ABSTRACT
The present work concerns the analgesic effects of zerumbona, obtained from Zingiber zerumbet L. Smith cultivated in Manaus/Amazonas. The compound has been studied for decades because it has potent cytotoxic activity against liver and prostate tumor cells, colon, and breast. The plant is rich in sesquiterpenes, glycosylated flavonoids that present important pharmacological activities; standing out cytotoxic activity against neoplastic cells of cancers. The objective of this study was to test the antinociceptive activity of zerumbone (ZER) using chemical and thermal nociception models, that is, writhing test induced by acetic acid and hot plate test. ZER administered orally and intraperitoneally produced significant and dose-dependent analgesic activity against the pain, acetic acid, formalin, and capsaicin models. In addition, ZER significantly increased the dormancy of the animals in the hotplate test pain model (49-540C). It was demonstrated that intraperitoneal (i.p.) and oral (p.o.) administration of ZER sesquiterpene in doses of 150 to 500 mg/kg i.p. and 250 to 1500 mg/kg p.o. produced significant dose-dependent inhibition of acetic acid-induced abdominal writhing, as compared to fentanest (20 µg/kg). At the same intraperitoneally and orally doses, the ZER produced significant dose-dependent latency-time increases in the hot-plate test relative to control. It was concluded that ZER exhibits both central and peripheral antinociceptive activity, indicating it to hold therapeutic potential for the discovery of new antinociceptive drugs as an alternative for the discovery of new drugs in the control of neurogenesi. The ZER exhibited similar efficacy and strength via both oral and intraperitoneally routes. ZER is the major essential oil component, a new sesquiterpene responsible for its nociceptive effect, when compared with other antinociceptive sesquiterpenes described in literature.
Key words: Zingiber zerumbet, zerumbone, Zingiberacea, antinociceptive activity.
Zerumbone (ZER) is the main component extracted from the essential oils of Zingiber zerumbet, a plant belonging to family Zingiberaceae, which is much used in the Malaysian traditional medicine (Dev, 1960; Koshimizu et al., 1988). However, in Amazonia, it is only used as an ornamental plant with no therapeutical ends, being popularly known as bitter ginger. The results obtained with the essential oils extracted by steam distillation provided the lowest, yet very pure, yield percentage (99.95%) purer than that of the previous method (97%), postulated in the patent number PI0505343 -9/28/11/2007. Dichlorometahne (DCM), methanol (MEOH) and ZER extracts phytochemical screening findings revealed the presence of the following compounds: terpenoids, xanthones, flavonoids, phenols, tannins and alkaloids. EM and DCM TLC indicated a stain revealed in iodine and ceric sulfate with the retention factor of approximately 0.7 cm similar to that of Zerumbona (0.8) and MEOH showing stains in iodine and UV 254 nm. Of all parts of the plant, the RZZ has been the subject of extensive chemical investigations because of its high medicinal values. Various reports have been published regarding the phytochemical content of RZZ. Attempts to isolate and identify bioactive compounds from the RZZ started since 1944 with the identification of humulene (Eddy and Leimback, 1987); monoterpenes (Carter, 1991; Hwang and Wilcox, 1987), and zerumbone (2,6,10-cy-cloundecatrien-1-one, 2,6,9,9-tetramethyl-, (E,E,E)-) (Carter, 1991) from the essential oil of RZZ (EOZZ). It is a substance, presenting antiinflamatory (Murakami et al., 2002), antiproliferative (Takada et al., 2005), antimicrobial (Abdul et al., 2008), antibacterial (Kitayama et al., 2001) and antitumoral (Murakami et al., 2004) activities, being recommended for the treatment of liver, colon and skin cancer (Sulamain et al., 2009). Zerumbone is a monocyclic sesquiterpene compound isolated from rhizomes of Z. zerumbet. Recently some scientific research on the bioactivities of zerumbone, which was identified as a major compound of Z. zerumbet, reported that zerumbone possesses many pharmacological activities, such as chemoprevention Yob et al. (2011), antiinflammatory and antiallergic activities. Somchit et al. (2005) have earlier reported on the antinociceptive profiles of AEZZ and EEZZ administered intraperitoneally into rats and assessed using the 0.6% acetic acid-induced writhing test.
