ABSTRACT
Lignocellulosic biomass has become a major ally for production of biofuels as a clean and renewable energy source. The manufacturing of cellulolytic enzymes capable of hydrolyzing lignocellulose and producing fermentable sugars has been, and still is a great challenge. There is a wide variety of microorganisms, both bacteria and fungi, capable of producing cellulolytic enzymes. These microorganisms can be grown by solid state fermentation (SSF) or submerged fermentation (FS). In this study, the production of endoglucanase and β-glycosidase by the bacterium IM32-90 were investigated using a 168-h submerged fermentation. The DNA was extracted, purified and subjected to molecular identification, and IM32-90 was classified as a Xanthobacter flavus species. The bacterium IM32-90 produces cellulolytic enzymes, with activities of 0.214 U.mL-1 for endoglucanase and 0.056 U.mL-1 for β-glucosidase.
Key words: Bacteria, biofuels, cellulase, molecular, renewable sources.
The depletion of non-renewable fossil fuels coupled with increased pollution has raised the need to search for alternative sources of renewable energy. Biofuels such as bioethanol derived from different raw materials can serve as an alternative source of energy (Mohapatra et al., 2018). The lignocellulosic biomass consists mainly of cellulose, hemicellulose and lignin; cellulose being a polysaccharide of glucose with β-1,4-glycosidic bonds. Enzymatic saccharification of biomass is a non-polluting process that produces fermentable reducing sugars (Guo et al., 2018). Cellulases are of great interest for the development of biofuels, since they are able to saccharify cellulose from lignocellulosic materials, releasing glucose that can be converted to ethanol by fermentation (Marques et al., 2018).
Cellulases are produced by a large number of organisms including bacteria, filamentous fungi as well as plants and animals. Production by microorganisms has been performed using solid state fermentation (SSF), a process using a solid material as carrier; or by submerged fermentation (SF) in a liquid culture medium (Passos et al., 2018).
Sreena et al. (2016) reported that the cellulolytic potentials of bacteria belongs to different genera, such as Bacillus, Cellulomonas, Streptomyces, Cytophaga, Cellvibrio, and Pseudomonas. Samantha et al. (1989) reported the production of cellulases by Xanthobacter flavus when studying 17 N2-fixing microorganisms isolated from different sources. Study by Chen et al., (2016) has revealed that this species of bacteria presents potential for biodegradation of organic compounds from the environment.
Several DNA-based identification assays can be used according to the design, test and primer objectives, allowing DNA or RNA to be detected. DNA extraction and detection is more common and technically easier than RNA due to increased DNA stability. Polymerase chain reaction (PCR) is an in vitro amplification of the target DNA of a single organism using specific primer or oligonucleotide (DNA) primer sequences and DNA polymerase. Selected primers must have a unique sequence that specifically and selectively binds to the previously defined DNA target sequence. The application of modern molecular tools can provide accurate DNA sequence comparison data (El-Sayed et al., 2017). In this work cellulase production as represented by endoglucanase and β-glucosidase activities of the bacterium IM32-90 and its molecular identification were evaluated.
Bacterial strain
The strain IM32-90 was obtained from the microorganism bank of the Forest Biotechnology and Genetics Laboratory (LGBF) of the Forest Engineering Department of the Federal University of the Jequitinhonha and Mucuri Valleys (UFVJM).
Inoculum preparation and culture for the production of cellulases
Bacteria of strain IM32-90 were inoculated into a Petri dish with culture medium of the following composition (g.L-1): MgSO4.7H2O, 0.2; KH2PO4, 0.4; K2HPO4, 0.1; NaCl, 0.1; yeast extract, 0.4; carboxymethylcellulose (Synth®), 10; agar, 15; distilled water to 1 L; pH 6.0. After inoculation, the plates were incubated in BOD incubator (SL 200/334 SOLAB ®) at 28°C for 96 h. The microorganisms were collected from the plate and inoculated into 12 ml of culture medium with composition similar to that mentioned above, but without agar, contained in a 15 ml Falcon tube, incubated in a shaker (SL 222 SOLAB®) at 28°C and at 150 rpm for 96h. The content of the falcon tube were inoculated into an Erlenmeyer flask (500 ml) containing 190 ml of the same medium described previously (without the agar) and incubated in shaker (SL 222 SOLAB®) at 28°C, 150 rpm for 240 h. 2 ml samples were withdrawn at 24 h intervals and centrifuged at 10,000 rpm for 10 min for evaluation of endoglucanase and β-glycosidase activities.
