ABSTRACT
This work investigated the best composition of a substrate for cultivation of Pleurotus ostreatus CCIBt 2339 using brewer's spent grain (SG) and cocoa pod shells (CP) complemented with hot trub (HT) and/or residual brewer’s yeast (RY). The residue HT was detrimental for cultivation and a substrate composition (%, w/w) of 58% SG + 40% CP + 2% RY – with a C/N ratio of 25.77 g/g – resulted in the best values of biological efficiency (BE = 1.204.0 g/kg) and productivity [Pd = 32.5 g/(kg.day)]. The crude multi-enzymatic extract, obtained as a result of the mycelial growth in this substrate was a good source for: laccases (7.644.4 U/g), xylanases (110.9 U/g) and amylases (277.4 U/g). The obtained results demonstrate the biotechnological potential for the proposed substrate for edible mushrooms production as much as for the obtaining of enzymes with industrial application.
Key words: Carbon/Nitrogen ratio, cellulases, pectinase, simplex-centroid design, tannase.
Mushrooms are excellent natural agents for degradation of lignocellulosic compounds besides all of their nutritional and/or medicinal value (Stamets, 2005). A good example of versatility and efficiency, is the edible mushroom Pleurotus ostreatus, which is a basidiomycete highly appreciated in different countries and has been cultivated on a wide variety of vegetable-based substrates (Ergun and Urek, 2017; Silva et al., 2019). Considering the current context when it is necessary to promote the valorization of different residues for different purposes, four agro-industrial residues generated from the production lines of beer [brewer’s spent grain (SG), hot trub (HT) and residual brewer’s yeast (RY)] and chocolate [cocoa pod husks (CP)] were selected as potential substrates for cultivation of edible mushrooms. The two main residues selected, SG and CP, can be considered good sources of carbon. In general, SG contains around 530 g/kg of polysaccharides and 100 g/kg of lignin (Hassan et al., 2020; Rojas-Camorro et al., 2020), while CP can contain from 430 to 490 g/kg of carbon with about 210 g/kg of lignin (Adjin-Tetteh et al., 2018; Antwi et al., 2019). To balance the ratio between carbon and nitrogen, HT and RY were selected, basically due to their protein content. For HT, levels of 200 to 700 g/kg of protein were estimated (Mattioli et al., 2020) and, for RY, around 25.3 g/kg of nitrogen (Puligundla et al., 2020). Considering the available data for 2018, over 5 million tons of cocoa beans are produced worldwide (FAOSTAT, 2020) and CP can represent 70 to 75% of the total weight of the fruit (Shet et al., 2018). Additionally, China, United States and Brazil were the three largest beer producers reaching more than 730 million hectoliters in 2018 (STATISTA, 2020) and SG is estimated to be around 85% of the total by-products generated in the beer-brewing process (Hassan et al., 2020). Thus, the four residues proposed, which have a rich nutritional composition, are generated in significant quantities and can be potentially suggested (with low environmental impact) for the production of edible mushrooms. Therefore, this present work investigated the best composition between SG and CP supplemented with HT and/or RY as a substrate for P. ostreatus CCIBt 2339 cultivation, and, consequently, to obtain multi-enzymatic extracts.