ZER presents high pharmacological potential, holding great relevance in the pharmaceutical industry application, and it can be utilized in natural medicine manufacturing and cosmetics formulation (Koshimizu et al., 1988). Recent studies using the rhizome essential oil of Z. zerumbet showed it to possess both central and periphereal antinociceptive activity when tested using chemical and thermal models of nociception (Takada et al., 2005) and to produce significant antinociceptive effect in all nociception models in mice when given via intraperitoneally route, presenting antinociceptive effect in the peripheral region (Perimal et al., 2011).
In another recent study, ZER produced its antinociception through the activation of nitric oxide (NO)/cyclic guanosine monophosphate (cGMP)/protein kinase C (PKC)/K+ channel pathways (Lapa et al., 2003). However, it is believed that Z. zerumbet essential oils antinociceptive activity is linked to its main component, the sesquiterpene ZER. The present work verified this believe using animal models, thereby revealing its potential in the search for new analgesic drugs.
Plant
The rhizome of Z. zerumbet (Zingiberaceae) was collected in the Tarumã, and two exsicats were forwarded to the herbarium at INPA for botanical determination. They were identified by Prof. Dr. Paul Maas (Departament of Plant Ecology and Evolucionary Biology) - herbarium at the University of Utrecht. A voucher specimen (no. 186913) was deposited at the Herbarium of the INPA.
Essential oil extraction and compound isolation
Attaining ZER from Z. zerumbet rhizomes essential oils was accomplished utilizing a steam drag distillator. Twenty of fresh and dried rhizomes were weighed on a digital scale, then ground in an electric grinder followed by hand grater. The ground rhizome was placed in a 12 L Mariott flask, coupled to a 20 L semi industrial pressure pot, and 15 L of water or more were poured into it. Temperature control (100°C) and pressure in essential oils destilation was recorded in a Tecnal digital water circulator connected to a condensator (temperature and pressure gauges coupled to a manometer adapted to the pot’s lid) linked to the Clevenger device. The graduated sorter was utilized for collecting and verifying the yields of the essential oils. The initial temperature of distilation by vaporisation was 80°C, being later reduced to 70°C and the condensation temperature was 5 to 150°C. Extraction took 4 h. The obtained essential oils were submitted to re-crystallization. The ZER was otained through the national patent deposit process number PI0505343-9 with a purity grade of 97%.
Chemical analyses
Thin layer chromatography analyses (TLC) were conducted on a Merck sílica-gel G 60 plate and developed with iodine vapors, ultraviolet radiation (254 and 366 nm), ceric sulphate and Dragendorff reagent.The chromatographic profile of the samples was also determined through sorting analytical technique by High Efficiency Liquid Chromatography (HELC) in liquid solid stationary phase. The chromatograms were analyzed using a Shimadzu chromatograph, model QP010, column µ-Bondapack CN, 100×8 mm having as mobile phase in water:metanol (80:20) at 6 mL/min and detected at 254 nm. The Hydrogen Nuclear Magnetic Ressonance spectra (1H NMR) and Carbon thirteen Nuclear Magnetic Ressonance (13C NMR were carried out at the Biotechnology Center of Amazonia). 1H NMR analyses spectra were recorded in Varian - Mercury 500 MHz sptectrometer with samples dissolved in CDCl3 (deuterared chloroform) and CD3OD.D2O (monohydrated methanol). The 13C NMR spectra were registered in Brüker mod. AC - 200 spectrometers operating at 50.3 MHz, the CDCl3 was the solvent utilized and TMS was the inner standard utilized in both resonances. The infrared region (IR) spectra were performed in Perkin-Elmer 1420 spectrometer using potassium bromide (KBr) tablets. Mass spectra (MS) were registered in Finnigan, 4020 model spectrometers.
