ABSTRACT
Abundance and unmanaged agriculture residues lead to unutilized resource waste and environmental pollution. Application of microbial technology to manage agriculture waste could produce value-added product. A preliminary study on biodegradation of rice straw using different potential microorganisms was tested under controlled environment. Three different inoculant cultures were used to observe their efficiency in rice straw degradation. Combination cultures microorganisms coding AMB1 shows the potential degrading activity, which reduces the hemicellulose of rice straw by 50% from the raw material. The highest cellulase activity at 1.5 U/mL was also observed in rice straw treatment with AMB1 than single inoculant fungi and commercial microbial product. Overall, the results suggested that the biodegradation of rice straw could be improved by using combination culture. The ability of these cultures to enhance biodegradation shows potential to fasten the decomposing period and may be used to manage agriculture waste.
Key words: Cellulase, lignin, agriculture waste, biodegradation.
Paddy is planted as an annual crop, thus producing two times of waste for the same area in a year. The ratio of the crop product to residue for paddy rice straw produce is 1: 1.4 (IPCC, 1996). In 2014, an amount of 2.98 million tonnes of rice straw was produced in Malaysia. Generally, on-field burning and incorporation of rice straw was practiced by farmers to manage this waste. Incomplete combustion of rice straw due to burning causes disturbance to air quality in the environment, losses of soil nutrient which will impact soil fertility and release of greenhouse gases (GHG) (Rosmiza et al., 2014). During rainy season, farmers practiced incorporation of rice straw to field to manage the abundance of this waste. The impact contributes to the production of methane (CH4) during planting season due to the availability of organic matter in soil (Nishiwaki et al., 2015). Therefore, an efficient approach of managing rice straw which is environmental friendly with no impact to human health should be considered.
Rice straw consists of cellulose, hemicellulose and lignin with high silica content (Rashad, 2013). One of the approaches for eliminating this waste is by decomposition process of rice straw to produced improved product such as compost for the use in agriculture. On-field decomposition of rice straw occurs naturally, however the process is slow. The short period of gap between paddy planting seasons are insufficient for the decomposition to fully occur, thus led to the production of methane on flooded field during planting season. This decomposition of organic matter could be accelerated by microbial technology (Fang et al., 2012).
Decomposition involves microbial process where the substrate is broken down into more stabilize product (Mohammad et al., 2013). Different microorganisms may have different roles in the decomposition process. As an example, several fungi take place in degrading lignocelluloses meanwhile some bacteria act as cellulose degrading microorganisms (Singh and Nain, 2014). The celluloses and hemicelluloses contained in rice straw could be hydrolyzed by certain microorganisms using solid state as well as submerged fermentation to produce value added products (Thomas et al., 2016). Microorganisms have the ability to produce enzymes and metabolites which enhance the decomposition process of organic waste and increase humus quality in soil (Barker et al., 2006). This characteristic is useful in the process of managing rice straw waste. Besides, technology using microorganism for managing waste could be one of the alternatives in optimizing the used of available biomass besides being much more environmentally friendly. Therefore, the aim of this work was to identify and determine the microbial inoculant tested which led to fasten decomposition of rice straw.
Microbial inoculant
Microorganisms used in this experiment were isolated from soil. Inoculants from lactic acid bacteria and cellulase degrader group were made as a mixed culture coded as AMB1. Trichoderma species inoculant used was obtained from MARDI Microbial Culture Collection (MMCC). The inoculants were grown on nutrient broth (NB) for AMB1 and potato dextrose broth (PDB) for Trichoderma spp. Commercial microbial product was used and obtained from the market which is known as Biodecomposer.
Rice straw
Rice straw used in the study was acquired from Tanjung Karang, Selangor, Malaysia; coordinates at 3.4627° N, 101.2208° E. Paddy planted in Selangor is located under the authority of Integrated Agriculture Development Area (IADA) in Barat Laut Selangor, Peninsular, Malaysia. IADA is one of the authorities which cover the granary area in Selangor. Granary areas refer to major irrigation schemes (areas greater than 4,000 ha) and are recognized by the government in the National Agricultural Policy (NAP) as the main paddy producing areas. Rice management cultivation applied in this area is under continuous flooding with two planting seasons per year.
