ABSTRACT
Anthuriums are among the most attractive ornamental plants; however, the commercial production of these plants is limited by the slow propagation methods presently in use. This situation can be resolved with the application of in vitro culture techniques which allow massive plant propagation through morphogenic processes. Plant growth regulators (PGR) and the composition of the basal media comprising the culture medium are among the factors influencing the induction of morphogenesis. Optical and electron microscopy analysis suggested that the morphogenic routes induced were organogenesis and somatic embryogenesis. This report presents three protocols of morphogenesis, two for adventitious shoot organogenesis and one via somatic embryogenesis. The treatments which induced adventitious shoot organogenesis were Murashige and Skoog medium at half ionic strength supplemented with 0.2 μM of thidiazuron (TDZ) and 2.2 μM of 6-benzylaminopurine (BAP); 0.2 μM of TDZ and 6.6 μM of zeatin (ZEA); the treatment which induced somatic embryogenesis was 0.4 μM of TDZ and 0.5 μM of 2, 4-dichlorophenoxyacetic acid (2, 4-D).
Key words: Anthuriums andreanum, morphogenesis, thidiazuron, 2, 4-dichlorophenoxyacetic acid (2, 4-D), organogenesis, somatic embryogenesis, electron microscopy.
Anthurium andreanum is a member of the Araceae family and the species is native to the tropics of Central and South America (Gantait and Mandal, 2010). The high demand of A. andreanum for its commercialization as cut flowers, potted plants and garden plants, requires highly efficient propagation methods. In vitro culture is an attractive alternative for the multiplication of cultivars with high commercial values, allowing the production of high quality planting material in large quantities (Desai et al., 2015). The micro-propagation of ornamental plants is performed mainly through tissue culture in commercial laboratories all over the world, producing approximately 200,000 in vitro plantlets a week (Bhowmik and Matsuiz, 2001). In vitro culture permits the establishment of morphogenesis in plants. Morphogenesis refers to the development of organs (shoots, roots or flowers) giving rise to the form and general structure of the plant (Ramage and Williams, 2002). The development of efficient regeneration protocols through morphogenic processes is also important for the genetic transformation and development of transgenic plants with new features, such as new shapes of leaves and flowers. The aim of this work was to develop morphogenic processes for the rapid and massive propagation of A. andreanum cv. Calypso. Morphological and histological evaluations of cultures were performed during the induction and the development using optical microscopy and scanning electron microscopy.
Explant source
Leaves were obtained from six-month-old plants of A. andreanum var. Calypso for the induction of morphogenesis. Anthurium plants were sprayed with a fungicidal and bactericidal solution over a period of 15 days prior to cutting the leaves. The selected explants were young leaves which were cut after the light green color had disappeared and the leaves presented a light brown color; the leaf was cut leaving a petiole of 5 to 8 cm. The freshly cut leaves were submerged in a fungicidal and bactericidal solution for 2 h, after which they were washed under running tap water and Extran ®. In a laminar flow station, the leaves were sprayed with a solution of ethanol at 96% (v/v) and rinsed once with distilled water; they were then soaked in a solution of NaOCl at 1% followed by two rinses with sterile distilled water. Once disinfected, the leaves were cut transversely to 0.5 cm2.
Preparation of the medium and culture conditions
All the treatments were supplemented with sucrose at 3%. The pH of the culture medium was adjusted to 5.8 using potassium hydroxide (1 N) or hydrochloric acid (1 N), before the addition of gel rite. The culture medium was subjected to autoclaving at a pressure of 1.05 kg cm2 at 121°C for 20 min.
For the induction of morphogenesis, the cultures were maintained at 25 ± 2°C, in darkness. For regeneration, the cultures were allowed to grow in the culture room at 25 ± 2°C under a 16/8-h (light/dark) photoperiod; the light was administered with white LED tube lamps with an irradiation of 60 μmol m-2s-1.
Experimental design and statistical analyses
The morphogenic treatments were designed using a factorial design with two factors. The first factor was the ionic strength of the basal culture medium: Murashige and Skoog medium (MS) at 2.2 gL-1 (1/2 MS) and 4.4 gL-1 (MS); the second factor was the combination of plant growth regulators (PGR). With respect to the PGR, thidiazuron (TDZ) at three concentrations was used, combining it with three auxins: naphthalene acetic acid (NAA), indoleacetic acid (IAA) and 2, 4-D; TDZ at three concentrations combined with three cytokinins: 6-benzylaminopurine (BAP), zeatin (ZEA), and Kinetin (KIN). The design gave a total of 60 treatments (Table 1). The concentrations evaluated varied, taking into consideration the range of action of each PGR. The controls were evaluated using MS and ½ MS without PGR. The results were analyzed with the program of statistical graphs XVI and the significance level was determined as P = 0.05. The average values of the treatments were compared with the Tukey HSD intervals.