Pleurotus ostreatus CCIBt 2339 P. ostreatus CCIBt 2339, originally from the Instituto de Botânica de São Paulo (São Paulo, SP, Brazil), was made available by the Comissão Executiva do Plano da Lavoura Cacaueira (CEPLAC, Itabuna, BA, Brazil). The strain was preserved in penicillin tubes (Castellani, 1967) and maintained in potato-dextrose-agar (PDA). Periodically, cultivation in a bio-oxygen-demand incubator (BOD) (SL-200, SOLAB Científica) was conducted at 25°C in Petri dishes with PDA until complete coverage of the surface by the vegetative mycelium (around 20 days). Preparation of residues and substrates Brewer's spent grain (SG), hot trub (HT) and residual brewer’s yeast (RY) were acquired at the Microbrewery of the Universidade Estadual de Santa Cruz (Ilhéus, BA, Brazil) after a production of a Witbier beer with: wheat and barley malts, cardamom, nutmeg and hop pellets (U.S. Golding Hops). SG was dried in an oven (MA-035, MARCONI) at 60°C until constant weight; HT and RY were autoclaved at 121°C/15 min (CS50, Prismatec), frozen (-80°C/24 h) and dried in a lyophilizer (LS3000, TERRONI). Cocoa pod shells (CP) were obtained from local producers from Ilhéus (Bahia, Brazil) and were manually chopped before drying, as performed for SG, and then crushed to a particle size of 3 to 4 cm. The compositions (g/kg, dry base) of carbon (C) and nitrogen (N), for each residue, were estimated (IAL, 2008); for technical limitations, only humidity (% w/w, dry base) was possible to be determined in triplicate (MB-120, OHAUS). Table 1 presents these compositions which were applied in the calculations of the C/N ratio (g/g) and the amount of water necessary to reach the substrate initial humidity of 70% (w/w).
The substrates were prepared, according to each composition to be evaluated, by weighing the pre-treated residues and mixing them with water. After 1 h, 100 g of substrate were transferred to polypropylene bags (50 × 30 cm), which were closed with an acrylic fabric and rubber bands (to allow aeration) and autoclaved (121°C/20 min) twice within 48 h (Marino and Abreu, 2009; Oliveira et al., 2007).
Spawn preparation
Based on Oliveira et al. (2007) and Shibata and Demiate (2003), with some modifications, the spawn (seeds) were produced using wheat grains purchased from local business (Ilhéus, BA, Brazil); the grains were cooked in boiling water [ratio of 1:2 (kg:L)] for 15 min; the excess water was drained and, after cooling down, 30 g/kg of a mixture [1:4 (w/w)] of CaCO2 (for pH adjustment) and plaster (to prevent particle agglomeration) was added. The wheat grains were transferred to polypropylene bags (50 × 30 cm) until about â…” of its volume (200 g), the bags were closed with an acrylic fabric and rubber bands and then autoclaved (121°C/30 min). After cooling down, ¼ of a Petri dish with complete mycelial growth of P. ostreatus CCIBt 2339 was inoculated on the surface of the wheat grains and, once closed, the bags were incubated at 25°C for 10 to 15 days (or until complete colonization). A tray with water was placed at the bottom of the BOD in order to maintain a high humidity (80 – 90% w/w) and it was constantly renewed.
Inoculation, mycelium running, fruiting and harvesting
The substrate inoculation proceeded with 10% (w/w) of spawn and incubation (mycelium running) occurred as described for spwan preparation but for 15 to 20 days (or until complete colonization). Only then, the bags were moved to a refrigerator (4°C/24 h) to promote a temperature shock to induce the appearance of the primordia. After that, the plastic bags were removed to expose the substrate blocks which were individually hung with a hook in boxes in the “fruiting room”. The room conditions were maintained as 80 to 90% (w/w) of humidity and 23 to 25°C and a direct artificial light was maintained with fluorescent lamps (55 W, G-LIGHT PREMIUM). After a period of around 15 to 20 days, the fully developed fruiting bodies (first flush) were harvested with a slight twist and pull and were weighed (fresh weight) and measured (diameter).
Study of substrate composition
The best compositions for the residues: SP, HT, RY and CP were investigated based on a 3-component Simplex-Centroid design, totaling 10 different runs, with SP varying between 10 and 90% (w/w) and HT and RY, individually, from 0 to 5% (w/w); CP was used to complement the weight to 100 g. Each run from the matrix was performed in triplicate and the statistical analysis was performed considering the individual (and valid) responses. The main responses analyzed were: biological efficiency (BE, g/kg) and productivity [Pd, g/(kg day)], the secondary responses were: pileus diameter (dpil, cm) and periods (days) of each phase: mycelium running (tmr), appearance of primordia (tap) and colonization and harvesting (tch). Two compositions (%, w/w) were selected and performed in triplicate for experimental validation: S5 (50% SG + 2.5% RY + 47.5% CP) and S11 (58% SG + 2% RY + 40% CP).