Evaluation of the production of endoglucanase and β-glucosidase
Analysis of endo-1,4-β-glucanase activity was performed according to the method described by Ghose (1987), which consists of the hydrolysis of a 1% solution of CMC (Synth), followed by the quantification of reducing sugars soluble solutions released over a given time interval. The reducing sugars concentration was determined according to the 3,5-dinitrosalicyclic acid (DNS) method described by Miller (1959).
The β-glycosidase activity assay was according to the method of Ghose (1987), with hydrolysis of a 1% solution of cellobiose in 100 mmol.L-1 citrate buffer at pH 4.8 in the presence of the enzyme extract. Glucose quantification was performed using the standard enzymatic procedure employing glucose oxidase / peroxidase (GOD-POD) (Lloyd and Whelan, 1969).
Extraction of genomic DNA
Total DNA extraction from bacterial cells was performed in LGBF, according to the protocol described by Ausubel et al. (1992). After extraction, DNA quality and integrity were assessed by 0.8% (m/v) agarose gel electrophoresis in 1x TAE buffer (Tris-Acetate-EDTA) previously stained with ethidium bromide (0.2 mg.ml-1) and visualized under ultraviolet light.
16S DNA amplification
The previously extracted DNA sample was amplified by PCR using the pair of oligonucleotide primers specific for the 16S rDNA region of bacteria. The Proteobacteria (ProteoD F) and Deferribacteres (ProteoD R) primers 5’- AGACTCCTACGGGAGGCAGCAGTC-3’ (foward) and 5’- GCTGACGACAGCCATGCAGCACCT-3’ (reverse), described by Ramos et al. (2010) were used.
Purification of DNA
The DNA sample was purified using MinElute reaction cleanup kit, according to the manufacturer's recommendations. After purification, the DNA was subjected to 0.8% (m/v) agarose gel electrophoresis with a voltage of 70 volts for 1 h in 1 x TAE (Tris-Acetate-EDTA) run buffer previously stained with ethidium bromide, 2 mg.ml-1). The gel was photographed under ultraviolet light and the remaining volume of the DNA sample was subjected to sequencing reaction.
Sequencing reaction
To perform the sequencing, the DNA sample was submitted to PCR in two sequencing reactions with the bigdye terminator sequencing kit, one containing the primer forward and the other containing the primer reverse. The cycle program was performed in a MyCycler Bio-Rad thermocycler. At the end of the thermocycler process, the samples were sequenced on the ABI 3730 XL DNA analyzer sequencer (Applied Biosystems, Foster City, California CA), as recommended by the manufacturer. The generated genetic sequence was submitted for molecular identification.
Molecular identification
The genetic sequence was subjected to analysis in the BioEdit sequence alignment editor software, where a consensus sequence was generated. The consensus sequence was aligned and compared to BLAST sequences (Basic Local Alignment SearchTool - www.blast.ncbi.nlm.nih.gov) and to the Ribosomal Database Project (http://rdp.cme.msu.edu). The sequences with identities closest to the consensus sequence were selected and submitted for assembly of the phylogenetic tree using the MEGA6 software (Tamura et al., 2013). It was constructed using the neighbor-joining method with multiple methods of maximum parsimony (MP) with 1000 bootstraps (Saitou and Nei, 1987; Satapute and Kaliwal, 2016).
Production of endoglucanase and β-glycosidase from the selected lineage in CMC-containing medium for 240 h was evaluated; the values of the enzymatic activities are shown in Figure 1. In Figure of 1A and B, it can be observed that the optimal activities of endoglucanase (CMCase) and β-glycosidase were observed after 96 h of culture, with values of 0.214 U.ml-1 and 0.056 U.ml-1 respectively.
DNA integrity analysis was performed on 0.8% agarose gel, as shown in Figure 2. The preparation contains whole DNA. After DNA extraction, the PCR reaction was performed using the ProteoD F/ProteoD R primer pairs. The amplification results are shown in Figure 3. The DNA sample was subjected to purification using the MinElute Reaction Cleanup kit. The purified DNA was subjected to a 0.8% agarose gel to verify the quality of the purification.
The result of IM32-90 purification was positive, as shown in Figure 4.
The purified DNA sample was subjected to sequencing on the ABI 3730 XL DNA analyzer sequencer (Applied Biosystems, Foster City, California CA), as recommended by the manufacturer. The gene sequence was submitted for molecular identification. Sequences generated were used to generate a consensus sequence with the aid of BioEdit sequence alignment editor software. Molecular identification of 16S rDNA of the cellulolytic enzyme-producing bacterial strain was done by comparing the consensus sequence obtained from each microorganism with GenBank through the BLASTn program (NCBI - www.ncbi.nih.gov), as well as comparison with data from the RDP, as described below.
