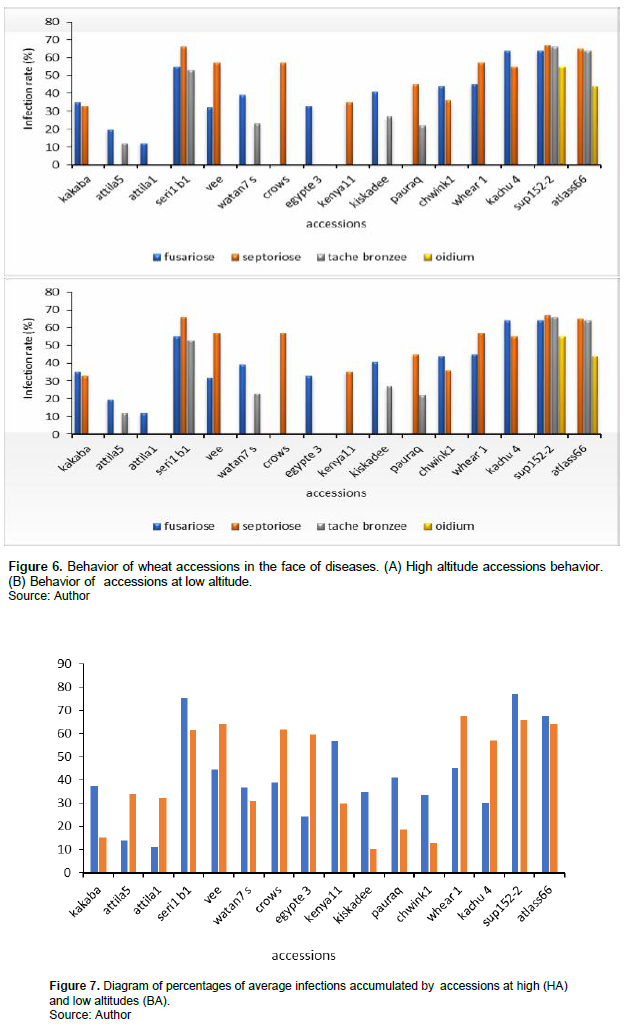
Concerning powdery mildew, microscopic observation showed ovoid, hyaline and unicellular conidia (Figure 4), with a thin, slightly transparent wall. The conidiophore was composed of a graduated series of conidia with progressive maturity.
Distribution of different diseases at both sites
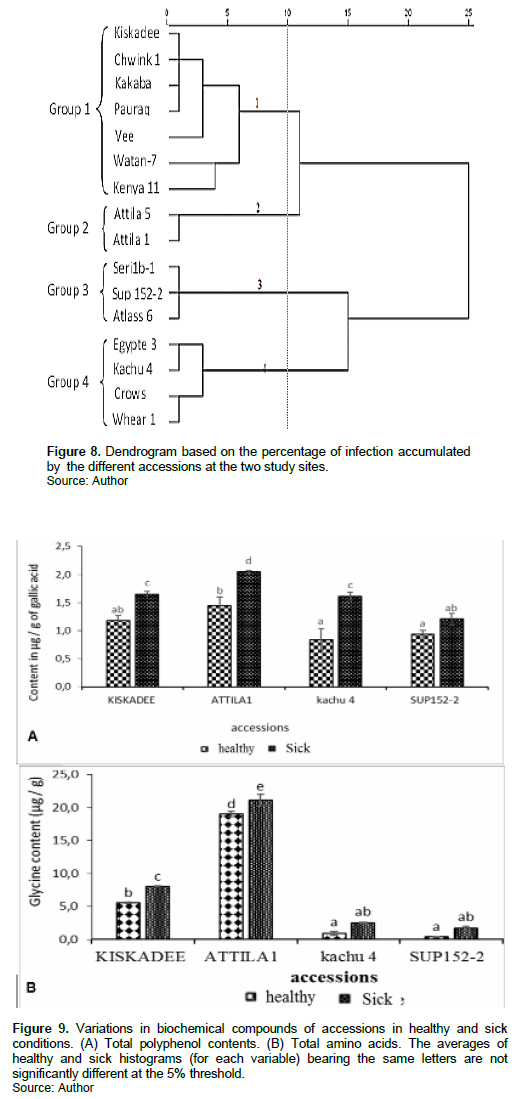
A high variability was observed in the distribution of diseases. Regarding the tanned spot, it was regularly distributed on both sites. Indeed, its prevalences were more or less similar and varied very little between 2 and 9% in Mbankolo and Nkolbisson respectively (Figure 5). All the same, the incidences were slightly higher in Mbankolo (23%) than in Nkolbisson (19.86%) and the severity was slightly higher in Mbankolo (19.7%) compared to Nkolbisson (16.55%). Fusarium wilt was prevalent at both sites but with a high prevalence in Mbankolo (31%) compared to Nkolbisson (23.3%). The disease was more severe in Mbankolo with average attack severity of 27.5% compared with 19 % in Nkolbisson. The incidences were also higher in Mbankolo (30 to 37%) contrary to Nkolbisson (23 to 28%). Like Fusarium wilt and blotch, septoria was also present at both sites with a marked predominance at Mbankolo. Indeed, it was present at 37% in Mbankolo against 21.12% in Nkolbisson. Its incidence is also higher in Mbankolo fluctuating between 40 and 44% against 27.5 to 33% in Nkolbisson although at the level of some plots, they were 100%. As regards severity, it was higher in Mbankolo than in Nkolbisson (31.15 and 22.86%, respectively) (Figure 5). In the case of powdery mildew, it was not observed at all sites. Indeed, during the surveys, it appeared only in Mbankolo with a low prevalence (2%), a low severity (1.5%), but also a low average incidence compared to other diseases (Figure 5).

Behavior of accessions to fungal diseases