Characterization of mushrooms and cultivation
Biological efficiency (BE, g/kg) expresses the relationship between the weight of fresh mushrooms obtained for each kilogram of initial dry substrate and productivity [Pd, g/(kg day)] expresses BE by the total time of cultivation until harvesting (tch, days) (Fonseca et al., 2015; Oliveira et al., 2007). The diameters (dpil, cm) of the harvested fruiting bodies were measured using a (millimeter) ruler. Following the order of cultivation phases, the periods (days) of: mycelium running (tmr), appearance of primordia (tap) and cultivation until harvesting (tch) were determined.
Obtaining and characterizing the crude multi-enzymatic extract
Four compositions (%, w/w) were selected and prepared in Petri dishes (30 g, triplicate): S5, S11, S1 (90% SG + 10% CP) and S9 (23.6% SG + 0.85% HT + 3.35% RY + 72.2% CP). The mycelial phase was conducted as previously described and the myceliated substrates were macerated with distilled water [ratio of 1:10 (g:mL)] and stirred in a shaker for 1 h (25°C/200 rpm). The solids were separated by vacuum filtration followed by centrifugation at (4,000 g/15 min/5°C) and the supernatant was identified as the crude multi-enzymatic extract (CME) which was investigated for the enzymes: laccase, xylanase, CMCase, FPase, amylase, pectinase and tannase. The spectrophotometric methodologies applied for each enzyme were described previously by Ghose and Bisaria (1987), Lu et al. (2013), Sharma et al. (2000), Umsza-Guez et al. (2011) and Vasconcelos et al. (2013) and the enzymatic activities (U) were expressed per gram of initial dry substrate (U/g).
Statistical analysis
The best adjustment of the obtained responses to a mathematical model (linear, quadratic, special cubic) was selected based on the Analysis of Variance (ANOVA) performed with at least 90% of confidence and the Contour Curves were generated with a statistical software (STATISTICA v.8, StatSoft). Also, the Tukey test was applied to compare mean values of specific conditions with 95% of confidence.
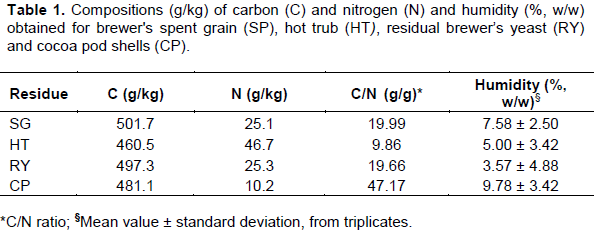
Study of the substrate composition Table 2 presents the Simplex-Centroid matrix, the C/N ratios for each substrate and the mean responses obtained. Some of the replicates performed were disregarded since they did not result in fruiting bodies during the entire period of cultivation (30 - 45 days), while the other replicates of the same condition did. According to the results, higher C/N (between 30 and 39 g/g) were obtained when CP > 60% w/w (S2, S3, S6, S8, S9 and S10) (Table 2). The two highest values of BE (> 550 g/kg) and Pd [> 16 g/(kg.day)] were obtained with the substrates S1 and S5 (Table 2), with S5 presenting the best performance and a more balanced composition between SG and CP in relation to S1 which, had the highest SG content and the lowest C/N among all the substrates. Based on the ANOVA for the responses BE and Pd it was possible to adjust quadratic models with p-values < 0.05, however, both mathematical models will not be presented because they also resulted in R2 and Radj2 not higher than 0.65 and a statistically significant Lack of Adjustment (p-value < 0.05). It should be noted that this does not invalidate the study and the Contour Curves were analyzed together with the experimental data in order to make decisions about the compositions, as described in the following. As Pd is calculated from the BE value, only the Contour Curve for Pd (Figure 1a) will be presented and discussed, since both curves are remarkably similar. The analysis of Figure 1a indicates a narrow range area in which the best results were obtained close to zero for HT, above 0.25 for SG and below 0.75 for RY where S1 and S5 are located (C/N between 21 and 28 g/g). The results also allowed to identify that the increase in HT was detrimental to Pd, probably due to astringent compounds (such as tannins) normally found in this residue (Mattioli et al., 2020), which could act as an anti-nutritional factor (Luz et al., 2013). This effect can be observed when comparing runs S4 and S5 (Table 2), both with the same values for SG and CP and similar C/N, however, S4 contained HT and none of its replicates presented primordia. Thus, considering S5, the relationship between the predicted values of Pd and the coded components values (Figure 1b) suggests maximum responses around SG = 0.7 and RY = 0.3 (which are indicated with arrows in Figure 1b). However, this theoretical condition is remarkably close to run S7 (Table 2) which did not presented primordia. Consequently, a new codified composition was selected and identified as S11 (SG = 0.60 + RY = 0.40, C/N = 25.77 g/g).
