ABSTRACT
Burkea africana and Erythrophleum africanum are characterized by seed coat-imposed dormancy that prevents water entry and gaseous exchange, which are essential for the germination process. The objective of this study was to determine the best possible pre-sowing treatment method that maximizes seed germination of the two species. Seeds of both species were subjected to four experiments, containing 10 levels of presowing treatments: The control, mechanical scarification, soaking in concentrated sulphuric acid (for 15, 30, 45 and 60 min), immersion in boiling water (for 1, 3 and 5 min), and soaking in boiling water (and cooling down for 24 h). The germination data were subjected to ANOVA followed by Tukey’s HSD Test to separate significantly different treatment means. The most promising results showed that seeds treated with mechanical, sulphuric acid and boiling water scarification had significantly higher mean percent germination than the controls for B. africana; whereas for E. africanum, mechanical scarification, exposure to sulphuric acid, boiling water (1 min) and immersion in boiling water (and cooling down for 24 h) had higher percent germination than the controls.
Key words: Burkea africana, Erythrophleum africanum, germination percentage, pre-sowing treatment, seed dormancy.
Over the past few years, Botswana has put considerable efforts into forest conservation and afforestation programmes, such as the annual national tree planting day. This day dates back to 1985 when the then President Sir Ketumile Masire launched the first national tree planting day and has since been commemorated on the last Saturday of November each year (BOPA, 2013). At inception of the tree planting day, exotic tree species were planted in community woodlots and distributed for planting by individuals. Exotic species were promoted because they establish easily, grow fast and are highly productive, especially on harsh sites where native tree species do not perform well (Dodet and Collet, 2012). They are highly productive because pests from their native habitats are absent (Nair, 2001). These characteristics contribute to their ability to invade local ecosystems (Dodet and Collet, 2012) and are a threat to native biodiversity (Bellard et al., 2016). Afforestation using exotic species has long been beneï¬cial to the environment, and the aim of using exotic species was to repair damaged ecosystems (Richardson, 1998).
The use of indigenous tree species in afforestation and reforestation programmes is increasing world-wide (McNamara et al., 2006; Shono et al., 2007a; Raman et al., 2009). Similarly, Botswana has also been promoting their use in recent years (Rasebeka et al., 2014) because they cope well with prevailing harsh environmental conditions. However, the use of indigenous species in planting programmes is limited by the availability of quality planting materials (Elliott et al., 2002; Meli et al., 2014). There is need to identify indigenous tree species with readily available seed and propagation techniques that are suited to local environments (Shono et al., 2007b; Doust et al., 2008; Lamb, 2011; Meli et al., 2014).
Burkea africana Hook. also known as monato, mosheshe, Ohehe, nkalati in Botswana (Setshogo, 2002), burkea red syringa, Rhodesia ash, sand syringa, wild seringa and wild syringa (English) (Setshogo, 2002; Maroyi, 2010) belongs to the family Fabaceae (Caesalpinioideae) (Palmer and Pitman, 1972; Palgrave, 2002; Neya et al., 2004; Maroyi, 2010). The species is distributed throughout tropical Africa (Neya et al., 2004; Mair et al., 2018), from Senegal to Sudan and as far as South Africa (Maroyi, 2010). It has a flat-top and grows up to 61 cm in diameter and 20 m high (Fanshawe, 1972). The species grows naturally in open, wooded grassland and open woodland (Maroyi, 2010; Tanko et al., 2011) on sandy soil and lower slopes on rocky hills in the high rainfall areas, occasionally in miombo woodland (Mulofwa et al., 1994). The wood of B. africana is hard, heavy and is used in constructional work such as bridges, sleepers, furniture, firewood, charcoal, fences and tool handles (Neya et al., 2004). The heartwood is very resistant to fungi (Neya et al., 2004). The bark, roots and leaves are used as medicine (Mulofwa et al., 1994; Mathisen et al., 2002). The bark has been used in medicine to treat colds, coughs, and constipation, gonorrhoea and syphilis (van Wyk and Gericke, 2007). B. africana is planted as a roadside tree and ornamental (Maroyi, 2010). It is host to caterpillars of Saturnid moths (Cirina forda and Rohaniella pygmaea), which are eaten by local people. The flowers produce nectar collected by honeybees (Mulofwa et al., 1994). The bark and leaves are eaten by elephants and the tree yields a semi-translucent gel or green gum of high quality (Roodt, 1998).
Erythrophleum africanum (Welw. ex Benth.) Harms is known as mmako, mobaku, ununza, mopombo and mokong ochi in Botswana (Setshogo, 2002) as well as African blackwood and ordeal tree in English and belongs to the family Fabaceae (Caesalpinioideae) (Burkill, 1995; Setshogo, 2002). It is a medium-sized to large tree growing up to 15 m high (Palmer and Pitman, 1972; Palgrave, 2002). It has a straight and cylindrical stem, up to 120 cm in diameter, and a dense and spreading crown (Kawanga, 2008). The bark is grey in colour and smooth in young trees and becoming red-brown, rough and fissured with age (Kawanga, 2008; Maroyi, 2019). The leaves are alternate, egg-shaped to oblong, finely velvety, particularly when young and on the under surface. The apex of the leaf is broadly tapering to rounded or notched and the base is broadly tapering with entire margins (Kawanga, 2008; Maroyi, 2019). Flowers are cream to yellow in colour, sweetly scented, occurring in dense spikes and often grouped together in large heads. The fruit is a pod, splitting along both sides simultaneously and each section curving backwards (Kawanga, 2008; Maroyi, 2019). It is indigenous to tropical Africa (Lock, 1989; Burkill, 1985; Germishuizen and Meyer, 2003; Smith and Allen, 2004; Hyde et al., 2020). The species grows naturally in hot and dry deciduous woodlands at 600 to 1400 m above sea level, and is absent from riparian woodlands and the dry savanna of the Sahel (Kawanga, 2008). It is indigenous to tropical Africa (Lock, 1989; Burkill, 1985; Germishuizen and Meyer, 2003; Smith and Allen, 2004; Hyde et al., 2020). The wood is used for furniture, heavy and light construction, posts, poles and tool handles. In addition, it is used for firewood and making high quality charcoal. The bark, roots and leaves are used in medicine. An infusion of the bark is drunk to treat stomach-ache or dysmenorrhoea. Steeped in water, the bark is applied externally and internally to cure cardiac diseases and epilepsy. The powdered root bark, mixed with urine, is applied to the skin to treat leprosy and a paste of root-bark is applied to the skin to cure scabies (Kawanga, 2008).