Animals
For the use of animals in vivo, this project was submitted to the Committee on Ethics and Use of Animals (CEUA)/INPA, being approved by the number of opinion 005/2012, in accordance with the current norms. The animals utilized in the experiments were albino mice (Mus musculus - albinus variety) weighing between 18 and 30 g and albino rats (Ratus norvegicus - Wistar variety) (male and female) with weight ranging from 150 and 300 g acquired from the Amazon Research Institute Bioterium.
Drugs and chemicals
The following drugs were used: fentanest, dipirone, indometacine, diclorometane (DCM) and methanol (MeOH), acetic acid (Sigma Chemical), and tween 20% (Sigma Chemical). All drugs were dissolved in 0.9% saline solution. The ZER (LTQPN/COTI/INPA) was dissolved in 1% (v/v) Tween 20. Respective controls received only 1% Tween 20% as a vehicle. All drugs, chemicals and ZER solutions were prepared just prior to experiments, administer orally, and intraperoneally route to mice.
Experiments
Acetic acid-induced abdominal writhing
The procedure used was similar to the one described earlier (Koster et al., 1959). Mice were pretreated orally with ZER (50, 250, 500, 1000, 1500 mg/kg, p.o.), 1 h prior to i.p. injection of 1% acetic acid (v/v). The control group received a similar volume of vehicle (0.01 mL/100 g; i.p). Fentanest (20 µg/kg s.c.), was used as the reference drug and administered 30 min before the nociceptive agent. Following the i.p. injection of acetic acid, the animals were immediately placed into a perspex chamber and the number of writhings was recorded for 15 to 120 min, starting from 10 min post injection, as test standards (Rocha and Silva, 1968).
Hot plate
The hot plate test was performed to assess the central antinociceptive properties of ZER according to the method described previously (Koster et al., 1959) with minor modifications. In this test, the hot plate (HOT PLATE F361-INSIGHT) was maintained at 50°C. Animals were placed in the perspex cylinder on the heated surface, and the latency to a discomfort reaction (licking paws or jumping) was determined before and after ZER or drug administration. The ZER (250, 500, 1000, 1500 mg/kg, p.o. and 150, 200, 220, 250, 500 mg/kg, p.i.), vehicle (saline 0.9%; tween 20%) and fentanest (20 µg/kg s.c.) were administered 30 min prior to the beginning of the experiment. Animals were observed before and 30, 60, 120, 180 and 360 min following the ZER or fentanest administration. The cut-off time was 25 s to avoid tissue injury.
Analgesímeter test
Four to five groups of mice, each one of them with about five animals, were studied. Both paws basal volume of all animals was measured, utilizing the LE 7306 Analgesimeter (Koster et al., 1959) apparatus. In the test of the induced ear edema, cróton and formalin capsaicina because of the zerumbona (1 mg ear, topical application) was evaluated for the inhibition of edema. According to the results, the zerumbone exhibited, in doses and routes tested analgesic effect in rats and mice in vivo and in vitro. The control group received 0.9% saline solution + Tween 20 (10 mL/kg) and the positive received fentanest synthetic opiate (20 µg/kg) and indomethacin (25 mg/kg). One hour following gavage (v.o) or i.p administration, the edema was induced on the animals hind paws, by injecting a 1% carrageenan solution and, the same volume of 0.9% + Tween 20 saline solution in the contra-lateral paw.
Acute toxicity
The method described by Rocha and Silva (1968) and Salustiano et al. (1996) was employed. Mice were separated into ten, six-mice groups. They were fasted overnight and then were administered with the ZER in 10, 100, 1000, 1500, 2500 and 5000 mg/kg doses via intraperoneal and oral routes, while the control group only received the vehicle (0.9% saline + Tween 20). The mice were observed during 180 min for any abnormal behavior such as sedation, respiratory distress, motor impairment and hyper excitability and left to be observed 24 h, 48 h and 14 days later. Activities general tests were repeated in mice injected with identical doses and via the same routes.