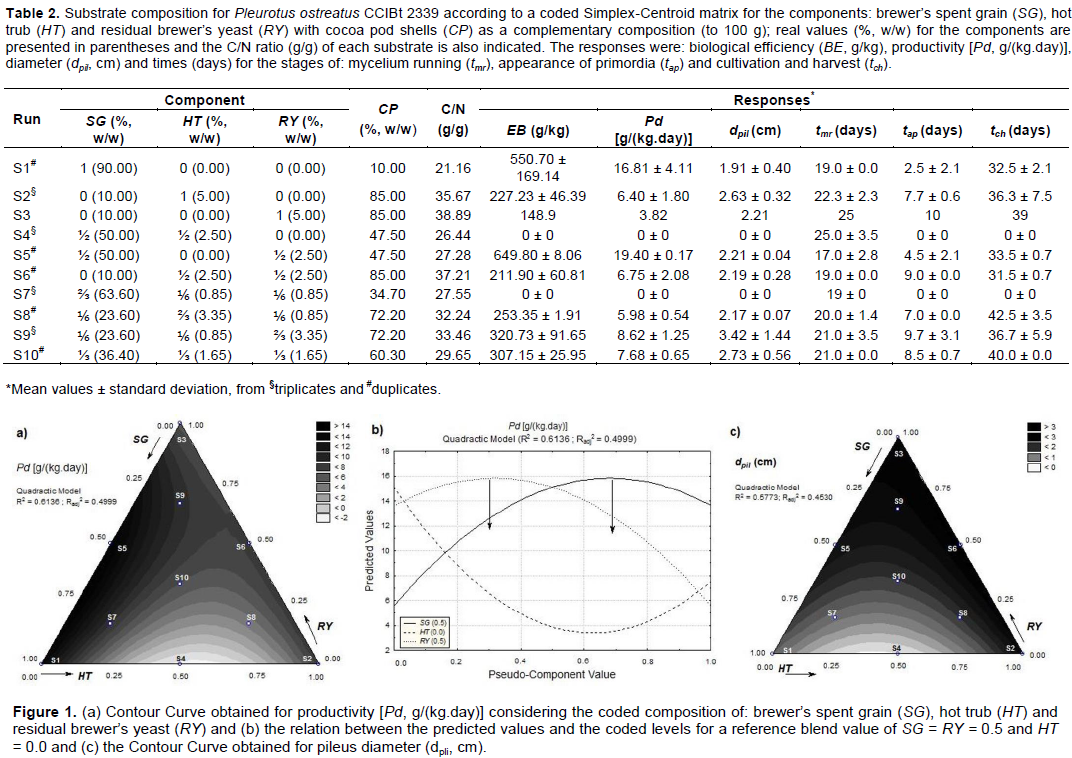
According to Kortei et al. (2018), mushrooms with larger pileus, besides having higher commercial value, will result in higher BE and Pd values; these researchers found a correlation of dpil with BE when cultivating P. ostreatus in composted sawdust. The results obtained in this study indicated, however, a different behavior since the highest dpil values were obtained with runs S6 and S9 (Table 2) in contrast to what was already discussed considering the best results for Pd (and BE, consequently). The adjustment of a quadratic model to the dpil data was statistically significant (p-value < 0.10), however, the R2 and Radj2 values were lower (< 0.45) and the Lack of Adjustment was statistically significant (p-value < 0.10), nevertheless, the analysis continued for the purpose of better understanding the effect of the residues over dpli.
