ABSTRACT
This study aims at characterizing new chicken feather-degrading bacteria isolated from soils of keratinic waste collected from four dumping sites of Ngaoundere. Fifteen proteolytic bacteria were isolated with three (D1, D2 and F1) showing the capacity to degrade feather in Basal Salt Media. Highest degradation rate (71.11 ± 3.05%) of feather was obtained from isolate D2 from Municipal slaughterhouse. The partial characterization of the strain D2 based on morphological and biochemical tests revealed that it is a Bacillus sp. The keratinolytic activity was positively correlated to the bacterial growth with the highest value (13.76 U.ml-1) obtained at the stationary phase after 120 h of incubation. The optimal conditions for the keratinolytic activity of this isolate were determined to be pH 8.0 and temperature of 45°C. Thus, the Bacillus strain (D2) isolated is a promising strain for the management and valorization of feather wastes.
Key words: Chicken feather-degrading bacteria, keratinolytic enzyme activity, characterization, Ngaoundere, Cameroon.
Proteases are enzymes consisting of one or more polypeptide chains that catalyze the hydrolysis of proteins by cleaving the peptide bond that binds two amino acids and belong to the class of peptidases (Drouin, 2005). They are the most produced and used industrial enzymes in the world, accounting for about 65% of the global industrial enzymes market (Sahoo et al., 2012). Proteases are divided into several groups among which keratinases. Most keratinases described are of microbial origin and are described to be produced only in the presence of a keratinous substrate (Bach et al., 2015). They possess a unique feature compared to other proteases, to effectively degrade keratinaceous materials found mainly in animal slaughter waste such as horns, hairs, hooves, nails and feathers.
One of the main sources of the keratins is poultry industry. Indeed, in the poultry industry, about 400 million chickens are processed every week in the world; which generates a total of about five million tons of feather (Godheja and Shekhar, 2014). Concerning Cameroon, there is about 50 million of chickens produced per year, which generates about 6000 tons of feather waste annually. With the improvement of local chicken breeding technics and growth production volume, there is a subsequent increase in production of feather waste (FAO, 2013; IPAVIC, 2016). This leads to the problem of waste disposal, and high risk of environmental pollution due to the recalcitrant nature of keratinaceous materials. Indeed, keratin contains in its structure disulfide bonds from a high level of cysteine, responsible for its three-dimensional stability, making it a non-degradable by common enzymes such as trypsin, pepsin and papain (Vidmar and Vodovnik, 2018). To solve the environmental pollution problem, chicken feathers have been valued for several years in the production of animal food after physical or chemical hydrolysis (Papadopoulos et al., 1986). But the high temperatures and pressures used in these treatments require large amounts of energy and the drastic conditions used also lead to the degradation of many essential amino acids (lysine, methionine, tryptophan), giving a product with poor and variable nutritional quality (Cai et al., 2008). This has prompted researchers to consider new possibilities, especially biological treatments in mild conditions through the use of microorganisms producing keratinases which will facilitate the disruption of disulfide bridges of keratins (Papadopoulos et al., 1986). It has also been shown that keratinases produced can have a variety of applications, particularly in production of animal feed (Suntornsuk and Suntornsuk, 2003), in the detergent (Gessesse et al., 2003), leather, textiles and wool industries (Alexandre et al., 2005).
The investigations carried out (including isolation, identification and characterization of keratinase-producing microorganisms) have shown that the soils of keratin waste dumpings constitute a reservoir of microbial strains with potential keratinolytic activities of which the most efficient are the species of the genus Bacillus; followed only by species of fungi (Mehta et al., 2013; Vidmar and Vodovnik, 2018). However, although several keratinolytic microbial strains have already been isolated worldwide, their effectiveness varies, according to the ecosystems in which they are isolated from and strain specific keratinolytic capacity. On this basis, the search of new keratinolytic microbial strains remains a challenge. In the Cameroonian ecosystem, such studies have not been done and the potential for finding new and even better bacterial strains for chicken feather degradation needs to be investigated. The aim of this study was to screen Cameroonian ecosystem where keratinous waste are dumped, to find, isolate and characterize bacteria having high ability to degrade the keratinous wastes, especially chicken feathers.
Chicken feathers samples were collected at a poultry processing plant in the Marza city (Ngaoundere, Cameroon). Soil samples were separately taken with sterile spatula at 5 cm depths from the surface of the soil of five different sites: the small market of Ngaoundere town (01 sample) and from municipal (04 samples), Gada-bini (02 samples) and Manwi (01 soils sample) slaughterhouses, Marza city (poultry processing plant)) and transported in sterile glass jars to the laboratory (Table 1). The selected sites are the main slaughtering points of Ngaoundere where the huge quantities of keratin-rich waste (wool, horns, hooves, feathers) are generated and dumped onto the soils.