Statistical analysis
All findings were acquired through statistical analyses of variance (ANOVA) with mean standard deviation and significance of p<0.05. The Student-Newman-Kelus test was adopted. The ID50 (dose that produced 50% inhibition in total time of paw licking) and 95% confidence intervals (CI) values were determined by using linear regression and graphs were drawn by using GraphPad Prism 4 software.
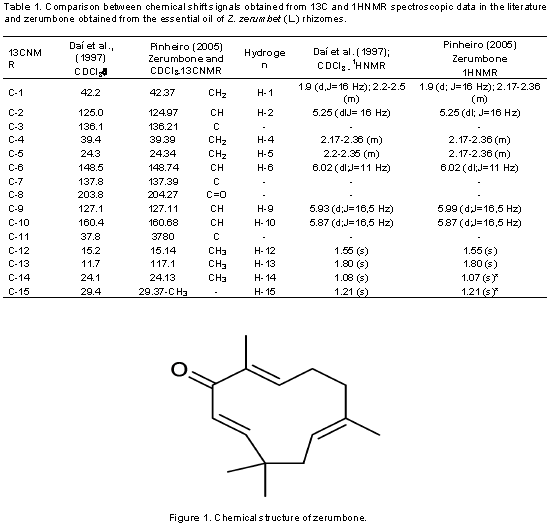
Structural determination of ZER from the essential oil of rhizomes Z. zerumbet (L.) Smith. The substance designated as zerumbone obtained from the recrystallization of essential oils from Z. zerumbet rhizomes was colorless, crystalline in appearance and the physico-chemical analysis of the substance showed a melting point of 64.5 to 64.57°C. Table 1 shows the 1 H NMR spectra exhibiting olefinic hydrogen signals at d 5.25, 6.02, 5.99 and 5.87 with similar multiplicities.The purity of zerumbone was determined by high-performance liquid chromatography (HPLC), and was shown to exceed 99.95%. 13C NMR spectra showed 15 carbon signals, as 11 hydrogenated carbons (4 CH3, 3 CH2 and 4 CH), data provided by the DEPT-135 spectrum. Z. zerumbet rhizomes essential oils analyses findings confirmed the presence of a major sesquiterpene component called Zerumbone (Figure 1), which was characterized by UV-visible spectrophotometry, high efficiency liquid chromatography (HELC), by gas chromatography and identified through 1H and 13C NMR. The 97% purity grade ZER was obtained through the national patent deposit process number PI0505343-9 (Pinheiro, 2005).
Pharmacological study
The effect of ZER on writhing response in mice is as shown in Figure 2. The ZER (150, 200, 220, 250, 500 mg/kg) given i.p. caused inhibition of dose-dependent acetic acid-induced writhing, with 98% of it being observed on the dose of 220 mg/kg (n=10, p<0.05) as compared to control. Such effects were also observed in mice pretreated with Fentanest (99%, p<0.05). Futhermore, the ZER (250, 500, 1,000, 1,500 mg/kg) given v.o. route 1 h before, ZER (1,000 mg/kg) caused a significant inhibition (98%) of the acetic acid-induced pain (Figure 2). The ED50 for ZER given via intraperoneal (i.p.) and oral (v.o.) routes, in this model, were 150, 6 (200-500) and (250-1,500 mg/kg), respectively. Through this test, it was established that ZER compound exerted a significant effect both via i.p. and v.o. by reducing the number of acetic acid-induced abdominal writhing contortions within 2 h. Essential oil from the rhizome of Z. zerumbet exhibited a significant antinociceptic effects on acetic acid-induced writing test in a dose-dependent manner, (Pinheiro, 2009).