On common wheat accessions, a classification analysis was carried out on the basis of the observation of the epidemiological characteristics (infection rates) presented by each accession on different sites (Figure 6). The result obtained showed that common wheat accessions on both sites were principally affected by four diseases, identified as: Tanned spot, fusarium, septoria, and powdery mildew. Indeed, we noted that all accessions of common wheat were sensitive to fungal diseases but to varying degrees. Thus, more sensitive accessions (Atlass 66, seri1b1, sup 152-2) recorded high infection rates (57.6, 60.66 and 62.5%, respectively) in Mbankolo and Nkolbisson sites. This sensitivity varied very strongly between accessions on a given site, but also on an intra- varietal scale at both sites. However, accessions Kabaka [Nkolbisson (10%) and Mbankolo (30%)] and Attila 5 [Nkolbisson (43%) and Mbankolo (11%)] showed depending on the site concerning infection (fungal disease). On the order hand, Watan-7-sehkra presented behavior substantial with infection rates in high (30%) and low (33%) altitude (Figure 6) in relation to others accessions.
Hierarchical cluster of accessions to tolerant level of disease
The analysis of the percentage of infection of accessions at high and low altitude revealed the existence of a great variability within these accessions (Figure 7). The dendrogram obtained made it possible to group these accessions according to their ability to be less or more attacked by pathogens. At 10 % similarity, analysis revealed that the 16 wheat accessions evaluated form four distinct groups (Figure 8). Indeed, each of these groups showed clear specificities for which the performances differ from those of the others.
Group 1 contained seven accessions (Kiskadee, Kakaba, Schwink1, Vee, Pauraq, Watan 7 and Kenya 11), characterized mainly by an average infection percentage of 31%. Group 2 was constituted of Attila 1 and Attila 5 accessions, which presented relatively low percentages of infections (23.17 % on average). Group 3 included the susceptible accessions (Seri1b-1, Sup-152-2 and Atlass 66) that presented significant infection percentage of 67.5%; however, it was less important (high infection percentage of 54%) in the fourth group embodied: Egypt 3, Kachu 4, Crows and Whear1 accessions (Figure 8).