Decomposition of rice straw using shake flask study
About 200 g of rice straw was added in sterile conical flask. AMB1 and Trichoderma spp. were added on flask containing rice straw, respectively. Commercial microbial product was added with rice straw following its usage instruction. Flask containing rice straw with water served as control. The simplified, treatment of shake flask study is as follow: T1 (Control); T2 (Rice straw treated with AMB1); T3 (Rice straw treated with Trichoderma spp.) and T4 (Rice straw treated with commercial microbial product). Each treatment was prepared in triplicate. Samples were collected at days 0, 2, 4, 8, 15, 20, 25, and 30 for physical and microbial analysis.
Microbial growth
Enumeration of each microorganism analysis was done for all treatment. Total aerobic culturable microorganisms were determined using dilution plate count technique. An initial of 5 g samples were diluted with 45 mL sterile phosphate sodium buffers and were preceded with serial dilutions till 105. About 1 ml of diluents were taken from dilution factors of 1:103, 1:104 and 1:104 and transferred into the required media. Cultures showing between 30 and 200 colonies were counted using colony counter (Rebollido et al., 2008).
Physical and enzymatic analysis
pH was analyzed using pH Eutech Instrument pH2700. Enzyme activity assay was done to test degradation of cellulose and reducing sugar assay. Endoglucasnase activity (CMCase) was determined using carboxymethyl cellulose solution in citrate buffer. Reducing sugar assay was analyzed using 3,5-di-nitorcyalicylic acid (DNS) method (Miller, 1959).
Cellulose, hemicellulose and lignin analysis
Cellulose, hemicellulose and lignin content from rice straw was analyzed on day 30 using neutral detergent fibre (NDF) and acid detergent fibre (ADF) method (Sarkar et al., 2012).
Statistical analysis
Data were subjected to one-way analysis of variance (ANOVA) using SAS software.
Microbial growth
Degradation of rice straw was observed by submerged fermentation with different microbial inoculant. Cell density of microbial growth for each treatment is as shown in Figure 1. Higher growth was observed in AMB1 with the range of 3.6 × 108 to 1 × 109 CFU/mL. The density of AMB1 was maintained throughout the degradation process and started to drop at day 30. This indicated that AMB1 are in a favorable environment for its growth. The same trend was observed on commercial microbial product where cell density was increased during day 2 and maintained till day 20. A drop of microbial population of commercial microbial product was also observed at day 30. Therefore, this might be linked to the reduction of microbial population since low substrates were available in the shake flask. This finding was similar to Jeude et al., (2006) where the study observed a constant of cell dry weight and a reduction of respiration activity after all carbon sources were exhausted. In contrast to other treatment, rice straw inoculated with Trichoderma spp. showed an increase of population number starting from day 4 to day 20 from 3.5 × 108 to 1.08 × 109. This may be explained by their slow growth characteristic which required being adapted in a new environment with rice straw as substrate. Approaching day 30 of the degradation process, only small pieces of rice straw were present and are compact with each other. The colour of the rice straw changed from light yellow to black indicating degradation process occurs.