From the Table 2, the administrations of the ZER (200-1500 mg/kg) v.o. and Fentanest (20 mg/kg) i.p. increased significantly the latency time to the nociceptive response in the hot plate test. In the antithermociceptive model (hyperalgesia), the treatment of animals with ZER (150-1500 mg/kg, p.i. or v.o.) altered the response to the stimulation up to 3 h following its administration (3.52 ± 0.21 min).
The application of fentanest (20 µ/kg, s.c) and ZER increased in 60% the time of reactivity (strength) of the stimulation application. Such outcomes demonstrate ZER to have central analgesic activity, similar to that presented by hypnoanalgesic drugs.
Formol-induced ZER analgesic activity test
The formalin test is one of the most widely used models to explain pain and analgesia mechanisms, with better results than those using mechanical or thermal stimuli. The model consists of two distinct phases. The first phase represents the irritant effect of formalin on the sensory C-fibers (Figure 3).
The formalin nociception model consists of the intraplantar injection of formaldehyde solution in the hind paw of the animal, which triggers intense nociception by direct stimulation of the nociceptors. Nociception caused by intraplantar injection of formalin and characterized by vigorous licking, biting and beating of the injected paw as an irritant. Most studies using this model use rodents, predominantly mice and mice. This test is characterized by two distinct stages of nociception, which seem to involve different mediators (Hunskaar et al., 1985; Hunskaar and Hole, 1987; Correa and Calixto, 1993). The first phase of nociception begins immediately after formalin injection, extending for the first 5 min, which is believed to be due to direct chemical stimulation of the nociceptors (Hunskaar et al., 1985), predominantly of type C afferent fibers and partly of type (Heapy et al., 1987) and is associated with the release of excitatory amino acids, nitric oxide and P-substances between others.
Figure 4 shows the second stage of this model occurs between 15 and 30 min after formalin injection and is mainly related to the release of several pro-inflammatory mediators such as bradykinins, histamine, prostaglandins and serotonins, among others (Hunskaar and Hole, 1987; Correa and Calixto, 1993).
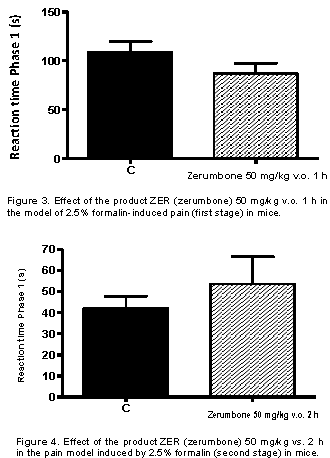
The present results from the present thesis showed that, when evaluated in the formalin test, the sequiterpenico ZER administered orally caused maximum antinociceptive activity in 1 h, being significant within 6 h after its administration, in relation to the second phase of the formalin induced nociception. In addition, the present data also demonstrated that the ZER compound, administered v.o, produced a significant antinociceptive effect in a dose-dependent manner in relation to both phases of formalin-induced nociception, but was more effective in relation to the second phase of this model. Thus, the results reinforce the hypothesis of the important antinociceptive and/or anti-inflammatory activity.
Analgesic activity produced by capsaicin
The treatment of animals with the ZER sesquiterpene (1-3%) caused significant reduction and dependent neurogenic pain induced by ear edema intraplaque injection of capsaicin. The results indicate that ZER sequiterpene at the dose of 50 mg/kg when compared with the control inhibited 70 + 5% of pain caused by capsaicin.
The results obtained in the model revealed that ZER sequiterpene, administered v.o, was able to inhibit neurogenic pain caused by capsaicin, thus opening up new perspectives for the therapeutic use of the ZER compound in these types of pains. Another important aspect in the present study was the fact that ZER showed the same efficacy and potency both administered orally and i.p. suggesting that this sesquiterpene has good availability, which makes this compound important for a future drug with analgesic activity (Figure 5).