According to the Contour Curve obtained (Figure 1c), in order to increase dpil it would be necessary to work with lower SG and higher HT and RY. Inside this region are located the runs S3, S6 and S9 and a narrow band around S2 (C/N = 33 - 39 g/g). This result suggests that, for P. ostreatus CCBIt 2339, higher HT and CP may induce the development of larger pileus, but with lower BE and Pd, as it can be observed when comparing runs S9 and S5 (Table 2).
Regarding the fact that it is always desirable to reduce the total time of a process to increase its productivity and lower the risks for contamination, tmr, tap and tch, were analyzed individually in a similar way as described so far, although, the obtained Contour Curves will not be presented and only the results will be discussed. Values of tmr between 15 and 19 days (Table 2) indicated a shorter mycelial running phase, in comparison to what was obtained by Kumari and Achal (2008) with P. ostreatus (20 days) and this response was favored by the same range of compositions that favored the responses Pd and BE. The substrates S2, S3 and S4 (Table 2) indicated to increase tcm to more than 22 days which is more similar to what was obtained for P. ostreatus (22 - 26 days) by Sharma et al. (2013).
It was observed that an increase in HT indicated an undesirable increase in tap values (similar to the analysis of dpil). Shorter tap values (1 – 5 days) were obtained with S1 and S5 (Table 3) which are around to the 3 days required by Pleurotus sajor-caju in onion juice waste (Pereira et al., 2017) but less than what was observed (9 to 17 days) with P. ostreatus in weeds (Das and Mukherjee, 2007). According to the results, tch varied from 30 to 45 days (Table 3) as a consequence of tmr and tap values.
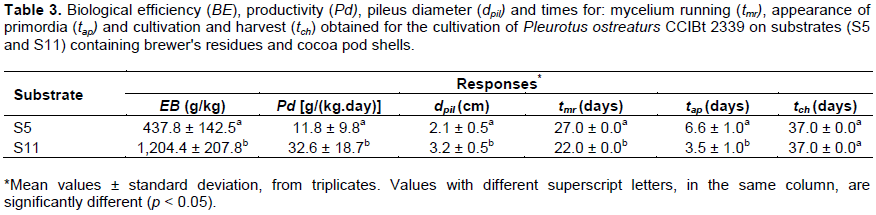
Ultimately, besides S11, S5 was also chosen for experimental validation, in triplicate, and the obtained responses are presented in Table 3. Considering that some operational difficulties were faced to maintain humidity inside the fruiting room for the first couple of days of cultivation and acknowledging the inherent variability of the experiments, substrate S11 (defined with the statistical analysis) was considered the best condition for P. ostreatus CCIBt 2339 cultivation. In comparison to S5, the increase in SG and the decrease in RY and CP resulted in a C/N ratio 6% lower and a Pd around 2.8 times higher (Table 3).
Enzymatic screening
The enzymatic screening for the obtained crude multi-enzymatic extracts (CMEs) is presented in Table 4, from which it is possible to identify the biotechnological potential of each CME, especially in relation to the activities of laccase, xylanase, pectinase and amylase. In general, the reduction of SG (%, w/w) from 90% (S1) to 58% (S11) associated with the increase of CP and RY (Table 2), indicated to be positive for the production of laccases and amylases. However, when considering to reduce SG even more, from 58% (S11) to 50% (S5) also associated with the increase of CP and RY, that indicated not to be beneficial for laccases, amylases and pectinases (Table 4). Regarding S9, the only condition with HT and the highest CP, the lowest laccase, xylanase and pectinase activities were obtained (Table 4). Between S1, S5 and S11, there were no significant changes in xylanase activities and, for all conditions evaluated, a similar CMCase production was obtained (Table 4).