Histological analysis
Samples of the foliar explants obtained from the treatments for morphogenesis induction were collected every three days and the sampling was conducted over a period of 60 days. For the stage of tissue fixation, the samples were placed in glass vials and submerged in formaldehyde/acetic acid/alcohol at 70% (v/v) under vacuum for 24 h, according to the protocol of Johansen (1940). Subsequently, a gradual dehydration of the samples was carried out in different concentrations of ethanol (30 to 100%), followed by paraffin inclusion, after which dewaxing was performed with xylene. The sample embedded in paraffin was serially sectioned, performing cuts of more than 5 μm using a KEEDE rotary microtome. The cut sections were placed on a microscope slide and were acidified with periodic acid × 20 min; they were then washed, dried and stained with the Schiff reactive × 15 min, after which naphthol blue was applied for 8 min; finally, the sections were washed and dried, and a solution of Permount TM mounting medium was applied. Observations and photographic records were registered with a Nikon microscope equipped with a camera and infinite analysis software 5.0.3.
Electronic microscopy
Samples of calluses from the cultures in the morphogenesis induction medium were collected. The samples were sectioned in 1 mm square-shaped pieces which were then fixed in glutaraldehyde at 3% and maintained at 4°C for one night. Subsequently, the samples were washed in potassium phosphate buffer 0.05 M (pH 7.0), dehydrated in a graduated series of ethanol: 40, 50, 70, 80, 95 and 100%; dried to a critical point with liquid carbon dioxide, fixed on aluminium plates and covered with gold/palladium. The samples were then placed in a metal support, using an adhesive, and were metalized with a thin film of gold. The callus was examined by scanning electron microscopy JSM6369LV. The results were observed in high resolution and the images were captured digitally.
Morphogenesis and plant regeneration
The induction of organogenesis from adventitious shoots in leaf explant of A. andreanum cv. Calypso was induced in two treatments: (1) 1/2 MS supplemented with 0.2 μ M TDZ and 2.2 μ M BAP; (2) 1/2 MS supplemented with 0.2 Μm of TDZ and 6.6 Μm of ZEA. In A. andreanum cv., the induction and development of the adventitious shoots and rooting ocurred in the same treatment. Similarly, Ramage and Williams (2002) reported that it is possible to use only one formulation for all the stages of morphogenesis; however, the formulation must be established depending on the species and variety.
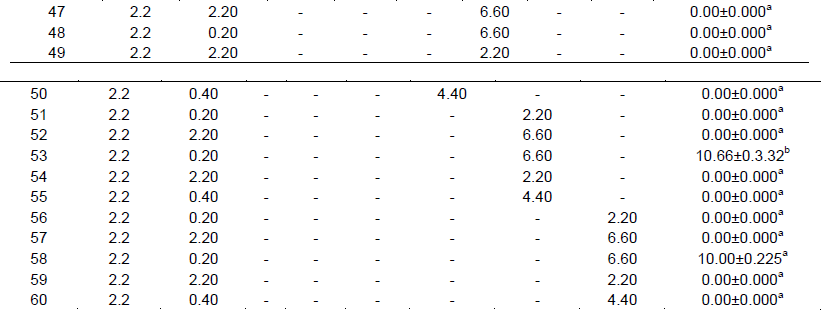
In A. andreanum cv. Calypso, after 45 days of culture in darkness, it was possible to observe the formation of yellow callus on the edge of the leaf explant in treatment with 1/2 MS supplemented with 0.2 μM of TDZ, 2.2 μM of BAP (Figure 1A to C). For the development and regeneration of the callus, the explants were cultured in photoperiod and after 60 days the formation of leaves and roots was observed (Figure 1D). Organogenesis was also obtained in the treatment with 1/2 MS supplemented with 0.2 μM of TDZ and 6.6 μM of ZEA. The treatment that allowed the greatest number of adventitious shoots per explant was 1/2 MS supplemented with 0.2 μM of TDZ and 2.2 μM of BAP (Table 1). In both treatments, the time required from the morphogenic induction to the procurement of completely developed plantlets was seven months.
