ABSTRACT
Identification of zygotic and nucellar seedlings of Harumanis mango by morphological approach is impossible or hard to be performed. Therefore, this research were to identify zygotic or nucellar seedlings from Harumanis mango polyembryonic seeds through simple sequence repeat (SSR) molecular markers in relation with germination sequence and vigour of seedlings for source of true-to-type cutting material. The results showed that there were 3 seedlings per seed on average. The result from the molecular analysis of 136 seedlings (45 seeds) showed four zygotic seedlings in seeds 1, 15, 29 and 43 representing 8.9% of seeds evaluated. Most of the zygotic seedlings were found towards the end of germination sequence except for seed number 15 and the rest were considered as nucellar seedlings. Based on the fitted logistic regression, the predicted sequence to obtain 90% nucellar seedlings is 5.47. This means that, the germination sequence of less than 6 has 90% chance of getting nucellar seedlings compared to zygotic seedlings. Morphological characters such as number of leaves, stem diameter and leaf area could also be used as references with germination sequence. The results showed that there were significant (p<0.01) relationships between germination sequence and all the growth variables. All growth variables were negatively correlated with germination sequence. This suggested that in order to have 90% chances of getting nucellar seedling (germination sequence below 6), the seedling needs to exhibit several morphology characters; big stem girth, tall plant, high leaf number and large leaf area. Therefore, choosing vigour seedling will increase chances of getting nucellar seedlings, which can be used as cutting source for true-to-type planting material or for breeding purposes.
Key words: Harumanis, molecular, morphology, nucellar, SSR markers, zygotic.
Harumanis mango (Mangifera indica) is a mango variety that is economically important and classified as one of the sought-after mango variety in Malaysia (Farook et al., 2013). The demand for Harumanis mango is increasing yearly due to the exquisite taste and aroma of the fruit (Khalid et al., 2017). However, the plant is only cultivated in the Northern region of Peninsular Malaysia such as in Perlis and Kedah since the weather is suitable for the growth of the Harumanis mango (Muhamad Hafiz et al., 2019; Rosidah et al., 2010). As recorded in 2019, there was about 6,373 ha of mango cultivation in Malaysia with 15,766 metric tons production (Department of Agriculture, 2019). This valuable crop is generally propagated by the means of grafting rather than via seedlings to ensure true-to-type planting material (Ahmad Hafiz et al., et al., 2020). This method of propagation however is laborious, costly, requires skilled worker, time-consuming and dependable on availability of seeds for rootstock; which explain the deficit in supply of Harumanis mango planting materials in Malaysia. An alternative method of mass production of true-to- type mango planting material is via cutting, which is reported to be more cost effective, efficient and uniform planting materials (Deependra et al., 2018). This method however is hindered in mango due to the polyembryonic attributes of the mango seed.
Harumanis mango is one of the several mango fruit trees that possess polyembryonic genotype (Mohd Asrul et al., 2018). Generally, cultivars originating from Southeast Asia, as well as tropical Latin America are polyembryonic, while those originating from Florida and India are largely monoembryonic (Vasanthaiah et al., 2007). Polyembryony is defined by the development of more than one seedling in a single seed, and one may be zygotic and the rest are nucellar (Simon et al., 2010; Ravishankar et al., 2004), in some reports all could be nucellar (Degani et al., 1993). This trait is genetically controlled, and in mangos, it is linked to a single dominant gene (Aron et al., 1998). The number of seedlings per seed varies with the cultivar and environmental conditions (Aline et al., 2014). The nucellar embryos in mango trees are developed in the nucellar tissue that covers the embryo sac, and the seedlings derived from these embryos are genetically identical to the parent plant (Aron et al., 1998). In contrast, the zygotic embryo is derived from fertilization by self- pollination or by cross-pollination. It is the objective in breeding programs for the selection of superior genotypes and variability achievement (Aline et al., 2014).