Germination of seed is important in propagating seedlings for mass planting of woody plant species. However, it can be a time-consuming process because seeds of some plants take a longer time to germinate, or may fail to do so under some culture regimes. According to Botumile et al. (2020), a high level of seed dormancy is a characteristic feature of many plants of dry regions, and it either completely prevents germination or allows very few seeds to germinate over a long period of time. Seed dormancy is an adaptive mechanism that blocks the germination of intact viable seeds under conditions when the chance of seedling survival and growth is low (Weibrecht et al., 2011; Smýkal et al., 2014; Long et al., 2015). Seeds of many leguminous plants have hard coats, which make it difï¬cult for the seeds to imbibe water and prevent gaseous exchange (Bolingue et al., 2010). In nature, hard seed coats are cracked or softened by fire (Mbalo and Witkowski, 1997; Walters et al., 2004), extreme temperatures, digestive acids in the stomachs of animals or the abrasion of blowing sand (Luna et al., 2009) that can promote germination.
Hard seed coat-imposed dormancy of leguminous species hinders their successful artificial regeneration (Teketay, 1996a, b; Mojeremane et al., 2017, 2018; Odirile et al., 2019; Setlhabetsi et al., 2019). Several pre-sowing treatments have been used to enhance germination of seeds characterised by hard coats. These include mechanical, acid, cold, hot and boiling water scarification (Teketay, 1996a, b, 1998, 2005; Alamgir and Hossain, 2005; Amri, 2010; Azad et al., 2011; Rasebeka et al., 2014; Fredrick et al., 2017; Kahaka et al., 2018; Opoku et al., 2018; Botumile et al., 2020), among others. These techniques can improve germination by overcoming seed dormancy within a relatively short period of time (Tadros et al., 2011; Mojeremane et al., 2017, 2018; Odirile et al., 2019; Setlhabetsi et al., 2019).
B. africana and E. africanum are among the excellent candidate species for introducing in planting programmes in dry regions, because of their multiple uses and adaptation to the local environment. The hard seed coat is seen as a hindrance to uniform and rapid germination of tree and shrubs species, hence, there is a need for pre-sowing seed treatments to enhance germination. Therefore, the objective of this study was to determine some of the best possible pre-sowing treatment methods that maximize the germination of B. africana and E. africanum seeds.
Study site
The experiment was conducted in the laboratory at the Botswana University of Agriculture and Natural Resources (BUAN) from January to February, 2019. The university is located at Sebele (23°34' S and 25°57' E, altitude of 994 m), approximately 10 km from the Centre of Gaborone, the capital city of Botswana along the A1 North-South highway.
Seed source
Seeds were collected from Kazuma Forest Reserve (18. 4259° S and 25.4970 E, altitude 997 m) in the Chobe district during August 2018. Mature and healthy fruits/pods were collected from the tree crown by shaking with a long-hooked stick. The mature dry pods were placed in paper bags and transported to the Department of Range and Forest Resources Laboratory, Botswana University of Agriculture and Natural Resources. Seeds were extracted by crushing the pods by hand, followed by winnowing to separate the husk. Seeds were kept refrigerated at 5°C for four months awaiting commencement of experiments. Prior to sowing, seeds were tested for viability using the floating method, in which the floated seeds were considered unviable and discarded.
Experimental design and treatments
In this study, four experiments, containing 10 levels of treatments, including the control, were carried out. The four experiments were mechanical scarification, exposure to sulphuric acid, exposure to boiling water and exposure to hot water for 24 h. The experiments were laid down in completely randomized design having four replications.
Experiment 1: Mechanical scarification
In this experiment, 100 seeds of each studied species, with four replications of 25 seeds, were used. In all these seeds, a pair of scissors was used to cut way 1 to 2 mm of the seed coat on a convex edge opposite where the embryo is located and avoiding removal of endosperm as much as possible.
Experiment 2: Exposure to sulphuric acid
In this experiment, four periods of exposure of seeds of the studied species using sulphuric concentrated sulphuric acid (98%), that is, 15, 30, 45 and 60 min, were used by employing the method described by Teketay (1996a). For each period of exposure, the four replications of 25 seeds were put into four 100-ml, heat-resistant, non-corrosive glass beakers containing sulphuric acid by making sure that all the seeds were covered by the acid. Seeds were hand stirred every 5 min during the specific treatment time to ensure their uniform exposure to the acid. After the specified periods of exposure, the seeds were sieved out of the acid using an acid-resistant sieve, while the acid was drained off simultaneously into another beaker. Seeds were, then, thoroughly washed and rinsed to remove acid using running water tap first and subsequently using distilled water, successively.