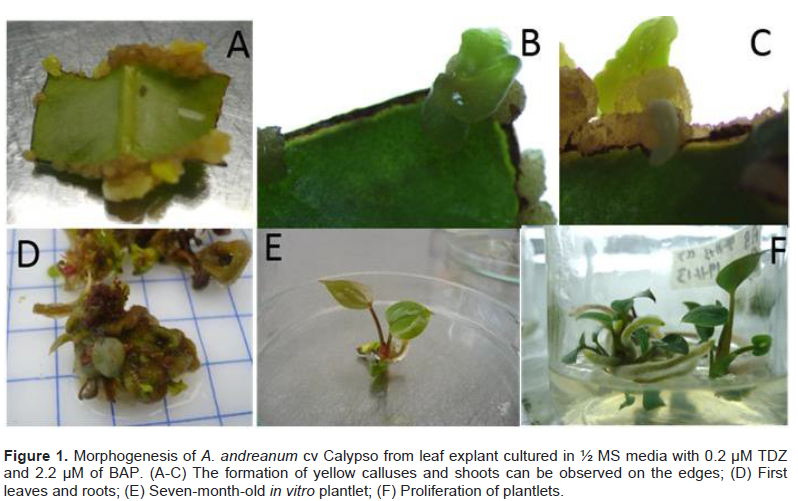
In this report, it was possible to observe that the leaf explant of A. andreanum cv Calypso has a high totipotent capacity given that it also acquired embryogenic competency. When the leaf explant was cultured in the treatment with 1/2 MS supplemented with TDZ 0.4 μM and 0.5 μM 2, 4-D in the dark, the formation of white embryogenic callus with a friable consistency was observed. Somatic embryogenesis has been described from nodal segments of A. andreanum cv. Eidibel through culture in Pierik medium supplemented with 10 µM α-naphthalene (ANA) (Pinheiro, 2014). In A. andreanum Calypso, the 2, 4-D induced somatic embryogenesis in combination with TDZ. It is known that 2, 4-D is a powerful PGR which has been reported in different species for the induction of somatic embryogenesis (Dhillon and Gosal, 2012; Pinheiro, 2013; Asthana et al., 2017). There are reports which indicate that 2,4-D alone or combined induces morphogenetic response, but it has an inhibitory effect on elongation or rooting (Nissen and Minocha, 1993; López-Puc et al., 2006); therefore, 2,4-D must be eliminated (Raghavan, 2004; Fehér, 2015). In this report, 2,4-D did not interfere in the development of morphogenesis, given that the same treatment that induced callus formation, also allowed the complete regeneration, forming somatic embryos which continued to develop until plantlets were obtained (Figure 2A to D).
The ionic strength of the basal medium MS used in this study had a significant influence on the morphogenesis and the regeneration response; this is due to the fact that the minerals are the main components of the culture media. A number of researchers have examined the process involved in the administration of minerals and the results suggest a complex network of interactions between the explant and the culture medium (Ramage and Williams, 2002). In A. andreanum, adventitious shoots of Calypso were obtained in 1/2 MS, a result which is similar to those obtained in Anthurium antioquiense (Murillo-Gómez et al., 2014), A. andreanum cv Tinora Red and Senator (Martin et al., 2003), A. andreanum cv flamingo (Viégas et al., 2007) and A. andreanum (Jahan et al., 2009). There are a number of reports in which the induction of shoots in A. andreanum has been possible using MS at 100% of its ionic strength (Sedaghati et al., 2012); this is possible due to the fact that the morphogenic response varies according to the genotype, which would suggest the need to develop protocols for each variety (Gantait and Mandal, 2010). It is well known that the success of morphogenesis is due, in part, to the correct selection of the type and concentration of the PGR. Teixeira et al. (2015) reported that the adequate concentration of PGRs or their combinations differed for different varieties, explants or culture stages, including, most importantly, the differentiation and proliferation of shoots for callus induction, formation of roots and other organogenic processes. In this report, TDZ was evaluated given that it has demonstrated its effectiveness in the induction of morphogenesis (Gopale et al., 2013). TDZ presents activity similar to that of a cytokinin and it has also been suggested that it may be a modulator of endogenous auxin levels. There is experimental evidence that TDZ stimulates the synthesis of de novo auxins, increasing the levels of IAA and its precursor, tryptophan (Murthy et al., 1995). In some cases, it is necessary to transfer the shoots to a treatment with a lower concentration of TDZ so as not to affect rooting (Cari and Preece, 1993). In this study, the presence of TDZ had no effect on the development for the formation of plantlets; this may be due to the fact that a low concentration was used. Gu et al. (2012) obtained 24 adventitious shoots in leaf explant of A. andreanum in the cultivars Alabama and Sierra when they were cultured in ½ MS supplemented with 1.82 μM TDZ and for the rooting, it was necessary to apply 0.98 μM of indole-3-butyric acid (IBA). In A. andreanum cv Calypso, the use of TDZ as the only growth regulator was unable to produce morphogenic response. However, the combined use of TDZ with another PGR allowed the formation of callus, regeneration of shoots and the formation of complete plantlets; although, it was necessary to combine TDZ with another regulator in A. andreanum Calypso, it is important to note the advantage in the fact that it the whole morphogenic process was carried out in the same treatment and there was no need to develop other formulations, as is usually the case in most of the reports on anthurium.