Variation of biochemical compounds
This study revealed that biochemical activities in the leaves of soft wheat accessions attacked with fungal diseases displayed significant variations among the sick and healthy accessions for total polyphenols and amino acid content between tolerant and susceptible accessions (Figures 9A and 9B). The highest total polyphenol content GE/mg, while Sup-152-2 accession recorded 2.1 ± 0.36 μg GE/mg (Figure 9B).
DISCUSSION
In this study, 11 microsatellite markers revealing 29 alleles allowed to discriminate three cultivars of hexaploid wheat introduced in Cameroon. The number of alleles per locus ranged from 2 to 3 with an average of 2.64. Röder et al. (2002) detected an average of 10.5 alleles per locus from 502 recent European wheat varieties, using 19 microsatellite markers. Khaled et al. (2015) used 17 SSR markers to assess genetic diversity of 33 genotypes of hexaploid wheat from Egypt and detected an average of 5.59 alleles per locus. In addition, the microsatellite markers used had an average PIC value of 0.59, which
means that these markers were highly informative in this study, since, Botstein et al. (1980) reported that a PIC value > 0.5 is considered to be a sign of a very high informative marker, while 0.5> PIC> 0.25 corresponding to a low informative marker. In previous studies, Röder et al. (2002) found an average PIC value of 0.67 in 500 genotypes. The choice of these SSR loci is therefore relevant for future study in wheat. On the other hand, a high variability in plant height was observed between different accessions in both study sites. These results can be explained by the genetic capacity of different accessions. Indeed, Hsissou (1994) underlined that the growth, development and production of wheat genotypes is the result of numerous morphological changes resulting from the biochemical and molecular expression of the plant. At the inter-site level, the high adaptation capacity observed in Mbankolo can be explained by the climatic conditions present on the site, which are closer to the conditions required for the cultivation of soft wheat, which is in line with results obtained by Ngo Ngom (2017), which states that Mount Mbankolo with its good humidity is ideal for growing wheat in Cameroon. These results also in conformity with the study made by Othmani and Tsimi who worked respectively on the performance of selected wheat lines in two ecosystems and on the potential of adaptation of soft wheat varieties in high and low altitudes conditions of the wet zone with bimodal rainfall. Indeed, these authors have also noted that the intense heat at low altitude negatively affects the genetic expression of varieties at the level of growth and development which is materialized by the reduction of the dimensions of the organs of the plant and the reduction of yield (Salawa et al., 2014).
The mycological analysis showed the presence of four diseases attacking wheat (septoria, fusariosis, tanned spot and powdery mildew). These diseases are the same as those reported by Siou (2013), Ayad et al. (2014) and Azoui (2015) who identified wheat pathogens according to standards previously defined by some authors such as Zillinsky (1983), Eyal et al. (1987) and Ezzahiri (2001). This fungal polymorphism may be due to the presence on the culture sites of inocula from the previous crops such as wheat in Mbankolo and corn in Nkolbisson. These works are in agreement with those of Pereyra et al. (2004), who observed that a disease-sensitive crop precedent during its cycle is a potential source of inoculum for the next crop through its residues.
For each disease, except the tanned spot, significant differences were observed between the two sites for prevalences, and incidences with marked dominance at Mbankolo. This suggests that in high altitude conditions, accessions of soft wheat are more prone to fungal fact invasions. Although not statistically significant, for some diseases it has also been shown that all the diseases observed were generally more severe in Mbankolo than in Nkolbisson. These results can be explained by the that Mbankolo's hot and humid climate favors the development and intensification of diseases. In fact, climatic factors (humidity and temperature) play a decisive role in the transmission and evolution of the disease. These results are consistent with those of Nguyen (2007), Moreau (2011) and Zahri et al. (2014) who state directly that the environment of the pathogen has an influence on its development and that the majority of fungal diseases prefer humidity and high temperatures. According to Mathieu et al. (2012), temperature and humidity are responsible for variations in disease. Indeed, a great variability in the distribution of diseases on both sites was observed. Powdery mildew was observed during surveys only at the Mbankolo site. Knowing that each pathogen has specific climatic requirements for its development, this contrast can be justified by the climatic conditions that prevailed at both sites. Mbankolo is characterized by a high hygrometry (15.53% higher than Nkolbisson) and lower temperatures (lower than 6.5°C, compared to Nkolbisson), thus appears very favorable to the development of pathogens.