Experiment 3: Exposure to boiling water
In this experiment, three periods of exposure of seeds of the studied species, that is, 1, 3 and 5 min, to boiling water were used. For each period of exposure, four replications of 25 seeds were put into four separate coffee filter papers and immersed into a cooking pot with boiling water for the specified period, after which they were removed and immersed in a small bucket containing room temperature distilled water to cool them down for a few minutes.
Experiment 4: Exposure to boiled water for 24 h
In this experiment, four replicates of 25 seeds were put into four separate coffee filters and placed into a 250 ml beaker. Boiling water was, then, poured into the beaker and left to cool with the seeds inside for 24 h.
Four replications of 25 untreated seeds were used as control for all the experiments. In all the experiments and the control, each replication, containing the 25 seeds, was placed in 8-mm closed Petri dishes lined with cotton wool. The cotton wool was continuously kept moist by adding distilled water whenever necessary until the end of the experiments. Seeds were considered to have germinated when the radicle penetrated the seed coat and reached 1 to 2 mm. The germinated seeds were counted and recorded on daily basis. The germinated seeds were removed from Petri-dishes after counting and recording. The experiments were terminated after 30 days.
Data analyses
Data collected on germinated seeds were used to calculate germination percentage (GP), for each treatment using the equation:
Germination percentage=(Total number of seeds germinated/Total number of seeds sown)×100
The data collected were subjected to both descriptive statistics and one-way analysis of variance (ANOVA) using Statistix Software, Version 10 (Statistix 10, 1984-2003). Before the ANOVA, the germination percentage data were arcsine transformed to meet the requirement of normality (Zar, 1996). Significant differences of means were tested using Tukey’s Honestly Significant Difference (HSD) at the significance level of P < 0.05.
Germination of seeds
The results indicated that seeds treated with mechanical scarification, sulphuric acid and boiling water had significantly higher mean germination percentages than the control in B. africana [(One Way ANOVA: (F (9, 39) = 15.86, P = 0.00001)]. For E. africanum mechanical scarification, sulphuric acid, boiling water (1 min) and hot water (boiling water allowed to cool for 24 h) had significantly higher mean germination percentages than the control [(One Way ANOVA: (F (9, 39) = 22.19, P = 0.00001)] (Table 1). The ANOVA also indicated that there were significant differences among the different treatment times and conditions further clarified by the HSD significant differences within and among the treatment means (Table 1) as explained more fully in the following paragraph.
The highest mean germination percentages (93 and 92%) for B. africana were found in sulphuric acid (45 and 60 min) treatments, followed by those exposed to mechanical scarification (80%), sulphuric acid for 15 (74%) and 30 (73%) minutes as well as boiling water for 5 (66%), 3 (62%) and 1 (50%) min, respectively. Results of seeds immersed in hot water for 24 hours showed no significant effect on the germination of seeds compared with the control (Table 1). For E. africanum, the sulphuric acid (30, 45 and 60 min) treatments had the highest mean germination (95-98%), followed by mechanical scarification (90%), those treated in boiling water (1 min) (72%), hot water (boiling water allowed to cool in 24 h) (65%) and sulphuric acid (15 min) (58%). Boiling water treatments (3 and 5 min) had no significant effect on the germination of seeds (Table 1).
Seed germination rate
The results showed that seeds of B. africana that were treated with sulphuric acid (for 45 and 60 min) exhibited the fastest and uniform germination, reaching > 90% cumulative germination within five days after sowing, followed by mechanical scarification, reaching > 78% within 8 days and those treated with sulphuric acid (for 30 and 15 min), reaching > 71% and > 65% within seven days, respectively (Figure 1). On the other hand, untreated seeds (control) and seeds treated with hot water exhibited, not only the lowest germination percentage, but also the slowest germination.
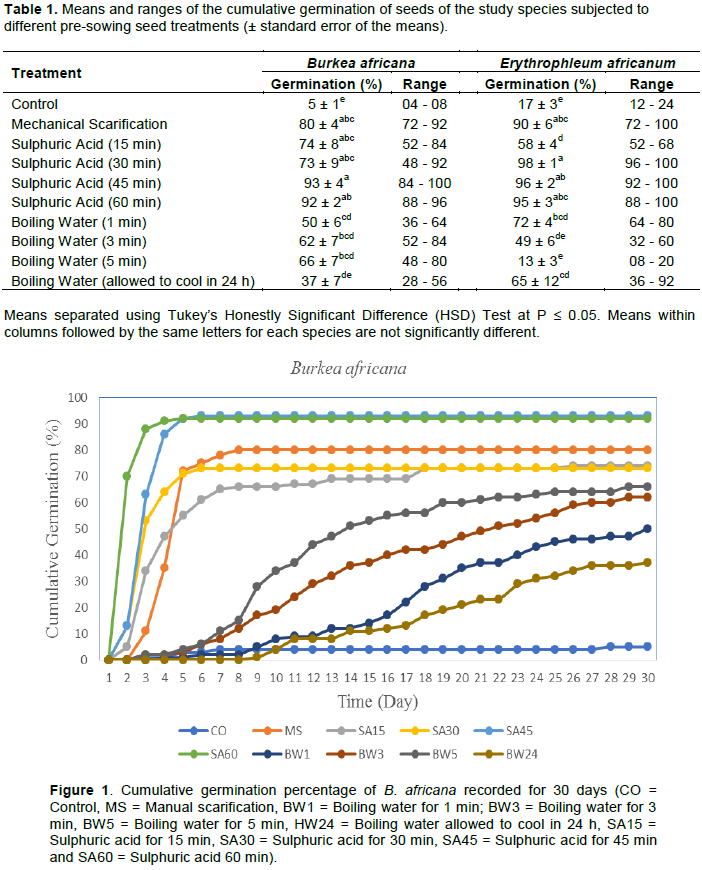
The results also showed that in the case of E. africanum, seeds treated with sulphuric acid (for 30, 45 and 60 min) and mechanical scarification exhibited the fastest and uniform seed germination, reaching > 95% and > 80% cumulative germination within five days, respectively (Figure 2). On the other hand, seeds treated with boiling water (5 min) and the control exhibited not only the lowest, but also the slowest germination.