The present study shows clearly the sesquiterpene ZER obtained from Z. zerumbet rhizomes essential oil to possess potent antinociceptive activity when administered via intraperitoneal or oral routes in the different neurogenic-induced nociception models in mice and rats. Moreover, the compound presented expressive antinociceptive activity when analyzed in the hot plate thermal sensitivity assessment nociception. Among the pain models used in this work, that of acetic acid intraperoneally-induced abdominal writhing is relatively simple with little specificity, but easy to be observed, quick and with good sensibility to several non-steroidal antiinflamatory and analgesic drugs, as well as to drugs similar to other centrally-acting analgesics.
Through this test it was possible to demonstrate, for the first time, that ZER administered via oral route, inhibits acetic acid induced writh
ing up to 98% within 2 h. In an
in vivo assay, an acetic acid-induced writhing response in mice was significantly reduced by treatment with zerumbone. Furthermore, zerumbone reduced paw edema and the pain response in a mono-iodoacetate (MIA)-induced rat osteoarthritis model (
Chien et al., 2016). Sulamain et al. (2010) tested ZER in doses of 10, 50 and 100 mg/kg with realized inhibitions of 19.3, 40.4 and 64.8%, respectively as compared to the control (acetylsalicylic acid). It was concluded that the direct acetylsalicylic acid induction can liberate endogenous mediators, with prostaglandins E2 (PgE2) and PGF2 in peritoneal fluids. While ZER inhibits abdominal writhing through the lipoxygenase and/or cycloxygenas mechanism by reducing the afferent nociceptors primary transduction. Expriments carried out with pharmacological agonists and antagonists suggest that the analgesic effect caused by substance ZER is probably related with an interaction with the opiate or glutaminergic systems without involving the L-arginine/nitric oxide pathway and sedative or muscle relaxing effects on the central and/or peripheral nervous system.
Another major aspect being analyzed in the present study is the possibiliy of the sesquisterpene ZER to produce analgesic effect in the supra-spinal mechanism in a nocicepuion model, which utilizes harmful thermal stimulation in the experiments using the hot plate. Doses that showed to be effective in the hot plate test, at 50°C, were similar to those that were necessary and efficient for the analgesic effect in chemical stimulation model. Furthermore, the present study demontrates the positive control, fentanest (synthetic opiate), to be effective in also lengthening the time of latency of the animals in the thermal nociception stimulation, regardless the temperature being used. That might be a reflex either from the great difference of the stimulations, or from the integration of the responses to different temperatures (Hwang and Wilcox, 1987).
Sulamain et al. (2010) investigated the effects of ZER through the test of thermal stimilations (hot plate) following the protocol for intraperitoneal administration (10, 50 and 100 mg/kg) with a special significance in the dose of ZER having a prolonged latency in the heat stimulation. The ZER effective time in mice using the hot plate test also confirmed its anti-nociceptive activity through its action on the central mediators. Perimal et al. (2011) demonstrated that zerumbone possesses significant peripheral and central antinociceptive effects.
Given that neurogenic nociception is very complex and that there are no satisfactory therapeutic alternatives for its treatment, as of yet, these findings may open new perspectives for the development of molecules presenting therapeutic potential for treating pain (Carter, 1991; Julius and Basbaum, 2001; MacFarlane et al., 1997).
The findings of the present study suggest ZER in the models of acetic acid-induced nociception, thermal and mechanical tests: hot plate and hyperalgesia to present therapeutic potential, contributing to the discovery of new drugs possessing analgesic effect, so much so as to be referred to for the control of the neurogenic nociception. Another important feature in the present study was the fact of ZER presenting the same efficacy and power, when administered in dependent dose both via oral and intraperitonial route, suggesting this substance to present good availability.
The autors thank Prof. Dr. Paul Maas (Departament of Plant Ecology and Evolucionary Biology) – herbarium, University of Utrecht and Biotechnology Center of Amazonia (CBA) for analysis.
The authors declare that they have no conflict of interest.