Based on the results of the biochemical markers of tolerance, it is noted that at the intra-varietal level, the polyphenol and total amino acid contents were appreciably higher in sick leaves for all the accessions analyzed. This result can be explained by the fact that in response to the fungal infection, plants synthesize specific substances that are phenolic compounds and amino acids to defend themselves. These results are similar to the work of Lattanzio et al. (2006), and Patil et al. (2011) who reported that during fungal invasion, one of the most important aspects of the defense strategy is the stimulation of phenolic compounds as well as the increase of the amount of amino acid from 20 to 25% in infected wheat tissues.
At the varietal scale, the results obtained indicate that the highest levels of total polyphenols and amino acids were observed in Attila 1 (highly tolerant accessions to diseases), and the lowest in Sup-152-2 which is characterized by a high sensitivity to diseases. This difference can be explained by several factors including the intrinsic factors of accession including the genotype. Indeed, the quantitative and qualitative variability of these metabolites is the result of the expression of the genes of the different accessions, which can be linked to the growth metabolism. In addition to their defense functions, these metabolites are involved in the growth of the plant. Several studies (Benbrook, 2005, Beta et al., 2005, Zouaoui, 2012) have reported that the concentration of polyphenols and amino acids is generally higher in wheat-resistant genotypes than in disease-susceptible genotypes fungal. These results are consistent with those of Manga et al. (2016) and Effa et al. (2017) which emphasize that these compounds act as barriers against pathogen invasion and hence constitute part of host resistance mechanisms of cocoa (Theobroma cacao L.).
Nevertheless, a significant negative correlation found between total polyphenol, amino acid and level of tolerance (percentage of infection) has been observed. These results are consistent with those of Bhuiyan et al. (2009) who also reported a correlation between the levels of these compounds and resistance to fungal infection, state that a high polyphenol and amino acid content prevents the development of pathogens and therefore, reduces the percentage of infection. Moreover, these findings agree with that of Effa et al. (2017) and Manga et al. (2018) observations who reported that tolerant genotype accumulates high amount of biochemical substances. These can be used as markers for selection of tolerant genotypes. Tolerant plants, when subjected to biotic stress, showed elevated levels of free phenolics and amino acid (Djocgoue et al., 2011).
CONCLUSION
The results obtained in this study provided new information about characterization of wheat cultivars for fungal disease tolerance in Cameroon. The set of the used microsatellite markers showed a high level of polymorphism and sufficient information to discriminate the cultivars of hexaploid wheat introduced in Cameroon. In this study, Mbankolo represents the most suitable site for wheat cultivation. However, specificity for the Nkolbisson site has been noted in accessions such as Kakaba and Kiskadee suggesting a possible adaptation of wheat at low altitude. Four diseases have been identified in wheat, namely Septoria, Fusarium, Tanned Spot and Powdery Mildew. In addition, the influence of the site on the evolution and intensity of the disease was determined. With a hygrometry higher than 15.53%, the site of Mbankolo is more conducive to the development of diseases. The percentage of infection, the total polyphenol content and the amino acid content were discriminating factors that could serve as selection criteria for the accessions to be introduced according to the sites. Of these criteria, 2 accessions to Mbankolo (Attila 1 and Attila 5), 2 to Nkolbisson (Kakaba and Kiskadee) and 1 common to both sites (Watan-7-sehkra) were among the 16 as high-performance and can then be recommended for growing wheat in Cameroon. For future investigation, a thorough mycological study could be carried out to identify the strains of the various pathogens responsible for the diseases observed and make a molecular characterization of the different pathogens.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
ACKNOWLEDGMENTS
This collaborative work was supported by «MIC-CERES» project jointly supported by Agropolis Fondation (grant AF Project ID 1301-003) through the "Investissements d'avenir" programme (with reference number ANR-10- LABX-0001-01) and Fondazione Cariplo (grant FC Project ID 2013-1888) and European Commission through PAFROID projects (INTRA-ACP Program, lot Africa for an action N°2013- 4644 / 001 – 001 with the reference: 384201-EM-1-2013-1-MGINTRA_ACP).