Different techniques of breaking seed dormancy, in order to improve germination rate and speed up the germination process, have been suggested by other authors (Airi et al., 2009; Azad et al., 2010). Results of this study indicated that B. africana and E. africanum are characterized by physical seed dormancy imposed on the seeds by a water-impermeable seed coat. Mechanical scarification proved to be one of the most effective methods to break dormancy for both the two study species compared with the controls (Table 1). Removing 1-2 mm of the seed coat allows the seed to imbibe water, hence promoted radicle emergence. Once the seed imbibed water, the cumulative germination was improved significantly, and became more rapid and uniform. This result is consistent with work carried out on other leguminous plant species (Teketay, 1996a, 1998; Tigabu and Odén, 2001; Sy et al., 2001; Alamgir and Hossain, 2005; Rodrigues-Junior et al., 2014; Naim et al., 2015; Boateng, 2017; Fredrick et al., 2017; Mojeremane et al., 2017, 2018; Odirile et al., 2019; Botumile et al., 2020). Teketay (1996a) reported that mechanical scarification enhanced seed germination for most leguminous species. Tigabu and Odén (2001) recorded 100% germination in Albizia gummifera seeds and 80% in Albizia grandibracteata compared with <10% germination of the untreated seeds. Mackay et al. (1995) also recorded 100% germination for mechanically scarified Lupinus havardii seeds. Botumile et al. (2020) obtained 96% germination for Vachellia robusta (Burch.) Kyalangalilwa and Boatwright and 88% for Senegalia galpinii (Burtt Davy) Seigler and Ebinger seeds. Mechanical scarification is a safer and more practical technique for scarifying few seeds. The technique is simple and effective in promoting rapid and uniform germination (Odirile et al., 2019). However, it requires a lot of time, especially if scarifying many seeds (Mapongmetsem et al., 1999; Himanen et al., 2012; Baskin and Baskin, 2014; Müllera et al., 2017). According to Mmolutsi et al. (2020), it is also possible to damage the endosperm, cotyledons or embryo during nicking, which could result in low germination.
Sulphuric acid enhanced germination in B. africana and E. africanum compared with the controls (Table 1). Sulphuric acid is one of the most effective pre-sowing treatments for seeds with very hard coats. The acid wears out the thick seed coat and allows water to enter the seeds and trigger germination, which is more rapid and uniform. The results of the sulphuric acid treatments on the two study species are supported by similar studies conducted on other leguminous species elsewhere (Teketay, 1996a, 1998; Sy et al., 2001; Rincón-Rosales et al., 2003; Cirak et al., 2004; Phartyal et al., 2005; Aref et al., 2011; Nasr et al., 2013; Fredrick et al., 2017; Mojeremane et al., 2017; Odirile et al., 2019). Although the sulphuric acid treatments are more effective methods for many tropical leguminous trees, the sulphuric acid used is expensive and a very dangerous and abrasive chemical to people and materials (Doran et al., 1983) as well as a potential pollutant of the environment unless properly disposed of. The acid needs to be handled with great care observing safety rules (Schmidt, 2007). Safety glasses, gloves and other protective clothing must be worn, and if possible, a fume cabinet used because inhaling the fumes is very harmful (Luna et al., 2009). There is also a possibility of damaging seeds by over soaking (Nasr et al., 2013). Disposing the waste acid safely can be serious challenge in some areas.
Hot water (boiling water allowed to cool for 24 h) increased the germination of E. africanum seeds compared with that of the control (Table 1). Soaking of seeds of E. africanum in hot water might have softened the seed coats and allowed for the imbibition of water. In contrast, B. africana seeds soaked in hot water (boiling water allowed to cool for 24 h) did not differ significant from the control. These contrasting results have been reported in other studies elsewhere (Albrecht, 1993; Teketay, 1996a, 1998; Sharma et al., 2008; Mwase and Mvula, 2011; Botsheleng et al., 2014; Fredrick et al., 2017; Mojeremane et al., 2017; Botumile et al., 2020). Studies have shown that the effectiveness of hot water in improving seed germination vary with species (Tigabu and Oden, 2001; Teketay, 2005). For seeds treated in hot water at 100°C, Sharma et al. (2008) reported germination of 94 to 100% in Albizia lebbeck (L.) Benth.), Albizia procera (Roxb.) Benth., Peltophorum pterocarpum (DC.) Backer ex Heyne, Acacia auriculiformis A. Cunn. ex Benth. and Leucaena leucocephala (Lam.) de Wit. Albrecht (1993) reported that treating seeds for 24 h in hot water at 100°C enhanced percent germination of Adansonia digitata L., Calliandra calothyrsus Meissner and Sesbania sesban (L.) Merr. Botumile et al. (2020) also reported that hot water improved percent germination in Vachellia karroo (Hayne) Banfi & Galasso compared with the control. According to Mwase and Mvula (2011), hot water softens hard seed coats, leaches out chemical inhibitors and allows imbibition and gaseous exchange. Mojeremane et al. (2017) found that hot water was not effective in improving percent germination of Vachellia rehmanniana Schinz just like those of B. africana in the present study. According to Teketay (1996a) the degree of the seed coat hardness among different species is the cause of different responses to various treatments. The poor performance of B. africana in the hot water treatment could be due to the thickness of the seed coat, which failed to break before the water cooled down. The fact that boiling water treatments (experiment 3 in this study) improved germination is evidence that the species is characterised by hard coat-imposed dormancy.