REFERENCES
|
Abdul AB, Abdelwahab SI, Al-Zubairi AS, Elhassan MM, Murali SM (2008).Anticancer and Antimicrobial Activities of Zerumbone from the Rhizomes of Zingiber zerumbet. International Journal of Pharmacology 4(4):301-304.
Crossref
|
|
|
|
Carter RB (1991). Differentiating analgesic and non-analgesic drug activitiers on rat hot plate: effect of behavioral endpoint. Pain 47(2):211-220.
Crossref
|
|
|
|
Correa CR, Calixto JB (1993). Evidence for participation of Bi and B2 kinin receptors in formalin-induced nociceptive response in mouse. British Journal of Pharmacology 110(1):193-198.
Crossref
|
|
|
|
Chien TY, Huang SK, Lee CJ, Tsai PW, Wang CC (2016). Antinociceptive and Anti-Inflammatory Effects of Zerumbone against Mono-Iodoacetate-Induced Arthritis. International Journal of Molecular Sciences 17(2):249
Crossref
|
|
|
|
Dai JR, Cardellina JH, McMohan JB, Boyd MR (1997). Zerumbone, an HIV innibitory cytotoxic sesquiterpene of Zengiber aromaticum and Zingiber zerumbet. Natural Product Letter 10(2):115-118.
Crossref
|
|
|
|
Dev S (1960). Studies in sesquiterpenes-XVI. Zerumbone, a monocyclic sesquiterpene ketone. Tetrahedron 8(3-4):171-180.
Crossref
|
|
|
|
Eddy NB, Leimback D (1987). Synthetic analgesics: II. Dithienylbutenyl- and dithienylbutylamines. Journal Pharmacology Experimental Therapy 107(3):385-393.
|
|
|
|
Heapy CG, Jamieson A, Russell NJW (1987). Afferent C-fibre and A delta activity in models of inflammation. British Journal Pharmacology 90:164-166.
|
|
|
|
Hunskaar S, Fasmer OB, Hole K (1985). Formalin test in mice, auseful techniqu for evaluation mild analgeia. Journal of Neuroscience Methods 14(1):69-76
Crossref
|
|
|
|
Hunskaar S, Hole K (1987). The formalin test in mice: dissociation between inflammatory and non - inflammatory pain. Pain 30(1):103-114.
Crossref
|
|
|
|
Hwang AS, Wilcox GL (1987). Analgesic properties of intrathecally administered heterocyclic antidepressants. Pain 28(3):343-355.
Crossref
|
|
|
|
Julius D, Basbaum AI (2001). Molecular mechanisms of nociception. Nature 413(6852):203-210.
Crossref
|
|
|
|
Kitayama TK, Yamamoto R, Utsumi M, Takatani RK, Hill Y, Kawai S, Sawada, Okamoto T (2001). Chemistry of zerumbone. 2. Regulation of ring bond cleavage and unique antibacterial activities of zerumbone derivatives. Bioscience Biotechnology Biochemistry 65(10):2193-2199.
Crossref
|
|
|
|
Koshimizu K, Ohigashi H, Tokuda H, Kondo A, Yamaguchi K (1988). Election of plants edible of meeting to the activity promoting antitumor. The Cancer Letters 39:247-257.
Crossref
|
|
|
|
Koster R, Andersons MD, Debeer EJ (1959). Acetic Acid Analgesic Screening. Federation Proceedings 18:412-417.
|
|
|
|
Lapa AJ, Valle JR, Rezende AM, Ribeiro FB, Neto AA (2003). Methods of Evaluation of the Pharmacological Activity of Medicinal Plants. São Paulo Editor, 220 p.
|
|
|
|
MacFarlane M, Ahmad M, Srinivasula SM, Fernandes-Alnemri T, Cohen GM, Alnemri ES (1997). Identification and molecular cloning of two novel receptors for the cytotoxic ligand TRAIL. Journal Biological Chemistry 272(41):25417-25420.