Chicken feather processing and feather meal preparation
Chicken feathers were washed thoroughly with distilled water, oven-dried (45°C) until constant mass and characterized for their chemical composition using standard methods: proteins (Devani et al., 1982), fat (Bourely, 1982) and ash (A.O.A.C method, 1975). One part of the dried feathers was cut into short fragments of 5 cm to be used in the basal cultivation medium. Another part was grinded in a blender to fine powder, sifted (? = 1 mm) and used as a the source of carbon and nitrogen in the preparation of Feathers Meal Agar (FMA) (composition (g.L-1): 0.5g of NH4Cl, 0.5 g of NaCl, 0.3 g of K2HPO4, 0.4 g of KH2PO4, 0.1 g of MgCl-6H2O; 0.1 g of yeast extract, 15 g of agar and 15 g of feather meal).
Isolation and screening of keratinolytic bacterial strains
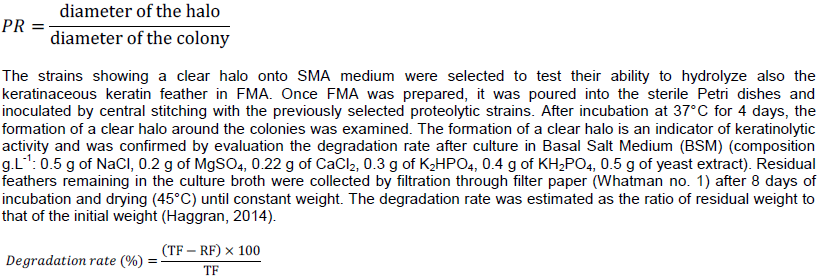
For the isolation of feather-degrading bacteria, 10 g of different soil samples from each location were suspended in sterile saline water and were shaken for 30 min (150 rpm). Serial dilutions were made up to 10-6. A volume of 0.1 ml of the different dilutions was spread on the surface of sterile FMA and incubated at 37°C for 48 h. Different bacterial colonies isolated were purified and aseptically inoculated for primary screening of proteolytic activity onto sterile Skim Milk Agar (SMA) (containing: peptone 1%, NaCl 0.5%, yeast extract 0.3%, agar 10%, sterile skim milk powder 10%). After incubation at 37°C for 24 h, colonies were examined for the formation of a clear halo (hydrolysis zone of casein) around their growth and the proteolysis ratio (PR) which is proportional to the amount of proteases released by the microbial cells into the medium during the incubation was calculated (Habbeche et al., 2014) as follows:
Where TF is the feather weight before bacterial activity and RF is the residual feather weight.
Identification of the high feather-degrading isolate
Bacteria were partially identified based on morphological and biochemical tests described in Bergey’s manual of systematic bacteriology (Bergey’s et al., 1974).
Keratinase production
For enzyme production, the mixture of 2.5 g of whole feather and 250 ml of BSM in Erlenmeyer was inoculated with 5% (v/v) of the inoculum 106 CFU.mL-1 of the selected bacteria (with the high feather-degrading potential), incubated at 37°C under stirring (150 rpm) up to 08 days. At 24 h intervals, a volume of 15 ml of culture broth was withdrawn, centrifuged at 4°C (5000 g for 20 min) and the supernatant containing crude extracellular keratinase blend was collected. The pH values of culture broths, the keratinolytic activities and the concentration of soluble proteins in the supernatants were investigated.
Effect of pH and temperature on bacterial keratinase activity
The effect of pH on keratinase activity was studied by adjusting the initial pH of the culture broth (BSM: Basal Salt Medium) with a solution of 1 N NaOH at different pH (pH 6, 7, 8, 9 and 10), followed by the inoculation with 5% (v/v) of 106 CFU.mL-1 and incubated at 37°C in an incubator under stirring (150 rpm) for 8 days. The optimum temperature for keratinolytic enzyme activity was determined by evaluating the enzyme activity at the optimum pH at different temperatures (25, 37 and 45°C) using the same culture broth and incubated up to 8 days.