Boiling water (for 1, 3 and 5 min) was effective in increasing percent germination in B. africana compared with controls (Table 1). Results indicated that percent germination in this species increased with exposure time, suggesting physical dormancy imposed by the hard seed coat. In the case of E. africanum, percent germination was increased by treating seeds in boiling water (1 min) compared with the control. There were no significant differences in percent germination among the boiling water (3 and 5 min) treatments and the control (Table 1). Results show that percent germination decreased with increase in exposure time to boiling water. This result is consistent with Botumile et al. (2020) who reported a decrease in percent germination with increasing exposure time up to 5 min with boiling water for Senegalia galpinii and Vachellia robusta. Similar results were also reported for Vachellia karroo (Mmolutsi et al., 2020). The decline in percent germination with increase in boiling time could be due to the sensitivity of seeds to the heat, which might have damaged the embryo.
Dormancy in the legume species is mainly caused by their hard seed coat covering which prevents water uptake and gaseous exchange. Therefore, the hard seed coat needs to be subjected to pre-sowing treatments before seeds can germinate. The study has shown that the hard seed coat in B. africana can be overcome by mechanical scariï¬cation, exposure to sulphuric acid and boiling water. Seed germination in E. africanum was significantly improved by mechanical scarification, exposure to sulphuric acid, boiling water (1 min) and hot water (boiling water allowed to cool for 24 h). The results also indicated that sulphuric acid and mechanical scarification treatments resulted in the highest, fastest and uniform germination percentages relative to the control and boiling water treatments. Therefore, extension agents and researchers that have plans to raise seedlings of B. africana should consider scarification treatments using mechanical scarification, sulphuric acid and boiling water before sowing. For E. africanum, they should subject seeds to mechanical scarification, sulphuric acid and boiling water (1 min) and hot water. Mechanical scarification and boiling water treatments are recommended for farmers and nurseries since they are safer and require less skill to administer; while sulphuric acid treatments can be used in research laboratories. When using mechanical scarification, care should be taken to ensure that the scariï¬cation treatment does not bruise the endosperm or the embryo since it could lead to fungal attack and death of the seed.
The authors have not declared any conflict of interests.
The authors would like to acknowledge the ï¬nancial support of the Research, Technology Development and Transfer Committee (RTDTC), Botswana University of Agriculture and Natural Resources. They would also like to thank the Department of Forestry and Range Resources, Ministry of Environment, Natural Resources Conservation and Tourism (MENT) for their permission to conduct research in Kazuma Forest Reserve and for granting the authors a Research Permit [Ref. ENT 8/36/4 XXXX (29)] to undertake the Research in KFR.
REFERENCES
|
Airi S, Bhatt ID, Rawal RS, Dhar U (2009). Variations in seed germination of Hippophae salicifolia with different presoaking treatments. Journal of Forest Research 20:27-30.
Crossref
|
|
|
|
Alamgir M, Hossain MK (2005). Effect of pre-sowing treatments on germination and initial seedling development of Albizia saman in the nursery. Journal of Forestry Research 16:200-204.
Crossref
|
|
|
|
|
Albrecht J (1993). Tree seed handbook of Kenya. GTZ Forestry Seed Centre Muguga, Nairobi, Kenya 264 p.
|
|
|
|
|
Amri A (2010). The effects of pre-sowing seed treatments on germination of snake bean (Swartzia madagascariensis): a reported medicinal plant. Research Journal of Agriculture and Biological Sciences 6(4):557-561.
|
|
|
|
|
Aref IM, Atta HAE, Shahrani TA, Mohamed AI (2011). Effects of seed pre-treatment and seed source on germination of five Acacia spp. African Journal of Biotechnology 10(71):15901-15910.
Crossref
|
|
|
|
|
Azad S, Manik MR, Hasan S, Matin A (2011). Effect of different pre-sowing treatments on seed germination percentage and growth performance of Acacia auriculiformis. Journal of Forestry Research 22(2):183-188.
Crossref
|
|
|
|
|
Azad MS, Musa ZA, Martin MA (2010). Effect of pre-sowing treatments on seed germination of Melia azedarach. Journal of Forest Research 21:193-196.
Crossref
|
|
|
|
|
Baskin C, Baskin JM (2014). Ecology, biogeography, and, evolution of dormancy and germination. 2nd Edition. Academic Press, London.1600 p.
|
|
|
|
|
Bellard C, Cassey P, Blackburn TM (2016). Alien species as a driver of recent extinctions. Biology Letters 12:20150623.
Crossref
|
|
|
|
|
Boateng SK (2017). Evaluation of pre-sowing treatments for seed germination enhancement of Chrysophyllum albidum g. Don. Ghana Journal of Agricultural Science 51:41-51.
|
|
|
|
|
Bolingue W, Ly Vu B, Leprince O, Buitink J (2010). Characterization of dormancy behaviour in seeds of the model legume Medicago truncatula. Seed Science Research 20(2):97-107.