REFERENCES
|
Ayad D, Sayoud R, Benbelkacem K, Bouznad Z (2014). La tache Septorienne du blé: Première signalisation de la présence en Algérie des deux Mating types du téleomorphe Mycosphaerella graminicola (Fuckel) Schröter, (anamorphe: Septoria tritici Rob. ex Desm.) et diversité phénotypique de l'agent pathogène. Revue « Nature and Technologie pp. 34-35. |
|
|
Azoui H (2015). Etude du comportement d'une collection de blés cultivés en Algérie vis- à - vis de quelques stress biotiques. Master, UNIV. El Hadj Lakhdar de Batna. 94 p. |
|
|
Benbrook CM (2005). Elevating Antioxidant Levels in Food through Organic Farming and Food Processing. An Organic Center State of Science Review. pp. 39-40. |
|
|
Bendif N (1994). La situation actuelle des maladies des céréales en Algérie. ITGC. Céréaculture 27:9. |
|
|
Beta T, Nam S, Dexter JE, Harry D (2005). Phenolic content and antioxidant activity of pearled wheat and roller-milled fractions. Cereal Chemistry 82:390-393. |
|
|
Bhuiyan NH, Selvaraj G, Wei Y, King J (2009). Gene expression profiling and silencing reveal that monolignol biosynthesis plays a critical role in penetration defence in wheat against powdery mildew invasion. Journal of Experimental Botany 60:509-521. |
|
|
Boizot N (2006). Méthode rapide d'évaluation du contenu en composés phénoliques des organes d'un forestier. Agris, cahier des techniques de l'INRA. pp. 79-82. |
|
|
Botstein D, White RL, Skolnick M, Davis RW (1980). Construction of a genetic linkage map in man using restriction fragment length polymorphisms. American Journal of Human Genetics 32:314-331. |
|
|
Boutraid J (1982). Une agriculture sans paysans: la grande culture du blé au Cameroun Economie rurale. pp. 49-80. |
|
|
Bushnell WR (1984). Structural and physiological alterations in susceptible host tissue. In: The cereal rusts Vol. I: Origins, Specificity Structure, and physiology. (Bushnell et Roelfs eds.) Academic Press, New York. pp. 477-528. |
|
|
Dajoz R (1985). Précis d'écologie. 5ème Ed. Dunod, Paris 505 p. |
|
|
Dice LR (1945). Measures of the amount of ecologic association between species. Ecology 26:297-302. |
|
|
Djocgoue PF, Mbouobda HD, Boudjeko T, Effa OP, Omokolo ND (2011). Aminoacids, carbohydrates and heritability of resistance in the Theobroma cacao/Phythophthora megakarya interaction. Phytopathologia Mediterraneae 50:370-383. |
|
|
Doyle JD, JL Doyle (1990). Isolation of plant DNA from fresh tissue. BRL Focus 12:13-15. |
|
|
Effa OP, Manga NJ, Ondobo ML, Djoko KJC, Djocgoue PF (2017). Virulence Test of Some Phytophthora Megakarya Isolates on Cocoa (Theobroma cacao L.) Hybrid Pods. Journal of Biotechnology and Biochemistry 3(1):73-81. |
|
|
Eyal Z, Scharen AL, Prescott JM, Van Ginkel M (1987). The Septoria diseases of wheat: concepts and methods of disease management. CIMMYT, Mexico 52 p. |
|
|
Ezzahiri B (2001). Les maladies du blé : identification, facteurs de développement et méthodes de lutte. Bulletin de transfert de technologie en agriculture 77:1-4. |
|
|
Gorji AH, Zolnoori M (2011). Genetic diversity in Hexaploid Wheat Genotypes using Microsatellite Markers. Asian J. Biotechnol. Knowledgia Review, Malaysia. ISSN 1996-0700/DOI,10.3923. |
|
|