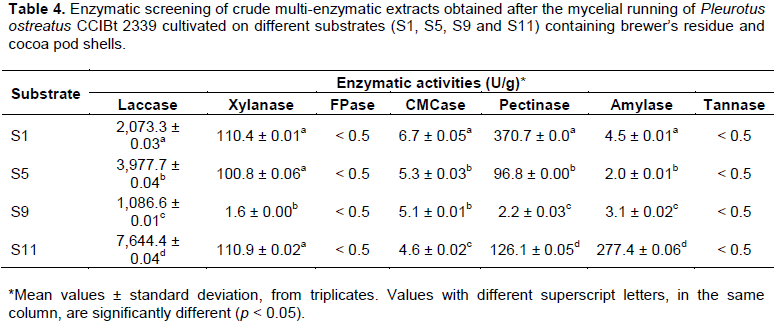
Laccases, xylanases, FPases and CMCases are enzymes investigated for the degradation of lignocellulosic substrates. It was reported for P. ostreatus, for instance, that nitrogen supplementation of soybean hulls could result in an increase in laccase production, in this case, the highest activities (60 - 80 U/g) were reached at a much lower C/N (5.0 g/g) (D´Agostini et al., 2011). In another example, a co-cultivation of P. ostreatus MTCC 180 and Penicillium oxalicum SAUE-3.510 in sugarcane bagasse and bean husk resulted in a higher yield of xylanase (8,205.31 U/g) (Dwivedi et al., 2011). With cultivation in sesame straw and wheat bran (C/N = 27 g/g), it was possible to obtain a lower activity of CMCase (1.75 U/g) with a substrate with a very similar C/N to S5 (Kurt and Buyukalaca, 2010). Pectinases and amylases are enzymes capable to hydrolyze, for example, mucilages and starches that are naturally found in different vegetable parts. In the spent substrate of P. sajor-caju in onion residues (with a much higher C/N of 266.22 g/g) a pectinase activity of around 96 U/g was obtained (Pereira et al., 2017), close to what was obtained with S5 (Table 4). Considering amylases from Pleurotus, there are only a few reports in literature, for example, around 9 U/g was reported in a spent substrate of undeclared composition (Nakajima et al., 2018).
All four compositions investigated presented FPase and tannase activities close to the control conditions of the enzymatic methodologies (0.43 < U/g < 0.01), indicating that the extracts obtained did not present expressive activities for these two enzymes. In order to obtain a better quantification, it could be suggested a methodology of greater sensitivity (such as liquid chromatography). However, FPase (22 U/g) and tannase (1 U/g) activities have been reported for P. ostreatus when cultivated, respectively, in banana pseudostem supplemented with Tween 80 (Silva et al., 2019) and jatropha biodiesel residues (Luz et al., 2013).
When considering the world production of mushrooms and truffles, which was almost 9 million tons in 2018 (FAOSTAT, 2020), it is possible to understand that the spent substrate produced, accumulated or sub-utilized still has a great biotechnological value that needs to be better explored. For that reason, the enzymatic profiles obtained (Table 4) can indicate the potential for the spent substrates as a source of important enzymes (Nakajima et al., 2018; Pereira et al., 2017).
When working with different residues to compose a substrate for mushroom cultivation, it is important to investigate the best composition since it can modulate different responses related to growth. In this study it was possible, with the help of a statistical tool, to detect the advantages of balancing the compositions of brewer’s spent grain and cocoa pod shells and it also permitted to choose the residual brewer’s yeast over the hot trub in order to improve, for example, productivity and laccase activity. Thus, it is reinforced that the proper use of agro-industrial residues to produce edible mushrooms is a viable, necessary and a low-impact practice since it is possible to produce a nutritional food and obtain important enzymes from the spent substrate.
The authors have not declared any conflict of interests.
The authors are grateful for the financial support of the Fundação de Amparo à Pesquisa do Estado da Bahia (FAPESB, Brazil) and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil) and for the experimental support from the Centro de Inovação do Cacau (CIC, Ilhéus, BA, Brazil).
REFERENCES
|
Adjin-Tetteha M, Asiedua N, Dodoo-Arhin D, Karam A, Amaniampong PN (2018). Thermochemical conversion and characterization of cocoa pod husks a potential agricultural waste from Ghana. Industrial Crops and Products 119:304-312.
Crossref
|
|
|
|
Antwi E, Engler N, Nelles M, Shüch A (2019). Anaerobic digestion and the effect of hydrothermal pretreatment on the biogas yield of cocoa pods residues. Waste Management 88:131-140.