Crossref
|
|
|
|
|
Botswana Press Agency (BOPA). (2013). Kgatleng commemorates tree planting day. Botswana Daily News, Tuesday 12 November 2013. Botswana Press Agency, Gaborone Botswana.
|
|
|
|
|
Botsheleng B, Mathowa T, Mojeremane W (2014). Effects of pre-treatments methods on the germination of Pod mahogany (Afzelia quanzensis) and mukusi (Baikiaea plurijuga) seeds. International Journal of Innovative Research in Science Engineering and Technology 3(1):8108-8113.
|
|
|
|
|
Botumile A, Teketay D, Mojeremane W, Mathowa T (2020). Overcoming seed dormancy of Senegalia galpinii and Vachellia robusta through scarification pre-sowing treatments. Agriculture and Forestry 66(1):157-173.
Crossref
|
|
|
|
|
Burkill HM (1985). The useful plants of West Tropical Africa. Royal Botanic Gardens, Kew 981 p.
|
|
|
|
|
Cirak C, Kevseroglu K, Saglam B (2004). Physical and physiological dormancy in black henbane (Hyoscyamus niger L.) seeds. Journal of Plant Biology 47(4):391-395.
Crossref
|
|
|
|
|
Dodet M, Collet C (2012). When should exotic forest plantation tree species be considered as an invasive threat and how should we treat them? Biological Invasions 4:1765-1778.
Crossref
|
|
|
|
|
Doran JC, Turnbull JW, Boland DJ, Gunn BV (1983). Handbook on seeds of dry-zone acacias: A guide for collecting, extracting, cleaning, and storing the seed and for treatment to promote germination of dry-zone acacias. Food and Agriculture Organization of the United Nations, Rome 92 p.
|
|
|
|
|
Doust SJ, Erskine PD, Lamb D (2008). Restoring rainforest species by direct seeding: Tree seedling establishment and growth performance on degraded land in the wet tropics of Australia. Forest Ecology and Management 256(5):1178-1188.
Crossref
|
|
|
|
|
Elliott S, Kuarak C, Navakitbumrung P, Zangkum S, Anusarnsunthorn V, Blakesley D (2002). Propagating framework trees to restore seasonally dry tropical forest in northern Thailand. New Forests 23:63-70.
Crossref
|
|
|
|
|
Fanshawe DB (1972). Useful trees of Zambian for the agriculturist. Ministry of Lands and Natural Resources, Lusaka, Zambia. 126 pp.
|
|
|
|
|
Fredrick C, Muthuri C, Ngamau K, Sinclair F (2017). Provenance and pretreatment effect on seed germination of six provenances of Faidherbia albida (Delile) A. Chev. Agroforest Systems 91:1007-1017.
Crossref
|
|
|
|
|
Germishuizen G, Meyer NL (2003). Plants of Southern Africa: An Annotated Checklist, Strelitzia 14, National Botanical Institute, Pretoria 1231 p.
|
|
|
|
|
Himanen K, Nygren M, Dumroese RK (2012). Boiling water scarification plus stratification improves germination of Lliamna rivularis (Malvaceae) seeds. Native Plants 13(3):245-254.
Crossref
|
|
|
|
|
Hyde MA, Wursten BT, Ballings P, Coates Palgrave M (2020). Flora of Zimbabwe: Species information: Erythrophleum africanum.
View retrieved 20 April 2020.
|
|
|
|
|
Kahaka P, Mojeremane W, Teketay D, Mathowa T (2018). Effects of scarification treatments on seed germination of three leguminous species from Botswana. Silva Lusitana 26(1):115-131.
|
|
|
|
|
Kawanga V (2008). Erythrophleum africana (Welw. Ex Benth.). Harms. In: Schmelzer GH, Gurib-Fakim A (Eds.), Plant Resources of Tropical Africa 11(1). Medicinal Plants 1, Backhuys Publishers, Leiden Netherlands / CTA, Wageningen, Netherlands pp. 244-245.
|
|
|
|
|
Lamb D (2011). Ecological restoration. In Regreening the Bare Hill. In Lamb D (editors): Tropical Forest Restoration in the Asia-Pacific Region. Springer, New York, NY, USA 8:325-358.
Crossref
|
|
|
|
|
Lock JM (1989). Legumes of Africa: A checklist. Royal Botanic Gardens, Kew, London 619 p.
|
|
|
|
|
Long RL, Gorecki M, Renton M, Scott JK, Colville L, Goggin DE, Commander LE, Westcott DA, Cherry H, Finch-Savage WE (2015). The ecophysiology of seed persistence: a mechanistic view of the journey to germination or demise. Biological Reviews 90(1):31-59.
Crossref
|
|
|
|
|
Luna T, Wilkinson K, Dumroese RK (2009). Seed germination and sowing options-In Nursery manual for native plants: A guide for tribal nurseries, Vol. 1: Nursery management. Agriculture Handbook 730. Department of Agriculture, Forest Service. Washington, D.C, USA. pp.163-183.
|
|
|
|
|
Mackay WA, Davis TD, Sankhla D (1995). Influence of scarification and temperature treatments on seed germination of Lupinus havardii. Seed Science and Technology 23:815-821.
|
|
|
|
|
Mair CE, Grienke U, Wilhelm A, Urban E, Zehl M, Schmidtke M, Rollinger JM (2018). Anti-influenza triterpene saponins from the bark of Burkea africana. Journal of Natural Products 81(3):515-523.
Crossref
|
|
|
|
|
Mapongmetsem PM, Duguma B, Nkongmeneck BA, Selegny E (1999). The effect of various seed pre-treatments to improve germination in eight indigenous tree species in the forests of Cameroon. Annals of Forest Science 56:679-684.