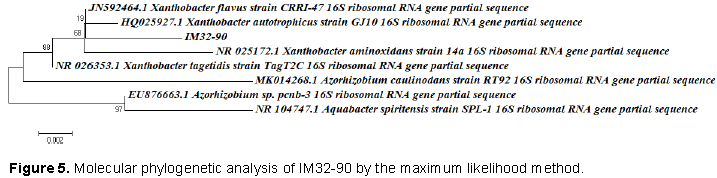
The alignment of the contiguous sequence of the IM32-90 microorganism showed 99.62% identity with the genotype registered for the X. flavus species; 99.43% with Xanthobacter autotrophicus and Xanthobacter tagetidis; 99.05% with Xanthobacter aminoxidans; 98.49% with Azorhizobium sp.; 98.12% with Azorhizobium caulinodans and 97.74% with Aquabacter spiritensis. Phylogenetic tree was constructed using the distance method, using the neighbor-joining algorithm, using Mega 6.0 software. The phylogenetic relationship of the nucleotide sequence analyzed aggregated IM32-90 to the monophyletic group with the species Xanthobacter flavus, as shown to Figure 5. The evolutionary history was inferred by using the Maximum Likelihood method based on the Kimura 2-parameter model (Kimura, 1980). The tree with the highest log likelihood (-906.9952) is shown. The percentage of trees in which the associated taxa clustered together is shown next to the branches. Initial tree(s) for the heuristic search were obtained by applying the Neighbor-Joining method to a matrix of pairwise distances estimated using the Maximum Composite Likelihood (MCL) approach. A discrete Gamma distribution was used to model evolutionary rate differences among sites (5 categories (+G, parameter = 0.1000)). The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. The analysis involved 8 nucleotide sequences. All positions containing gaps and missing data were eliminated. There were a total of 532 positions in the final dataset. Evolutionary analyses were conducted in MEGA6 (Tamura et al., 2013).
In general, the enzymatic activity of endoglucanases in this study was similar to that of other organisms already studied, as shown in Table 1. The extracellular production of β-glucosidase by bacteria is not a highly occurring process. The production values of β-glycosidase 0.056 (U.ml-1) were satisfactory compared to those found by Vyas and Chhabra (2017), who cultivated the yeast Cystobasidium oligophagum in medium containing CMC as carbon source under the same conditions and obtained 0.031 U.mL-1 after 72 h of incubation.

The analysis of the phylogenetic tree suggests that IM32-90 belongs to the genus Xanthobacter, with a 99% similarity of its genome with three species: Xanthobacter flavus, X. autotrophicus and X. tagetidis. A comparison of 16S rRNA sequences between X. flavus and A. caulinodans shows that these are strongly related (98%) (Lee et al., 2008). The genus Xanthobacter are bacteria found in moist soil and mud containing organic material in degraded wood around the roots and in the roots of plants, suggesting that it is also a species associated with nitrogen fixation (Chen et al., 2016; Samanta et al., 1989).
Until 1992, the genus Xanthobacter consisted of three species: X. autotrophicus, X. flavus and Xanthobacter agilis. Strains of X. autotrophicus and X. flavus were described as being without motility, whereas strains of X. agilis were considered mobile. New isolates of Xanthobacter have been classified into one of three species of Xanthobacter based on the motility and requirement of biotin. In general, growth in the presence of tricarboxylic acid cycle intermediates produces cells without motility, and growth in alcohols or lack of tricarboxylic acid cycle intermediates results in motile cells. Growth in H, -CO, glutamate or glutamine also produces cells without motility (Reding et al., 1992).
Other species of Xanthobacter can now be found as described in this work, such as X. aminoxidans, X. autotrophicus and X. tagetidis. The analysis of the phylogenetic tree suggests an evolutionary proximity between the species of Xanthobacter with bacteria of the genus Azorhizobium. The production of cellulolytic enzymes using bacteria of the genus Xanthobacter is not well documented. Cellulase production by this genus was reported by Samanta et al. (1989), with production of cellulase, α-amylase, protease, pectinase and lipase by these bacteria (Samanta et al., 1989). Although there was 99% similarity with three species, IM32-90 had superior score with the species X. flavus.
A cellulase producing strain IM32-90 was classified as X. flavus. This bacterium might be developed to a source for enzyme production to enable the economic manufacture of biofuels (bioethanol) from lignocellulose.
The authors have not declared any conflict of interests.