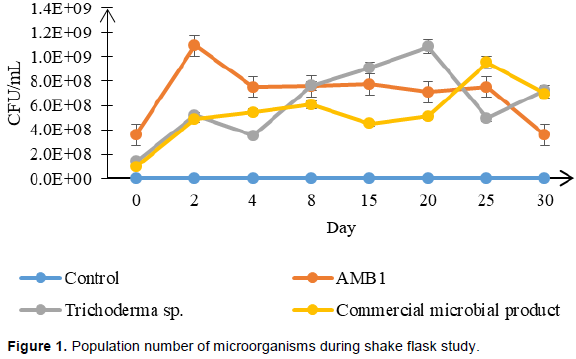
pH
The pH range varied by microbial inoculant used. In early degradation, all treatments except rice straw inoculated with AMB1 were at pH 6 (Figure 2). In contrast, rice straw treated with AMB1 shows much lower pH value at the range of 3.2 to 4.2 on early degradation and throughout the fermentation time. This is due to the condition of the microbial culture itself since one of the components of AMB1 was lactic acid bacteria. Along the fermentation period, treatment of rice straw inoculated with commercial microbial product and control treatment are in the range of 5.9 to 8.2. This is a similar characteristic for rice straw degradation as reported by Zhao et al., (2014). Treatment with Trichoderma spp. shows a lower value of pH from day 2 to day 15 with the range of pH 4.3 to 4.5. However, the Trichoderma spp., tend to overcome the acidification condition during day 20 with pH at 6.5. Trichoderma spp. are known to grow and adapt well at variation of pH 8, thus the drop of pH did not interfere with the fungi growth (Belal, 2013).
Cellulase and sugar reducing activity
Cellulase is one type of enzymes which is involved significantly in degradation process of organic material. Cellulase activities were analyzed throughout the degradation period in order to understand the reaction of each inoculum towards the rice straw degradation. Higher amount of cellulase activity was observed in treatment AMB1 as compared to other treatments (Figure 3).
The highest value was observed at day 3 with 1.5 U/ml. Trend of cellulase activity in AMB1 starts dropping at day 8 when approaching 0.2 U/ml by day 30. The increase of abundance of AMB1 during early degradation promotes higher cellulase activity since more cells are available to hydrolyze cellulose composition in rice straw. This indicates that higher decomposition activity occurs in treatment with AMB1. This suggests that mixed culture of microorganisms used could improve the decomposition process of organic materials. Several researchers had also reported that rice straw decomposition process could be accelerated by using mixed culture of microbial inoculant (Zhao et al., 2014; Mohamed et al., 2016).
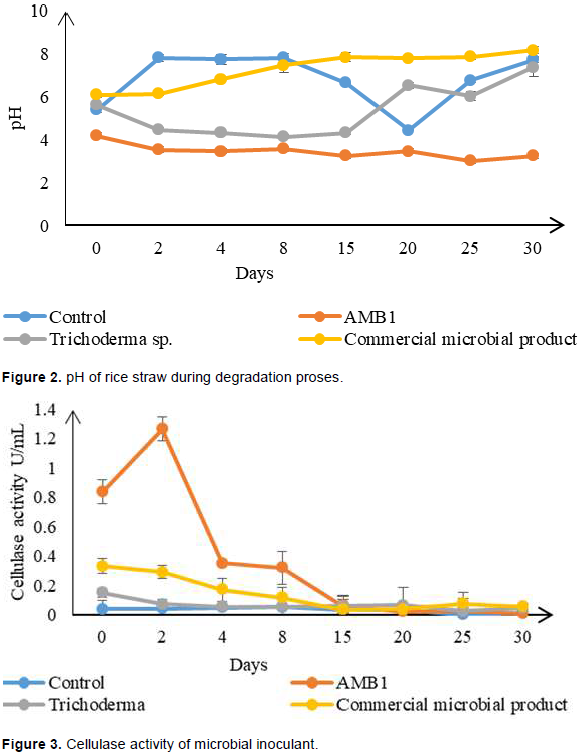
Rice straw inoculated with commercial microbial product shows the second highest inoculant to produce cellulase activity in the shake flask study. The range of cellulase activity for this microbial inoculant are at 0.2 to 0.6 U/ml. Lower cellulase activity was shown in Trichoderma spp. but an increase in trend is observed from days 8 to 30. This result is in line with the microbial growth where an increase of microbial population is observed at day 8 (Figure 1). Therefore, it is believed that the slow growth of Trichoderma spp. in shake flask condition is the cause of this condition. Previous research by Goyal and Sindhu (2011) observed that degradation of paddy straw with fungal cultures shows an increase and higher value of cellulase activity after day 30 of degradation process. Hence, a much longer period of degradation are needed to study and observed effects of fungal cultures on rice straw degradation. Control treatment showed the lowest activity which is expected since no inoculant were present to boost the degradation process.