Crossref
|
|
|
|
|
Castellani A (1967). Maintenance and cultivation of common pathogenic fungi of man in sterile distilled water. Further Researches. The Journal of Tropical Medicine and Hygiene 70:181-184.
|
|
|
|
|
D'Agostini EC, Mantovanti CE, Valle JS, Meirelles LD, Colauto NB, Linde G (2011). Low carbon/nitrogen ratio increases laccase production from basidiomycetes in solid substrate cultivation. Scientia Agricola 68(3):295-300.
Crossref
|
|
|
|
|
Das N, Mukherjee M (2007). Cultivation of Pleurotus ostreatus on weed plants. Bioressource Technology 98(14):2723-2726.
Crossref
|
|
|
|
|
Dwivedi P, Vivekanand V, Pareek N, Sharma A, Singh RP (2011). Co-cultivation of mutant Penicillium oxalicum SAUE-3.510 and Pleurotus ostreatus for simultaneous biosynthesis of xylanase and laccase under solid-state fermentation. New Biotechnology 28(6):616-626.
Crossref
|
|
|
|
|
Ergun SO, Urek RO (2017). Production of ligninolytic enzymes by solid state fermentation using Pleurotus ostreatus. Annals of Agrarian Science 15(2):273-277.
Crossref
|
|
|
|
|
FAOSTAT (2020). Food and Agriculture Data - Food and Agriculture Organization of the United Nation (FAO). Available at:
View - last consult on September 3rd, 2020.
|
|
|
|
|
Fonseca TRB, Alecrim MM, Cruz Filho RFC, Teixeira MFS (2015). Cultivation and nutritional studies of an edible mushroom from North Brazil. African Journal of Microbiology Research 9(30):1814-1822.
Crossref
|
|
|
|
|
Ghose TK, Bisaria VS (1987). Measurement of hemicellulase activities. Part 1: 472. Xylanases. Pure and Applied Chemistry 591:739-52.
|
|
|
|
|
Hassan SS, Ravindrana R, Jaiswal S, Tiwari BK, William GA, Jaiswal AK (2020). An evaluation of sonication pretreatment for enhancing saccharification of brewers' spent grain. Waste Management 105:240-247.
Crossref
|
|
|
|
|
Instituto Adolfo Luts (IAL) (2008). Métodos físico químicos para análise de alimentos. 4th Ed. Instituto Adolfo Lutz, São Paulo.
|
|
|
|
|
Kortei KN, Odamtten TG, Obodai M, Kwagyan-Wiafe M, Mensah NLD (2018). Correlations of cap diameter (pileus width), stipe length and biological efficiency of Pleurotus ostreatus (Ex.Fr.) Kummer cultivated on gamma-irradiated and steam-sterilized composted sawdust as an index of quality for pricing. Agriculture and Food Security 7:35.
Crossref
|
|
|
|
|
Kumari D, Achal V (2008). Effect of different substrates on the production and nonenzymatic antioxidant activity of Pleurotus ostreatus. Life Science Journal 5:73-76.
|
|
|
|
|
Kurt S, Buyukalaca S (2010). Yield performances and changes in enzyme activities of Pleurotus spp. (P. ostreatus and P. sajor-caju) cultivated on different agricultural wastes. Bioresource Technology 101(9):3164-3169.
Crossref
|
|
|
|
|
Lu L, Zeng G, Fan C, Ren X, Wang C, Zhao Q (2013). Characterization of a laccase like multicopper oxidase from newly isolated Streptomyces sp. C1 in agricultural waste compost and enzymatic decolorization of azo dyes. Biochemical Engineering Journal 72:70-76.
Crossref
|
|
|
|
|
Luz JMR, Paes SA, Torres DP, Nunes MD, Silva JS, Mantovani HC, Kasuya MCM (2013). Production of edible mushroom and degradation of antinutritional factors in jatropha biodiesel residues. LWT - Food Science Technology 50(2):575-580.