Crossref
|
|
|
|
|
Maroyi A (2019). A review of botany, medicinal uses, phytochemistry and biological activities of Erythrophleum africanum. Journal of Pharmaceutical Science and Research 11(11):3559-3564.
|
|
|
|
|
Maroyi A (2010). Burkea africana Hook. [Internet] Record from PROTA4U. In: Lemmens RHMJ, Louppe D, Oteng-Amoako AA (Editors). PROTA (Plant Resources of Tropical Africa. Wageningen, Netherlands.
View . Accessed 19 April 2020.
|
|
|
|
|
Mathisen E, Diallo D, Anderson ØM, Malterud KE (2002). Antioxidants from the bark of Burkea africana, an African medicinal plant. Phytotherapy Research 16(2):148-53.
Crossref
|
|
|
|
|
Mbalo BA, Witkowski ETF (1997). Tolerance to soil temperatures experienced during and after the passage of fire in seeds of Acacia karroo, A. nilotica and Chromolaena odorata: a laboratory study. South African Journal of Botany 63:421-425.
Crossref
|
|
|
|
|
McNamara S, Tinh DV, Erskine PD, Lamb D, Yates D, Brown S (2006). Rehabilitating degraded forest land in central Vietnam with mixed native species plantings. Forest Ecology and Management 233(2-3):358-365.
Crossref
|
|
|
|
|
Meli P, Martínez-Ramos M, Rey-Benayas JM, Carabias J (2014). Combining ecological, social and technical criteria to select species for forest restoration. Applied Vegetation Science 17(4):744-753.
Crossref
|
|
|
|
|
Mojeremane W, Mathowa T, Teketay D (2018). Effects of different pre-sowing treatments on germination of Peltophorum africanum Sond. seeds from two provenances in Botswana. Journal of Biodiversity and Environmental Sciences 12(2):230-236.
|
|
|
|
|
Mojeremane W, Mathowa T, Teketay D, Stimela T, Kopong I, Rampart M (2017). Pre-sowing seed treatment method to overcome dormancy in seeds of Vachellia rehmanniana Schinz. Agriculture and Forestry 63(2):171-181.
Crossref
|
|
|
|
|
Müllera FL, Raitta LM, Cupido CF, Chimphango SBM, Samuels MI, Boatwright JS (2017). Dormancy-breaking treatments in two potential forage crop legumes from the semi-arid rangelands of South Africa. South African Journal of Botany 113:133-136.
Crossref
|
|
|
|
|
Mmolutsi GG, Matsuane C, Mojeremane W, Mathowa T, Teketay D (2020). Application and use of pre-sowing treatment methods to improve germination of Vachellia karroo (Hayne) Banfi & Galasso. Agriculture and Forestry Journal 4(1):47-54.
|
|
|
|
|
Mulofwa J, Simute S, Tengas B (1994). Agroforestry manual for extension workers in the southern Province, Zambia. Technical Handbook No.4. Regional Soil Conservation Unit/SIDA.
|
|
|
|
|
Mwase WF, Mvula T (2011). Effect of seed size and pre-treatment methods of Bauhinia thonningii Schum. on germination and seedling growth. African Journal of Biotechnology 10(13):5143-5148.
|
|
|
|
|
Naim AH, El Hadi AA, El Gasim Ahmed F (2015). Evaluation of different pre-sowing seed treatments to break dormancy of Crotalaria senegalensis, a famous rangeland forage in Sudan. Asian Journal of Plant Science and Research 5(10):16-21.
|
|
|
|
|
Nasr SMH, Savadkoohi SK, Ahmadi E (2013). Effect of different seed treatments on dormancy breaking and germination in three species in arid and semi-arid lands. Forest Science Practice 15(2):130-136.
Crossref
|
|
|
|
|
Nair KSS (2001). Pest outbreaks in tropical forest plantations: is there a greater risk for exotic tree species? Centre for International Forestry Research, Jakarta, Indonesia 74 p.
|
|
|
|
|
Neya B, Hakkou M, Pétrissans M, Gérardi P (2004). On the durability of Burkea africana heartwood: evidence of biocidal and hydrophobic properties responsible for durability. Annals of Forest Science 61(3):277-282.
Crossref
|
|
|
|
|
Odirile O, Mojeremane W, Teketay D, Kashe K, Mathowa T (2019). Responses of seeds of Vachellia erioloba (E. Mey.) P.J.H. Hurter in Botswana to different pre-sowing treatment methods. International Journal of Biology and Biotechnology 16(1):181-188.
|
|
|
|
|
Opoku JA, Amissah JN, Essilfie ME, Norman JC (2018). Effect of pre-sowing treatments on seed germination and seedling growth of silver butterfly tree (Bauhinia rufescens). Current Agriculture Research Journal 6(3):344-354.
Crossref
|
|
|
|
|
Palgrave KC (2002). Trees of southern Africa. 3rd Edn., Struik Publishers, Cape Town 1212 p.
|
|
|
|
|
Palmer E, Pitman N (1972). Trees of southern Africa-covering all indigenous species in the R.S.A, SWA, Botswana, Lesotho and Swaziland. Vol. 2, A.A. Balkema Publishers, Cape Town 1497 p.
|
|
|
|
|
Phartyal SS, Baskin JM, Baskin CC, Thapliyal RC (2005). Physical dormancy in seeds of Dodonaea viscosa (Sapindaceae) from India. Seed Science Research 15(1):59-61.