Assay of Keratinase activity
Soluble keratin was prepared using the modified protocol of Wawrzkiewicz et al. (1987). The powder of feather (10 g) was mixed with 500 ml of Dimethyl Sulfoxyd (DMSO). The mixture was heated at 100°C for 120 min under reflux. Soluble keratin was then cold precipitated at 4°C for 2 h by adding 1000 ml of cold (-20°C) acetone, centrifuged at 4°C (5000 g for 20 min). The precipitate obtained was washed thoroughly, thrice, with distilled water, and dried at 40°C in a vacuum dryer. The dried precipitate (1 g) was dissolved in 20 ml of 0.05 N NaOH and the pH was adjusted to 8 using 0.1 N HCl. The resulting solution was finally diluted to 200 ml with 0.05 M Tris-HCl buffer pH 8 and used as keratin solution.
The keratinolytic activity was evaluated by mixing 1.0 ml of crude enzyme solution with 1 ml of keratin solution at 50°C in a water bath for 10 min. The reaction was stopped by adding 2.0 ml 0.4 mol.L-1 trichloroacetic acid (TCA) and the mixture centrifuged at 4°C (5000 g for 20 min). The absorbance of the supernatant was measured at 280 nm against a control prepared by incubating the enzyme solution with 2.0 ml TCA without the addition of keratin solution. One unit of keratinolytic activity was defined as an increase of corrected absorbance of 280 nm (A280) (Gradisar et al., 2005) with the control for 0.01 under the conditions described above. The enzyme activity was calculated as follows (Cai et al., 2008):
Where n is the dilution rate; 4 is the final reaction volume (ml) and 10 is the incubation time (min).
Protein measurement
Soluble proteins were measured by the method of Lowry et al. (1956) with bovine serum albumin (BSA) as a standard.
Statistical analysis
The results obtained were expressed as mean ± standard deviation. The DUNCAN test was performed using STATGRAPHICS Centurion XVI to compare the means with significant differences and the correlation tests to measure the links between the different variables.
The chemical composition of chicken feathers waste used in this study (proteins, fat and ash content) are presented in Table 1. Proteins appear as a major compound of feather with a value of 76.44%. Ash and fat content respectively represented 0.46 and 1.58%. The chemical composition of chicken feather indicated that it is a proteinaceous substrate, which can constitute a principal source of carbon and energy for many microorganisms. Therefore, these microorganisms do not require other C- and N- nutrients for growth in laboratory culture (Table 2).
Bacterial isolates with feather-degrading potential
A total of fifteen bacterial colonies (A1, B3, B5, C2, C3, D1, D2, E2, E4, F1, F2, F3, F4, H1, H2) were found to have a clear halo on SMA medium (Figure 1) and were considered as proteolytic strain. Their proteolysis ratio was ranged between 1.05 and 3.0 with four strains (D1, D2, E2 and F1) showing higher proteolysis ratio (total weight as compared to degraded weight) which are presented in Table 3.
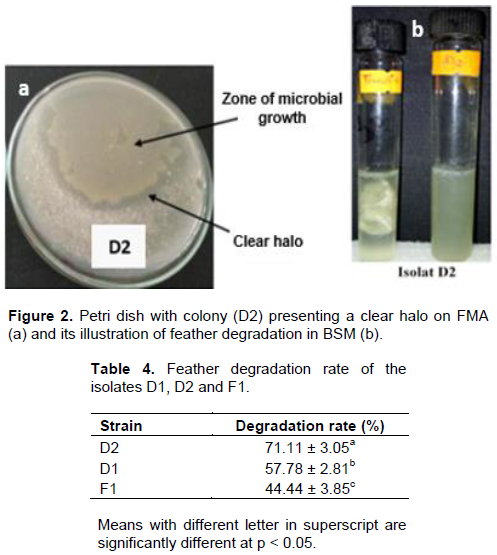
The isolates that showed higher proteolysis ratio were screened for their capacity to degrade feather. Three isolates namely D1, D2 and F1 were able to use feather as carbon and nitrogen sources and were thus able to grow on FMA and degrade feather in submerged fermentation (in BSM) (Figure 2). Out of the three isolates, D2 have presented the highest degradation rate (Table 3) and was selected for further analysis (Table 4).
Characteristics of the feather degrading isolate D2
According to biochemical parameters, isolate D2 belongs to the genus Bacillus (Table 5).
Keratinolytic activity
Keratinolytic activity and growth of the isolate D2 was investigated during cultivation in BSM containing feather as unique source of carbon and nitrogen (Figure 3). The keratinolytic activity increased during the 120 h of cultivation and showed the peak (13.76 U.mL-1) at the stationary microbial growth phase. During the production of keratinases, we also observed an increase in pH of the culture broth during the 144 h which decreases after this time (Figure 3). Likewise, the amount of soluble proteins released in the medium during the incubation increased up to 4.54 mg.mL-1 during the 144 h of incubation and decreased later on (Figure 3). The keratinolytic activity of isolate D2 was maximal at pH8 and at 45°C (Figure 4). As for her bacterial growth, it was maximal at pH 8 and at 37 to 45°C.