Crossref
|
|
|
|
Murakami A, Takahashi D, Kinoshita T, Koshimizu K, Kim HW, Yoshihiro A, Nakamura Y, Jiwajinda S, Terao J, Ohigashi H (2002). Zerumbone, a Southeast Asian ginger sesquiterpene, markedly supresses free radical generation, proinflammatory protein production, and cancer cell proliferation accompanied by apoptosis: the α,β- unsaturated carbonyl group is a prerequisite. Carcinogenesis 23(5):795-802.
Crossref
|
|
|
|
Murakami A, Tanaka T, Lee JY, Surh YJ, Kim HW, Kawabata K, Nakamura Y, Jiwajinda S, Ohigashi H (2004). Zerumbone a sesquiterpene in subtropical ginger suppresses skin tumor initiation and promotion stages in ICR mice. International Journal of Cancer 110(4):481-490.
Crossref
|
|
|
|
Perimal EK, Akhtar MN, Mohamad AS, Khalid MH, Ming OH, Khalid S, Tatt LM, Kamaldin MN, Zakaria ZA, Israf DA, Lajis N, Sulaiman MR (2011). Zerumbone-induced antinociception: Involvement of the L-arginine-nitric oxide-cGMP-PKC-K+ATP channel pathways. Basic & Clinical Pharmacology and Toxicology 108:155162.
Crossref
|
|
|
|
Pinheiro CCS (2005). Process of obtaining zerumbona isolated from the essentialoils of root of Zingiber zerumbet (l.). Request for national patent depository PI0505343-9.
|
|
|
|
Pinheiro CCS (2009). Process and potential analgesic, anti-inflammatory and pre-zerumbona clinictoxicity retrieved from Zingiber Zerumbet (l.) smith (Zingiberaceae), Doctoral thesis presented to Universidade Federal doAmazonas – UFAM. Postgraduate course in biotechnologymulti-institutional xiv, 119 p.
|
|
|
|
Rocha E, Silva M (1968). Statistical methods applied to Pharmacology. ch. 3. in: Fundamentals of Pharmacology and its applications to Therapeutics - volume1, 2a ed. Edart, Livraria, Editora Ltda., São Paulo.
|
|
|
|
Salustiano JK, Hocino E,Carlini EA (1996). Effects of Cannabis sativa and chlorpromazine on mice as measured by two methods used for evaluation of tranquilizing agents. Medical Pharmacology Experimental 15:153-162.
|
|
|
|
Somchit MN, Shukriyah MH, Bustamam AA, Zuraini A (2005). Anti-pyretic and analgesic activity of Zingiber zerumbet. International Journal of Pharmacology 1(3):277-280.
Crossref
|
|
|
|
Sulamain MR, Perimal EK, Zakaria ZA, Mokhtar F, Akhtar MN, Lajis NH, Israf DA (2009). Preliminary analysis of the antinociceptive activity of zerumbone. Fitoterapia 80:230-232.
Crossref
|
|
|
|
Sulamain MR, Tengku Mohamad TA, Shaik Mossadeq WM, Moin S, Yusof M, Mokhtar AF, Zakaria ZA, Israf DA, Lajis N (2010). Antinociceptive activity of the essential oil of Zingiber zerumbet. Planta Medica 76(2):107-112.
Crossref
|
|
|
|
Takada Y, Murakami A, Aggarwal BB (2005). Zerumbone abolishes NF-κB and IκBα kinase activation leading to suppression of antiapoptotic and metastatic gene expression, upregulation of apoptosis and downregulation of invasion. Oncogene 24(46):6957-6969.
Crossref
|
|
|
|
Yob NJ, Jofrry SM, Affandi MM, Teh LK, Salleh MZ, Zakaria ZA (2011). Zingiber zerumbet (L.) Smith: A review of its ethnomedicinal, chemical, and pharmacological uses. Evidence-Based Complementary and Alternative Medicine 543216.
Crossref
|