Hsissou D (1994). Sélection In vitro et caractérisation de mutants de blé dur tolérants à la sécheresse. Thèse de doctorat, Univ. Catholique de Louvain 306 p. |
|
|
Jabal RA, Trikoesoemaningtyas, Yudiwanti W, Amin N (2017). Contribution of genotype × environment interaction on the performance of wheat breeding lines in two tropical agro ecosystems. International Journal of Agronomy and Agricultural Research 10(2):13-21. |
|
|
Khaled FMS, Röder MS, Börner A (2015). Assessing genetic diversity of Egyptian hexaploid wheat (Triticum aestivum L.) using microsatellite markers. Genetic Resources and Crop Evolution 62(3):377-385. |
|
|
Khlestkina EK, Röder MS, Efremova TT, Börner A, Shumny VK (2004). The genetic diversity of old and modern Siberian varieties of common spring wheat as determined by microsatellite markers. Plant Breeding 123(2):122-127. |
|
|
Lattanzio V, Lattanzio VMT, Cardinali A (2006). Role of phenolics in wheat diseases, Bull. Soc. Pharm. Bordeaux. 29-38. management in Eritrea, ICARDA 84 p. |
|
|
Leslie J, Summerell B (2006). The Fusarium laboratory manual. First edition. Blackwell publishing, Toulouse 387 p. |
|
|
Liu K, Muse SV (2005). Power Marker: Integrated analysis environment for genetic marker data. Bioinformatics 21(9):2128-2129. |
|
|
Mahfoud A, Lasbuhani A (2015). Approche de lutte contre les maladies fongiques du blé: étude de l'efficacité de trois molecules antifongiques (in vitro et in situ) et l'effet antagoniste de certains microorganisms fongiques (In vitro). Master, Université des frères Mentouri 144 p. |
|
|
Manga NJ, Ondobo ML, Effa OP, Djoko KJC, Djocgoue PF (2018). Heterosis, heterobeltiosis, narrow-sense and broad sense heritabilities for Phytophthora megakarya tolerance in two populations of Theobroma cacao L. African Journal of Biotechnology 17(14):495-504. |
|
|
Manga NJ, Effa OP, Ondobo ML, Djoko KJC, Djocgoue PF (2016). Heritability of the tolerance to Phytophthora megakarya Bras. and Grif. of Theobroma cacao L. in terms of their necrosis length, phenolic contents and activity of enzymes. International Journal of Biosciences 8(5):249-261. |
|
|
Marigo G (1973). Sur une méthode de fractionnement et d'estimation des composés phénoliques chez les végétaux. Analusis 2:10-110. |
|
|
Mathieu CB, Nathalie S, Denis Pageau MS, Sylvie R (2012). Pour en savoir plus sur la Fusariose 7 p. |
|
|
Mohsen J, Zahra M, Naser S (2014). Multivariate statistical analysis of some traits of bread wheat for breeding under rainfed conditions. Journal of Agricultural Science 59(1):1-14. |
|
|
Moreau JM (2011). Lutte contre les maladies. Livre Blanc « Céréales » ULg Gembloux Agro-Bio Tech et CRA-W, Gembloux. |
|
|
Nda HA, Akanvou L, Kouakou K, Irié Bi ZA (2014). Diversité morphologique des variétés locales de maïs (Zea mays L.) collectées au centre et centre-ouest de la côte d'ivoire. European Scientific Journal 10(12):1-17. |
|
|
Nei M (1973). Analysis of gene diversity in subdivided populations. Proceedings of the National Academy of Sciences of the United States of America 70(12):3321-3323. |
|
|
Nei M, Li WH (1979). Mathematical model for studying genetic variation in terms of endonucleases. Proceedings of the National Academy of Sciences of the United States of America 76(10):5269-5273. |
|
|