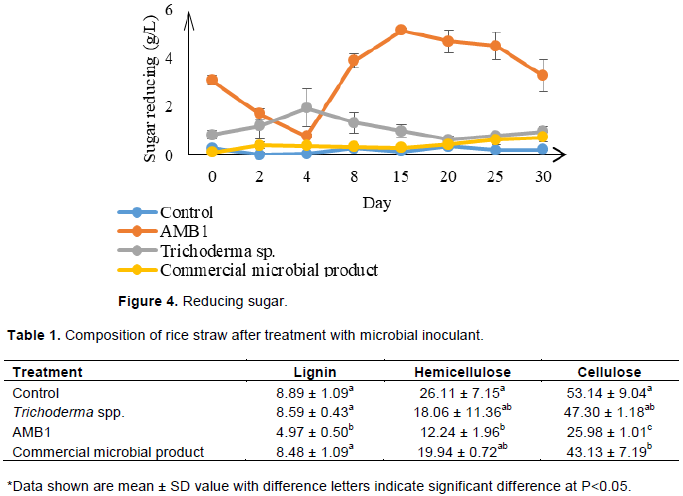
The reducing sugar that accumulates during rice straw fermentation period with different inoculant shows significantly difference of treatment with AMB1 from other treatment (Figure 4).
The highest amount was obtained in treatment with AMB1 at 5 g/L during day 15. Reducing sugar was present after degradation of cellulose (Prasertsung et al., 2017). Treatment with Trichoderma also shows the formation of reducing sugar with the highest amount of 1.9 g/L at day 4. The presents of this reducing sugar indicate that enzyme activity of microorganisms for biomass breakdown is present which converts carbohydrate to further reducing sugar (Peng et al., 2015).
Cellulose, hemicellulose and lignin of rice straw
During degradation period, the composition of rice straw undergoes changes to much stable fraction. The amount of lignin, hemicellulose and cellulose composition in treatment with microorganisms’ inoculant are much lesser than the control rice straw (Table 1).
Rice straw treated with AMB1 showed significantly lower amount of lignin at 4.97%, hemicellulose at 12.24% and cellulose at 25.98% indicating a rice straw hydrolysis. Significant differences were observed in AMB1 treatment for lignin, hemicellulose and cellulose. This is similar to the finding by Phutela et al. (2011) where the decrease in composition of rice straw occurrence indicated the results of breakdown or hydrolysis of complex sugar into fermentable sugars. Furthermore, it was parallel to the results of reducing sugar where AMB1 produced more amount of reducing sugar than other treatment (Figure 4). Composition of rice straw for treatment with Trichoderma spp. and commercial microbial product falls under the same grouping, which indicated that both treatments were at par for rice straw degradation. Both of these microbial inoculants, therefore, still show potential characteristic as rice straw degrader.
The present study suggests that usage of microbial input could aid in decomposition process of rice straw. A combination of microorganisms coded as AMB1 used has shown a performance of an efficient biodegradation of lignocellulosic material compared to single inoculum. Higher level of cellulase activity was observed in AMB1 as compared to other treatment which could lead to a benchmark strain for degradation of biomass. The reduction of lignin, hemicellulose, and cellulose percentage in rice straw treated with AMB1 showed the potential degrading characteristics of this inoculant towards rice straw. However, Trichoderma spp. Also show a figure of strong degradation process towards the end of experimental period. Therefore, further studies are required to determine the maximum potential degradation by these microorganisms and their ability to degrade waste biomass on field and under exact environmental condition.
The authors have not declared any conflict of interests.
REFERENCES
|
Belal E(2013). Bioethanol production from rice straw residues. Brazilian Journal of Microbiology 44(1):225-234.
Crossref
|
|
|
|
Fang R, Li J, Cheng X, Cui J (2012). Performance and spatial succession of a full-scale aerobic plant treating high concentration cassava bioethanol wastewater. Journal of Microbiology and Biotechnology 22(8):1148-1154.