A screening approach was employed to obtain feather-degrading bacteria able to produce keratinase. Three isolated strains obtained from two locations were able to grow on FMA and degrade feather in BSM. Among these strains, the isolate D2 showed the highest feather degradation efficiency. The degradation rate has been proposed by several authors as an indicator positively correlated to the keratinolytic activity of a microbial strain (Veenayohini and Sangeetha, 2016; Bach et al., 2015). The characterization revealed that isolate D2 is Gram- positive Bacillus sp. This result is similar to those reported by several authors who showed that Gram-positive bacteria, including Bacillus, Streptomyces can hydrolyze keratin (Gupta and Ramnani, 2006). Likewise, species of Bacillus is considered as the best producer of keratinase ((Govinden and Puchooa, 2012).
The increase in pH observed during the culture of Bacillus isolate D2 resulted from the release of alkaline molecules such as ammonia (NH3+) by deamination of peptides and amino acids. These are products of the keratinaceous feather protein degradation (Wilkesman and Kurz, 2009). These results corroborate with those of De Azeredo et al. (2006) and Kaul and Sumbali (1997), who showed that the increase in pH during culture is a consequence of the use of amino acids as sole source of carbon, since nitrogen has to be eliminated which involves deamination reactions and the keratinolytic potential of microorganisms especially Bacillus sp. However, the decrease in the pH during the culture after 144 h could result from the production of metabolites such as organic acids.
Many studies reported that most bacteria and fungi species produced keratinases in a large range of pH from 5 to 13 and the production is optimum at pH 7-8 (Sahoo et al., 2012; Gupta and Ramnani, 2006). The optimum temperature of keratinase production by most Bacillus strains has also been reported to be in the range of 30 to 80°C (Gupta and Ramnani, 2006). Similarly, the keratinolytic activity of isolate D2 was maximal at pH 8 at 45°C. Enzymes with optimum activity at alkaline pΗ and high temperature have advantages in application, both in degradation of chicken feathers, for production of animal feed and for management of poultry waste, as well as in leather industry (Godbole et al., 2017).
The soils of keratinous waste dumping in Ngaoundere have been shown to be a good source for isolation of microbial strains with keratinolytic activities. From the strains isolated, the Bacillus isolate D2 has its maximum keratinolytic activity in alkaline conditions (pH 8) and at rather high temperatures (optimum at 45°C). The strain D2 needs to be fully characterized about its taxonomic position, specifically in relation to relatedness to other Bacillus species known to be keratinolytic, such as B. licheniformis. If the D2 isolate appears to be novel, the following next two investigations would be highly interesting: Elucidation (at molecular level) of the enzyme composition of the D2 enzyme secretome; and testing of the end product of enzymatic (D2) bacterial degradation of the keratinaceous feather biomass for its nutritional value (in non-ruminant species, e.g. pigs). Furthermore, it would be of interest to adjust the protocol for the least energy requiring pretreatment and to test the resulting processing (pretreatment and microbial/enzymatic degradation) under upscaled conditions.
The authors have not declared any conflict of interests.
REFERENCES
|
Alexandre JM, Walter OB, Renata G, David D, Antonio PHJ, Carlos T (2005). Novel keratinase from Bacillus subtilis S14 exhibiting remarkable dehairing capabilities. Applied Environmental Microbiology 71:594-596.
Crossref
|
|
|
|
Bach E, Lopes FC, Brandelli A (2015). Biodegradation of α and β- keratins by Gram-negative bacteria. International Biodeterioration and Biodegradation 104:136-141.
Crossref
|
|
|
|
|
Cai C, Lou B, Zheng X (2008). Keratinase production and keratin degradation by a mutant strain of Bacillus subtilis. Journal of Zhejiang University Science B9:60-67.
Crossref
|
|
|
|
|
De Azeredo LAI, De Lima MB, Coelho RRR, Freire DMG (2006). Thermophilic protease production by Streptomyces sp. 594 in submerged and solid-state fermentations using feather meal. Journal of Applied Microbiology 100:641-647.