The identification of nucellar seedlings by morphological criteria is impossible or hard to be performed (Desai, 2004). Different morphological and biochemical markers had been used to distinguish nucellar from zygotic seedlings, but none was as efficient as molecular marker (Elisa Del et al., 2012). Thus, the use of molecular or isoenzymatic markers is necessary to observe the differences. Since it was difficult to select nucellar seedlings in seeds of polyembryonic mangos using morphological characteristics, several researchers had reported on the usage of genetic markers to identify zygotic and nucellar embryos. Some of the genetic markers used were Amplified Fragment Length Polymorphism (AFLP) (Eiadthong et al., 2000), Random Amplified Polymorphic DNA (RAPD) (Ravishankar et al., 2000; Elisa Del et al., 2012) and Inter Simple Sequence Repeat (ISSR) polymorphism (Aline et al., 2014). However, seedling industries find difficulties in identifying these markers and continuously choosing plants according to their morphological characteristics. To make identification of nucellar seedlings an easy task in selecting of cutting source, germination sequence and vigour of Harumanis seedlings were investigated in this study, to determine whether these characteristics exhibit relationship to their genetic origin. The objectives of this work were to identify the genetic origin, zygotic or nucellar of seedlings from Harumanis mango polyembryonic seeds by using SSR molecular markers and thereafter relating it to the seedling germination sequence and vigour.
Plant and growth conditions
Seeds for the experiment were collected from Harumanis mango fruits obtained from Malaysian Agriculture Research and Development Institute (MARDI) Station, Sintok, Kedah on May 2019. Forty-five mature fruits of Harumanis mango were chosen. The flesh and seed coat were removed and washed with clean water and soaked in 0.2% Benomyl before the seeds were sown in the sandy seedbed. The seedbed was shaded with black netting (70%) and watered daily.
Germination sequence and seedling vigour determination
Seedlings that germinated from each seed were colour-tagged according to their germination sequence until 30 days of germination period. At 30 days after germination, plant height, stem diameter, number of leaves and leaf area of each seedling were determined. Measurement of plant height was taken from the soil surface to the highest shoot tip using a measuring tape. Stem diameter was measured at the lowest part of stem using Electronic Digital Caliper (Model SCM DIGV-6) while the leaf number was manually counted based on fully expanded leaves. Leaves areas were measured using an automatic leaf area meter (MODEL LI-300, LI-COR) and recorded as a total leaf-area per plant (Figure 1).
DNA extraction
Genomic DNA was extracted following the method described by Mace et al. (2003) with some modifications in term of incubation time. Leaf samples of each seedling at 30 days after germination was ground using the Tissue Lyser (Qiagen, Netherlands) before incubated with extraction buffer (2% CTAB, pH 8, 100 mMTris-HCl, 20 mM EDTA, 1.4 M NaCl, 0.05% β-mercaptoethanol) at 65°C for 1 h. Then, an equal volume of cold isopropanol was used to precipitate the DNA before being washed with 70% ethanol. The DNA pellet was air-dried before being eluted in 50 μl of TE-RNase buffer. The DNA concentration and integrity was measured using Epoch Biotek (Thermo Scientific, USA), and a 0.8% agarose gel, respectively.
SSR genotyping
The SSR markers were selected from Ravishankar et al., (2011) for the assessment of nucellar and zygotic seedlings presented in Table 1. The genotyping process followed the protocol as suggested by Schuelke (2000) by concatenating each of the forward primer with M13 sequence. The PCR was conducted in final volume of 10 µl reaction mixtures containing 10 × Invitrogen PCR Buffer, 2.5 mM MgCl2, 1 μl genomic DNA (20 ng/μl), 2 μM dNTPs (0.2 μM/μl), 10 μM of each primer pair (0.5 μM/μl), 5 μM of fluorescent dye (FAM/NED/PET/VIC) (0.5 μM/μl) and 1 Unit of Taq polymerase (Invitrogen, California). The amplification of the target region was performed using the Applied Biosystem Gene Amp (Thermo Fischer Scientific, California). The PCR profile was set with an initial denaturation for 2 min at 95°C followed by 35 cycles of denaturation at 94°C for 30 s, annealing at 45 to 60°C for 30 s and extension at 70°C for 45 s before being terminated with a final extension for 5 min at 70°C. Then, the PCR products were resolved using an ABI3730xl DNA Analyzer (Thermo Fischer Scientific, California) with Gene Scan TM 500 LIZ (Applied Biosystems, California) used as the DNA ladder.