REFERENCES
|
Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhi K (1992). Current protocols in molecular biology. Vol I. New York: Greene Publishing Association; Wiley- Interscience.
|
|
|
|
Carvalho FP (2013). Cellulolytic and xylanolytic enzymes from yeasts isolated from the cerrado. Doctoral thesis- 118p. Lavras: UFLA.
|
|
|
|
|
Chen DZ, Jin XJ, Chen J, Ye JX, Jiang NX, Chen JN (2016). Intermediates and substrate interaction of 1,4-dioxane degradation by the effective metabolizer Xanthobacter flavus DT8. International Biodeterioration and Biodegradation 106:133-140.
Crossref
|
|
|
|
|
El-Sayed A, Awad W, Abdou N, Vásquez HC (2017). Molecular biological tools applied for identification of mastitis causing pathogens International Journal of Veterinary Science and Medicine 5(2):89-97.
Crossref
|
|
|
|
|
Ghose TK (1987). Measurement of cellulose activities. Pure and Applied Chemistry 59(2):257-268.
Crossref
|
|
|
|
|
Guo H, Chang Y, Lee DJ (2018). Enzymatic saccharification of lignocellulosic biorefinery: Research focuses. Bioresource Technology 252:198-215.
Crossref
|
|
|
|
|
Kimura M (1980). A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. Journal of Molecular Evolution 16(2):111-120.
Crossref
|
|
|
|
|
Lloyd JB, Whelan WJ (1969). An improved method for enzymic determination of glucose in the presence of maltose. Analytical Biochemistry 30(3):467-470.
Crossref
|
|
|
|
|
Marques NP, Pereira JC, Gomes E, Da Silva R, Araújo AR, Ferreira H, Rodrigues A, Dussán KJ, Bocchini DA (2018). Cellulases and xylanases production by endophytic fungi by solid state fermentation using lignocellulosic substrates and enzymatic saccharification of pretreated sugarcane bagasse. Industrial Crops and Products 122:66-75.
Crossref
|
|
|
|
|
Miller GL (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry 31(3):426-428.
Crossref
|
|
|
|
|
Mohapatra S, Padhy S, Mohapatra PKD, Thatoi HN (2018). Enhanced reducing sugar production by saccharification of lignocellulosic biomass, Pennisetum species through cellulose from a newly isolated Aspergillus fumigatus. Bioresource Technology 253:262-272
Crossref
|
|
|
|
|
Passos DF, Pereira Jr N, De Castro AM (2018). A comparative review of recent advances in cellulases production by Aspergillus, Penicillium and Trichoderma strains and their use for lignocellulose deconstruction. Current Opinion in Green and Sustainable Chemistry 14:60-66.
Crossref
|
|
|
|
|
Priyanka P, Yuvraj C, Farha S, Aranganathan V (2017). Isolation of cellulose degrading fungi from soil and optimization for cellulase production using carboxy methyl cellulose. International Journal of Life science and Pharma Research 7(1):56-60.
|
|
|
|
|
Ramos CG, Grilo AM, Sousa SA, Barbosa ML, Nadais H, Leitão JG (2010). A new methodology combining PCR, cloning, and sequencing of clones discriminated by RFLP for the study of microbial populations: application to an UASB reactor sample. Applied Microbiology and Biotechnology 85(3):801-806.
Crossref
|
|
|
|
|
Reding HK, Croes GLM, Dijkhuizen L, Wiegel J (1992). Emendation of Xanthobacter flavus as a motile species. International Journal of Systematic Bacteriology 42(2):309-311
Crossref
|
|
|
|
|
Saitou N, Nei M (1987). The neighbor-joining method: a new method for reconstructing phylogenetic trees. Molecular Biology and Evolution 4(4):406-425.
|
|
|
|
|
Samanta R, Pal D, Sen SP (1989). Production of Hydrolases by N2-fixing Microorganisms. Biochemie und Physiologie der Pflanzen 185(1-2):75-81.
Crossref
|
|
|
|
|
Satapute P, Kaliwal B (2016). Biodegradation of the fungicide propiconazole by Pseudomonas aeruginosa PS-4 strain isolated from a paddy soil. Annals of Microbiology 66(4):1355-1365.
Crossref
|
|
|
|
|
Sreena CP, Vimal KP, Sebastian D (2016). Production of cellulases and xylanase from Bacillus subtilis mu s1 isolated from protected areas of munnar wildlife division. Journal of Microbiology, Biotechnology and Food Sciences 5(6):500-504.
Crossref
|
|
|
|
|
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013). MEGA: Molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution 30(12):2727-2729.
Crossref
|
|
|
|
|
Vyas S, Chhabra M (2017). Isolation, identification and characterization of Cystobasidium oligophagum JRC1: a cellulase and lipase producing oleaginous yeast. Bioresource Technology 223:250-258.
Crossref
|
|