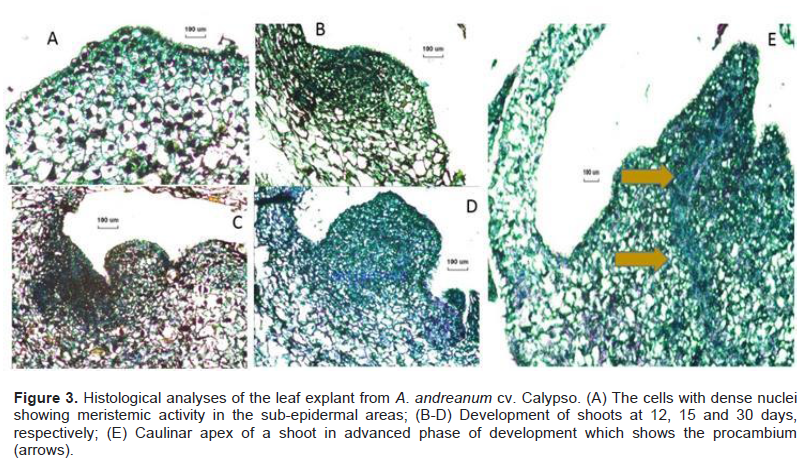
From the histological analyses, morphological changes during the morphogenesis were observed in the leaf explant of A. andreanum Calypso when it was cultured in the induction treatment with ½ MS with 0.2 μM of TDZ and 2.2 μM of BAP. The formation of small protrusions with dense nuclei was observed at 12, 15 and 30 days (Figure 3A to C). Emergent organogenic structures were observed at 30 days (Figure 3D) and apical formation of the meristems in the shoot was obtained at 60 days of induction (Figure 3E). Vargas et al. (2007) reported anatomic studies which showed green callus with organogenic potential and their results coincide with our findings; organogenesis was confirmed by the histological evaluations during the induction and the development. For genetic transformation studies, it is important to identify the areas where cellular divisions are produced, giving rise to morphogenesis.
With the use of scanning electron microscopy (SEM), structures regenerated from the leaf explant were visualized (Figure 4A to B). For the treatment in 1/2 MS supplemented with 0.2 μM TDZ and 2.2 μM BAP adventitious shoots of high resolution were observed. The samples of leaf explant cultured in ½ MS supplemented with 0.4 μM TDZ and 0.5 μM 2, 4-D presented somatic embryo formation (Figure 4C). Observations in the SEM revealed that the morphogenic calluses formed structures with organogenic and embryogenic potential.
Three successful protocols were established for the culture of A. andreanum cv Calypso tissue, two for shoot organogenesis and one through somatic embryogenesis, which would allow the clonal propagation of this plant for the floriculture market. Moreover, these protocols represent efficient methods for the regeneration of genetically transformed plants.
The authors have not declared any conflict of interests.
REFERENCES
|
Asthana P, Rai MK, Jaiswal U (2017). Somatic embryogenesis from sepal explants in Sapindus trifoliatus, a plant valuable in herbal soap industry. Ind. Crop Prod. 100:228-235.
Crossref
|
|
|
|
Bhowmik PK, Matsuiz T (2001). Novel micropropagation system: a review. Pakistan J. Biol. Sci. 4(2):117-120.
Crossref
|
|
|
|
|
Cari AH, Preece JE (1993). Thidiazuron: a potent cytokinin for woody plant tissue culture. Plant Cell Tiss. Org. 33:105-119.
|
|
|
|
|
Desai C, Inghalihalli R, Krishnamurthy R (2015). Micropropagation of Anthurium andraeanum-An important tool in floriculture. J. Pharmacogn. Phytochem. 4(3):112-117.
|
|
|
|
|
Dhillon NK, Gosal SS (2012). Effect of growth adjuvents on somatic embryogenesis in maize (Zea mays L.). J. Cell Tiss. Res. 12(2):3205-3211.
|
|
|
|
|
Fehér A (2015). Somatic embryogenesis-stress-induced remodeling of plant cell fate. Biochim. Biophys. Acta (BBA)-Gene Regulatory Mechanisms.1849(4): 385-402.
|
|
|
|
|
Gantait S, Mandal N (2010). Tissue culture of Anthurium andreanum: a significant review and future prospective. Int. J. Bot. 6(3):207-219.