Scoring and data analyses
The output files (fsa. file) from ABI3730 DNA Analyzer were analyzed using Gene Mapper 5.0 (Applied Biosystems). The allele peaks were identified and scored as suggested by Arif et al., (2010). The seedlings alleles were compared with the parental alleles. POWERMARKER (Liu and Muse, 2005) was used to calculate the genetic distance. Number of seedlings germinated in for each seed was analyzed using descriptive analysis while the responses (zygotic or nucellar) was analyzed using logistic regression. The logistic regression model was used to estimate the sequence of germination for a certain odd ratio of nucellar: zygotic seedlings. Pearson correlation coefficient (r) was determined between the sequence of germination and all the vegetative parameters at p ≤ 0.05%.
Polyembryony in Harumanis mango
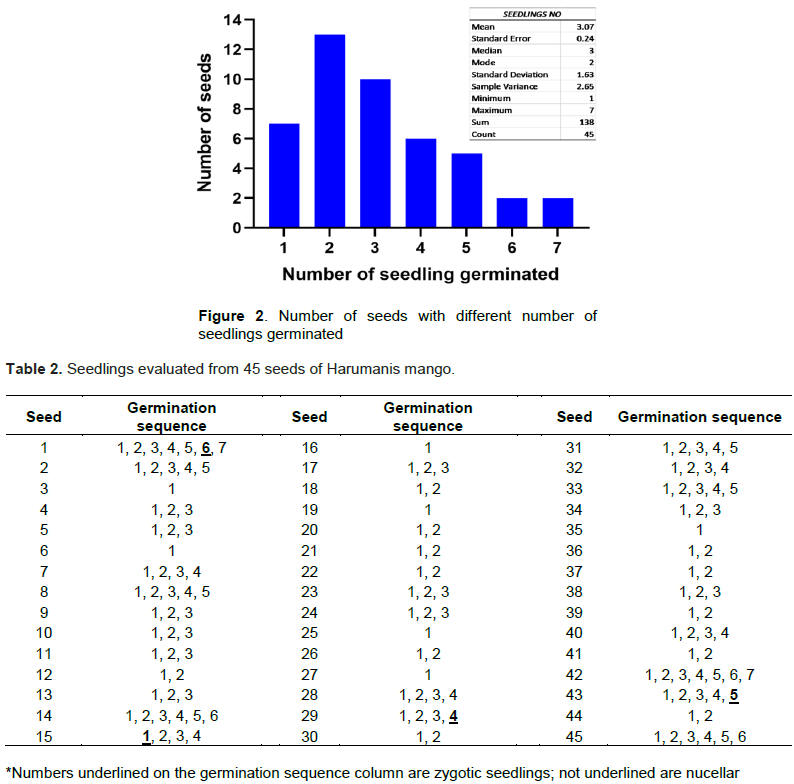
The results in this study revealed that there were 3.07 seedlings per Harumanis mango seed on average; ranging from 1 to 7 seedlings. Most of the seeds, 51.1% had 2 or 3 seedlings per seed while only 4.4% had 7 seedlings (Figure 2). The results in this study are in accordance with Zakaria et al. (2002) that reported 2 to 6 seedlings per seed in M. indica (cultivar Sala and Tangkai Panjang) while 1 to 2 seedlings per seed in Mangifera foetida and Mangifera caesia. The difference between the numbers of seedlings in species of Mangifera has been reported by Cordeiro et al. (2005) and is due to the genetic differences between the species.