Crossref
|
|
|
|
|
Marino RH, Abreu LD (2009). Cultivation of mushroom Shiitake in coconut wastes supplemented with bran or/and rice bran. Revista Brasileira de Ciências Agrárias 4(1):11-16.
Crossref
|
|
|
|
|
Mattioli S, Castellini C, Mancini S, Roscini V, Mancinelli AC, Cotozzolo E, Pauselli M, Bosco AD (2020). Effect of trub and/or linseed dietary supplementation on in vivo oxidative status and some quality traits of rabbit meat. Meat Science 163:108061.
Crossref
|
|
|
|
|
Nakajima VM, Soares FEF, Queiroz JH (2018). Screening and decolorizing potential of enzymes from spent mushroom composts of six different mushrooms. Biocatalysis and Agricultural Biotechnology 13:58-61.
Crossref
|
|
|
|
|
Oliveira MA, Donega MA, Peralta RM, Souza CGM (2007). Production of spawn for edible mushroom Pleurotus pulmonarius (Fr.) Quélet - CCB19 using agricultural wastes. Ciência e Tecnologia de Alimentos 27(1):84-87.
Crossref
|
|
|
|
|
Pereira GS, Cipriani M, Wisbeck E, Souza O, Strapazzon JO, Gern RMM (2017). Onion juice waste for production of Pleurotus sajor-caju and pectinases. Food and Bioproducts Processing 106:11-18.
Crossref
|
|
|
|
|
Puligundla P, Moka C, Park S (2020). Advances in the valorization of spent brewer's yeast. Innovative Food Science and Emerging Technologies 62:102350.
Crossref
|
|
|
|
|
Rojas-Chamorro JA, Romero I, López-Linares JC, Castro E (2020). Brewer's spent grain as a source of renewable fuel through optimized dilute acid pretreatment. Renewable Energy 148:81-90.
Crossref
|
|
|
|
|
Sharma S, Yadav RKP, Pokhrel CP (2013). Growth and yield of oyster mushroom (Pleurotus ostreatus) on different substrates. Journal of New Biological Reports 2(1):03-08.
|
|
|
|
|
Sharma S, Bhat KT, Dawara KR (2000). A spectrophometric method for assay of tannase using rhodanine. Analytical Biochemistry 279:85-89.
Crossref
|
|
|
|
|
Shet VB, Nisha sanil, Bhat M, Naik M, Mascarenhas LN, Goveas LC, Rao CV, Ujwal P, Sandesh K, Aparna A (2018). Acid hydrolysis optimization of cocoa pod shell using response surface methodology approach toward ethanol production. Agriculture and Natural Resources 52(6):581-587.
Crossref
|
|
|
|
|
Silva IF, Luz JMR, Oliveira SF, Queiroz JH, Kasuya MCM (2019). High-yield cellulase and LiP production after SSF of agricultural wastes by Pleurotus ostreatus using different surfactants. Biocatalysis and Agricultural Biotechnology 22:101428.
Crossref
|
|
|
|
|
Shibata C, Demiate I (2003). Cultivation and chemical analysis of the sun mushroom (Agaricus blazei Murril). Publication UEPG 9(2):21-32.
|
|
|
|
|
Stamets P (2005). Mycellium running - How mushrooms can help save the world. Ten Speed Press, New York.
|
|
|
|
|
STATISTA (2020). Leading 10 countries in worldwide beer production in 2018. Available at:
View- last consult on September 3rd, 2020.
|
|
|
|
|
Umsza-Guez MA, Díaz AB, Ory I, Blandino A, Gomes E, Caro I (2011). Xylanase production by Aspergillus awamori under solid state fermentation conditions on tomato pomace. Brazilian Journal of Microbiology 42:1585-1597.
Crossref
|
|
|
|
|
Vasconcelos NM, Pinto GAS, Aragão FAS (2013). Boletim de Pesquisa n. 88, Determinação de Açúcares Redutores pelo Ácido 3,5-Dinitrosalicílico: Histórico do Desenvolvimento do Método e Estabelecimento de um Protocolo para o Laboratório de Bioprocessos. EMBRAPA Agroindústria Tropical, Fortaleza.
|
|