Crossref
|
|
|
|
|
Goyal S, Sindhu S (2011). Composting of rice straw using different inocula and analysis of compost Quality. Microbiology Journal 1(4):126-138.
Crossref
|
|
|
|
|
Jeude M, Dittrich B, Niederschulte H, Anderlei T, Knocke C, Klee D, Bu D (2006). Fed-batch mode in shake flasks by slow-release technique. Biotechnology and Bioengineering 95(3):432-445.
Crossref
|
|
|
|
|
Miller L (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry 31(3):426-428.
Crossref
|
|
|
|
|
Mohamed AA, Mohamed NE, Bahgat MR, Essam HA, Emad EE, Hassan MAA (2016). Biotechnological application of thermotolerant cellulose-decomposing bacteria in composting of rice straw. Annals of Agricultural Sciences 61(1):135-143.
Crossref
|
|
|
|
|
Mohammad HAR, Ong, HK, Nurul AB, Fauzi J (2013). Application of agro-waste compositional data to predict composting efficiency. Journal of Tropical Agriculture and Food Science 41(2):329-339.
|
|
|
|
|
Nishiwaki J, Mizoguchi M, Noborio K (2015). Greenhouse gas emissions from paddy fields with different organic matter application rates and water management practices. Journal of Developments in Sustainable Agriculture 10(1):1-6.
|
|
|
|
|
Phutela G, Sahni N, Sooch S (2011). Fungal degradation of paddy straw for enhancing biogas production. Indian Journal of Science and Technology 4(6):660-665.
|
|
|
|
|
Peng X, Qiao W, Mi S, Jia X, Su H, Han Y (2015). Characterization of hemicellulase and cellulase from the extremely thermophilic bacterium Caldicellulosiruptor owensensis and their potential application for bioconversion of lignocellulosic biomass without pretreatment. Biotechnology Biofuels 8:131.
Crossref
|
|
|
|
|
Prasertsung P, Chutinate A, Watthanaphanit N, Saito S (2017). Conversion of cellulose into reducing sugar by solution plasma process (SPP). Carbohydrate Polymers 172:230-236.
Crossref
|
|
|
|
|
Rashad R, Hussien R (2013). Studying the use of cellulose, silica and lignin extracted from rice straw as sandy soil conditioners. International Journal of Agronomy and Agricultural Research 3(12):21-35.
|
|
|
|
|
IPCC (1996). IPCC Guidelines for National Greenhouse Gas Inventories: Reference Manual P 4.85.
|
|
|
|
|
Rosmiza Z, Davies W, Rosniza A, Mazdi M, Jabil J (2014). Farmers' knowledge on potential uses of rice straw: An assessment in MADA and Sekinchan, Malaysia. Malaysian Journal of Society and Space 10(5):30-43.
|
|
|
|
|
Sarkar N, Sumanta K, Satarupa B, Aikat K (2012). Bioethanol production from agricultural waste: An overview. Renewable Energy 37(1):19-27.
Crossref
|
|
|
|
|
Singh S, Nain L (2014). Microorganisms in the conversion of agricultural wastes to compost. Indian National Science Academy, 80(2):473-481.
Crossref
|
|
|
|
|
Zhao H, Yu H, Yuan X, Piao R, Li H, Wang X, Cui Z (2014). Degradation of lignocelluloses in rice straw by BMC-9, a composite microbial system. Journal of Microbiology and Biotechnology 24(5):585-591.
Crossref
|
|
|
|
|
Rebollido R, Martinez J, Aguilera Y, Melchore K, Koerner I, Stegmann R (2008). Microbial populations during composting process of organic fraction of municipal solid waste. Applied Ecology and Environmental Research 6(3):61-67.
Crossref
|
|
|
|
|
Thomas L, Binod P, Ashok P (2016). Hydrolysis of pretreated rice straw by an enzyme cocktail comprising acidic xylanase from Aspergillus sp. for bioethanol production. Renewable Energy 98:9-15.
Crossref
|
|