Crossref
|
|
|
|
|
Raman T, Mudappa D, Kapoor V (2009). Restoring rainforest fragments: Survival of mixed-native species seedlings under contrasting site conditions in the western Ghats, India. Restoration Ecology 17(1):137-147.
Crossref
|
|
|
|
|
Rasebeka L, Mathowa T, Mojeremane W (2014). Effect of seed pre-sowing treatment on germination of three Acacia species indigenous to Botswana. International Journal of Plant and Soil Science 3(1):62-70.
Crossref
|
|
|
|
|
Richardson DM (1998). Forestry trees as invasive aliens. Conservation Biology 12(1):18-26.
Crossref
|
|
|
|
|
Rincón-Rosales R, Culebro-Espinosa N.R, Gutierrez-Miceli FA, Dendooven L (2003). Scarification of seeds of Acacia angustissima (Mill.) Kuntze and its effect on germination. Seed Science and Technology 31(2):301-307.
Crossref
|
|
|
|
|
Rodrigues-Junior AG, Faria JMR, Vaz TAA, Nakamura AT, José AC (2014). Physical dormancy in Senna multijuga (Fabaceae: Caesalpinioideae) seeds: the role of seed structures in water uptake. Seed Science Research 24(2):147-157.
Crossref
|
|
|
|
|
Roodt V (1998). Trees and shrubs of the Okavango Delta: Medicinal uses and nutritional value. Shell Oil Botswana, Gaborone. 62p.
|
|
|
|
|
Schmidt L (2007). Tropical forest seed. Springer-Verlag, Berlin, Germany 399 p.
Crossref
|
|
|
|
|
Setlhabetsi OT, Mojeremane W, Mathowa T, Teketay D (2019). Breaking seed dormancy in Philenoptera violacea (Koltzsch) Schire using different pre-sowing treatment methods. Journal of Agriculture and Environmental Sciences 8(1):104-110.
Crossref
|
|
|
|
|
Setshogo MP (2002). Common names of some flowering plants of Botswana. Bay Publishing 144 p.
|
|
|
|
|
Sharma S, Naithani R, Vargtrese B, Keshavkant S, Naithani SC (2008). Effect of hot-water treatment on seed germination of some fast-growing tropical tree species. Tropical Ecology 24:49-53.
|
|
|
|
|
Shono K, Cadaweng EA, Durst PB (2007a). Application of assisted natural regeneration to restore degraded tropical forestlands. Restoration Ecology 15(4):620-626.
Crossref
|
|
|
|
|
Shono K, Davies SJ, Chua Y (2007b). Performance of 45 native tree species on degraded lands in Singapore. Journal of Tropical Forest Science 19(1):25-34.
|
|
|
|
|
Smith P, Allen Q (2004). Field guide to the trees and shrubs of the miombo woodlands. Royal Botanic Gardens, Kew, London 176 p.
|
|
|
|
|
Smýkal P, Vernoud V, Blair MW, Soukup A, Thompson RD (2014). The role of the testa during development and in establishment of dormancy of the legume seed. Frontiers in Plant Science 5:351.
Crossref
|
|
|
|
|
Sy A, Grouzis M, Danthu P (2001). Seed germination of seven Sahelian legume species. Journal of Arid Environments 49(4):875-882.
Crossref
|
|
|
|
|
Tadros MJ, Samarah NH, Alqudah AM (2011). Effect of different pre-sowing seed treatments on the germination of Leucaena leucocephala (Lam.) and Acacia farnesiana (L.). New forests 42(3):397-407.
Crossref
|
|
|
|
|
Tanko Y, Iliya B, Mohammed A, Mahdi M, Musa K (2011). Modulatory effect of ethanol stem bark extract of Burkea africana on castor oil induced diarrhea on experimental animals. Archives of Applied Science Research 3(5):122-130.
|
|
|
|
|
Teketay D (1996a). Germination Ecology of twelve indigenous and eight exotic multipurpose leguminous species from Ethiopia. Forest Ecology and Management 80(1-3):209-223.
Crossref
|
|
|
|
|
Teketay D (1996b). The effect of different pre-sowing seed treatments, temperature and light on the germination of five Senna species from Ethiopia. New Forests 11:155-171.
Crossref
|
|
|
|
|
Teketay D (1998). Germination of Acacia origena, A. pilespina and Pterolobium stellatum in response to different pre-sowing seed treatments, temperature and light. Journal of Arid Environments 38(4):551-560.
Crossref
|
|
|
|
|
Teketay D (2005). Seed and regeneration ecology in dry Afromontane forests of Ethiopia: I. Seed production - population structures. Tropical Ecology 46:29-44.
|
|
|
|
|
Tigabu M, Odén PC (2001). Effect of scarification, gibberellic acid and temperature on seed germination of two multipurpose Albizia species from Ethiopia. Seed Science and Technology 29(1):11-20.
|
|
|
|
|
Van Wyk BE, Gericke N (2007). People's Plants. A Guide to Useful Plants of Southern Africa. Briza Publications, Pretoria, Republic of South Africa 416 p.
|
|
|
|
|
Walters M, Midgley JJ, Somers MJ (2004). Effects of fire and fire intensity on the germination and establishment of Acacia karroo, Acacia nilotica, Acacia luederitzii and Dichrostachys cinerea in the field. BMC Ecology 4:1-13.
|
|
|
|
|
Weibrecht K, Müller K, Leubner-Metzger G (2011). First off, the mark: early seed germination. Journal of Experimental Botany 62(10):3289-3309.
Crossref
|
|
|
|
|
Zar JH (1996). Biostatistical Analysis. Prentice Hall, Englewood Cliff, New Jersey, USA 663 p.
|
|