Crossref
|
|
|
|
|
Gopale KD, Zunjarrao RS (2013). In vitro culture of Jatropha curcas L: A Biofuel plant. Int. J. Pure Appl. Sci. Technol. 16(2):46-54.
|
|
|
|
|
Gu A, Liu W, Chao M, Cui J (2012). Regeneration of Anthurium andreanum from leaf explants and evaluation of microcutting rooting and growth under different light qualities. Hortscience 47(1):88-92.
|
|
|
|
|
Jahan T, Islam MR, Khan R, Mamun ANK, Ahmed G, Hakim L (2009). In vitro Clonal Propagation of Anthurium (Anthurium andreanum L.) Using Callus Culture. Plant Tiss. Cult. Biotechol. 19(1):61-69.
|
|
|
|
|
Johansen DA (1940). Plant Microtechnique. Mac Graw Hill Book, New York.
|
|
|
|
|
López-Puc G, Canto-Flick A, Barredo-Pool F, Zapata-Castillo P, Montalvo-Peniche MDC, Barahona-Pérez F, Iglesias-Andreu L (2006). Direct somatic embryogenesis: A highly efficient protocol for in vitro regeneration of habanero pepper (Capsicum chinense Jacq.). HortScience 41(7):1645-1650.
|
|
|
|
|
Martin KP, Dominic J, Madasser J. Philip VJ (2003). Direct shoot regeneration from lamina explants of two commercial cut flower cultivars of Anthurium andreanum Hort. In vitro Cell. Dev. Biol. Plant. 39(5):500-504.
Crossref
|
|
|
|
|
Murashige T, Skoog F (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plantarum. 15:473-497.
Crossref
|
|
|
|
|
Murillo-Gómez PA, Naranjo E, Callejas R, Atehortúa L, Urrea A (2014). Micropropagation of the native species Anthurium antioquiense Engl. for conservation purposes. Agro. Col. 32(3):334-340.
Crossref
|
|
|
|
|
Murthy BNS, Murch SJ, Saxena PK (1995). Thidiazuronâ€induced somatic embryogenesis in intact seedlings of peanut (Arachis hypogaea): Endogenous growth regulator levels and significance of cotyledons. Physiol. Plantarum, 94(2):268-276.
Crossref
|
|
|
|
|
Nissen P, Minocha SC (1993). Inhibition by 2, 4-D of somatic embryogenesis in carrot as explored by its reversal by difluoromethylornithine. Physiol. Plantarum. 89(4): 673-680.
Crossref
|
|
|
|
|
Pinheiro MVM, Martins FB, Cruz ACFD, de Carvalho ACPPD, Oliveira EJD, Otoni, WC (2014). Somatic embryogenesis in anthurium (Anthurium andraeanum cv. Eidibel) as affected by different explants. Acta Sci. Agron. 36(1):87-98.
Crossref
|
|
|
|
|
Pinheiro MVM, Martins FB, da Cruz ACF, de Carvalho ACPP, Ventrella MC, Otoni, WC (2013). Maturation of Anthurium andraeanum cv. Eidibel somatic embryos from nodal segments. In vitro Cell. Dev. Biol. Plant. 49(3):304-312.
Crossref
|
|
|
|
|
Raghavan V (2004). Role of 2, 4-dichlorophenoxyacetic acid (2, 4-D) in somatic embryogenesis on cultured zygotic embryos of Arabidopsis: cell expansion, cell cycling, and morphogenesis during continuous exposure of embryos to 2, 4-D. Am. J. Bot. 91(11):1743-1756.
Crossref
|
|
|
|
|
Ramage CM, Williams R (2002). Mineral nutrition and plant morphogenesis. In vitro Cell Dev. Biol-Plant. 38:116-124.
Crossref
|
|
|
|
|
Sedaghati B, Babaeiyan NA, Bagheri NA, Salehiyan H, Khademian, R (2012). Effect of type and concentration of growth regulators on plant regeneration of Anthurium andreanum. Int. J. Agric. Res. Rev. pp. 998-1004.
|
|
|
|
|
Teixeira da Silva JA, Dobránszkib J, Winarto B, Zeng S (2015). Anthurium in vitro: A review. Sci. Hortic. 186:266-298.
Crossref
|
|
|
|
|
Viégas J, Rosa da Rocha, Ferreira-Moura D, Leira da Rosa J, Almedia da Souza MGS, Correa MGS, Texeira da Silva JA (2007). Anthurium andreanum (Linden ex André) culture: In vitro and ex vitro. Floricult. ornamental Biotechnol. 1:61-65.
|
|