Crossref
|
|
|
|
|
Drouin M (2005). Etude de production de protéases alcalines par Bacillus licheniformis en utilisant des boues d'épuration municipales comme substrat. Mémoire de Maître Ès Sciences, INRS - Centre Eau, Terre et Environnement.
|
|
|
|
|
FAO (2013). Food Outlook: Poultry Meat. Available on
View.
|
|
|
|
|
Gessesse A, Hatti-Kaul R, Gashe BA, Mattiasson B (2003). Novel alkaline proteases from alkaliphilic bacteria grown on chicken feather. Enzyme and Microbial Technology 32(5):519-524.
Crossref
|
|
|
|
|
Godbole S, Pattan J, Gaikwad S, Jha T (2017). Isolation, Identification and Characterization of Keratin degrading microorganisms from Poultry soil and their Feather degradation Potential. International Journal of Environment, Agriculture and Biotechnology 2(4):2060-2068.
Crossref
|
|
|
|
|
Godheja J, Shekhar SK (2014). Biodegradation of Keratin from Chicken Feathers by Fungal Species as a Means of Sustainable Development. Journal of Bioremediation and Biodegradation 5:232.
Crossref
|
|
|
|
|
Gradisar H, Friedrich J, Krizaj I, Jerala R (2005). Similarities and Specificities of Fungal Keratinolytic Proteases: Comparison of Keratinases of Paecilomyces marquandii and Doratomyces microsporus to Some Known Proteases. Applied and Environmental Microbiology 71(7):3420-3426.
Crossref
|
|
|
|
|
Gupta R, Ramnani P (2006). Microbial keratinases and their prospective applications an overview. Applied Microbiology and Biotechnology 70(1):21-33.
Crossref
|
|
|
|
|
Habbeche A, Saoudi B, Jaouadi B, Haberra S, Kerouaz B, Boudelaa M (2014). Purification and biochemical characterization of a detergent-stable keratinase from a newly thermophilic actinomycete Actinomadura keratinilytica strain Cpt29 isolated from poultry compost. Journal of Biosciences and Bioengineering 117(4):413-21.
Crossref
|
|
|
|
|
Haggran A (2014). Enhanced Keratinase Production and Feathers Degradation by a Mutant Strain of Bacillus subtilis. Journal of applied Sciences Research 10(1):46-52.
|
|
|
|
|
IPAVIC (2016).
View
|
|
|
|
|
Kaul S, Sumbali G (1997). Keratinolysis by poultry farm soil fungi. Mycopathologia 139:137-140.
Crossref
|
|
|
|
|
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1956). Protein measurement with the folin phenol reagent. Journal of Biology and Chemistry 193:265-275.
Crossref
|
|
|
|
|
Mehta RS, Riddhi JJ, Sawant CS (2013). Optimization of cultural conditions for extracellular keratinase production by Bacillus species isolated from poultry farm soil. International Journal of Pharma and Bio Sciences 4(2):B454-B463.
|
|
|
|
|
Papadopoulos MC, El-Boushy AR, Roodbeen AE, Ketelaars EH (1986). Effects of processing time and moisture content on amino acid composition and nitrogen characteristics of feather meal. Animal Feed Sciences and Technology 14(3-4):279-290.
Crossref
|
|
|
|
|
Sahoo DK, Das A, Thatoi H, Mondal KC, Mohapatra PKD (2012). Keratinase Production and Biodegradation of Whole Chicken Feather Keratin by a Newly Isolated Bacterium Under Submerged Fermentation. Applied Biochemistry and Biotechnology 167:1040-1051.
Crossref
|
|
|
|
|
Suntornsuk W, Suntornsuk L (2003). Feather degradation by Bacillus sp. FK 46 in submerged cultivation. Bioresource Technology 86:239-43.
Crossref
|
|
|
|
|
Veenayohini K, Sangeetha D (2016). Isolation and identification of keratinolytic bacteria from poultry waste and assessment of its keratinase activity on chicken feathers. International Journal of Applied Research 2(11):396-402.
|
|
|
|
|
Vidmar B, Vodovnik M (2018). Microbial Keratinases: Enzymes with Promising Biotechnological Applications, A review. Food Technology and Biotechnology 56:3.
Crossref
|
|
|
|
|
Wawrzkiewicz K, Lobarzewski J, Wolski T (1987). Intracellular keratinase of Trichophyton gallinae. Journal of Medical and Veterinary Mycology 25(4):261-268.
Crossref
|
|
|
|
|
Wilkesman J, Kurz L (2009). Protease Analysis by Zymography: A review on techniques and patents. Recent Patents on Biotechnology 3(3):175-184.
Crossref
|
|