Ngo Ngom KAB (2017). Evaluation du potentiel génétique des accessions de blé (Triticum aestivum L.) en zone forestière humide à pluviométrie bimodale du Cameroun. Master, Université Yaoundé I 60 p. |
|
|
Nguyen MT (2007). Identification des espèces de moisissures, potentiellement productrices de mycotoxines dans le riz commercialisé dans cinq provinces de la région centrale du Vietnam - Étude des conditions pouvant réduire la production des mycotoxines. Thèse de doctorat, Université de Toulouse. |
|
|
Notteghem JL, Andriatompo GM, Chatel M, Dechanet R (1980). Techniques of pearled wheat and roller-milled fractions. Cereal Chemistry 82(4):90-393. |
|
|
Patil LC, Lohithaswa HC, Nadaf HL, Kalappanavar IK, Megeri SN (2011). Biochemical relationship in resistant and susceptible cultivars of spot blotch infected tetraploid wheat. Karnataka Journal of Agricultural Sciences 24:520-522. |
|
|
Pereyra SA, Dill-Macky R, Sims AL (2004). Survival and inoculum production of Gibberella zeae in wheat residue. Plant Disease 88:724-730. |
|
|
Röder MS, Korzun V, Wendehake K, Plaschke J, Tixier MH, Leroy P, Ganal MW (1998). A microsatellite map of wheat. Genetics 149(4):2007-2023. |
|
|
Röder MS, Wendehake K, Korzun V, Bredemeijer G, Laborie D, Bertrand L, Isaac P, Rendell S, Jackson J, Cooke RJ, Vosman B, Ganal MW (2002). Construction and analysis of a microsatellite- based database of European wheat varieties. Theoretical and Applied Genetics 106:67-73. |
|
|
Salawa AR, Hammad A, Osama AM (2014). Physiological and biochemical studies on drought tolerance of wheat plants by application of amino acids and yeast extracts. Annals of Agricultural Sciences 59(1):133-145. |
|
|
Singh PP, Shin YC, Park CS, Chung YR (1999). Biological control of Fusarium wilt of cucumber by chitinolytic bacteria. Phytopathology 89(1):92-99. |
|
|
Siou D (2013). Développement épidémique de la fusariose des épis de blé et conséquences des interactions entre espèces du complexe fusarien. Doctorat, Université du Mentouri 198 p. |
|
|
Sneath PHA, Sokal RR (1973). Numerical Taxonomy. Freeman. San Francisco, 573p. |
|
|
Somers DJ, Isaac P (2004). SSRs from the Wheat Microsatellite Consortium. |
|
|
Tekeu H, Ngonkeu MEL, Djocgoué PF, Ellis A, Lendzemo V, Springfield L, Moulin L, Klonowska A, Diouf D, Botes WC, Béna G (2017). Genetic diversity of Cameroonian bread wheat (Triticum aestivum L.) cultivars revealed by microsatellite markers. African Journal of Biotechnology 16(36):1832-1839. |
|
|
Tesfay B, Araya A (2015). Grain and biomass yield reduction due to Russian wheat aphid on bread wheat in northern Ethiopia. African Crop Science Journal 23(2):197-202. |
|
|
Yemm EW, Cocking EC (1955). The determination of amino acid with ninhydrin. The Analyst 80:209-213. |
|
|
Zahri S, Farih A, Douira A (2014). Statut des principales maladies cryptogamiques foliaires du blé au Maroc en 2013. Journal of Applied Biosciences 77:6543-6549. |
|
|
Zillinsky FJ (1983). Les maladies des céréales à paille. Guide d'identification. Eds. CIMMYT, Mexico 142 p. |
|
|
Zouaoui N (2012). Effet des polyphénols sur la résistance à l'infestation fongique dans le grain de blé dur. Master, Université de Mentourie Constantine 104 p. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0