Molecular analysis
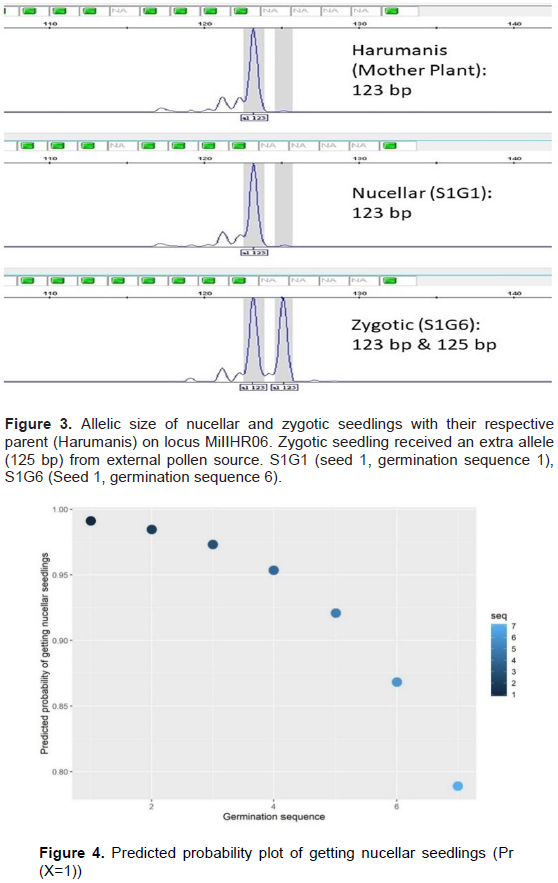
The analysis of 136 seedlings which were derived from 45 seeds using microsatellite markers showed a total of four zygotic seedlings and they were identified in seeds 1, 15, 29 and 43 representing 8.9% of seeds evaluated (Table 2). Most of the zygotic seedlings were found towards the end of germination sequence except for seed number 15. This was in agreement with the study conducted by Aline et al. (2014) on Uba cultivars. However, details and depth study are required to understand the sequence order and the occurrence of the zygotic seedling in Harumanis seed. Meanwhile, the remaining seedlings were considered as nucellar seedlings as the SSR based DNA profile match with the parent DNA profile of Harumanis mango. In the present study, seedlings that showed polymorphism at least by one primer were also considered as zygotic since this study was using codominant marker system. However, in this study, zygotic seedling from seed 15 showed the least polymorphic across all nine. As we were using codominant marker, we can directly and perfectly identified the heterozygote seedling which happened due to the pollination with the external pollen source. Hence, the seedlings with the presence of heterozygote allele form even at one marker were considered as zygotic seedling. Figure 3 describes the allelic segregation of nucellar and zygotic seedling on locus. The number of polymorphic marker was different between the zygotic seedlings which might be caused by receiving a different pollen donor. There is the successful study on identification of pollen donor in European Plum using microsatellite markers (Meland et al., 2020). Unlike previous studies which were using dominant marker system such as RAPD and ISSR, the researcher was required to set at least three polymorphic primers in order to consider the seedling is zygotic (Aline et al., 2014). Since the dominant marker system such RAPD and ISSR is unable to differentiate between homozygote and heterozygote of allele which lead to numerous number of marker is needed in order to identify the occurrence of zygotic and nucellar seedlings (Miah et al., 2013). The present study also showed there was no occurrence of double zygotic seedling in a single seed (Policaulismo).
Relationship of genetic material in accordance with germination sequence and vigour
In computing the probability of getting nucellar seedling for source of true-to-type cutting material based on germination sequence, the logistic regression model fitted from the data of germination sequence and genetic material (nucellar or zygotic) is:
Y = 5.29 - 0.57x
where Y is the outcome of nucellar or zygotic (nucellar (1) or zygotic (0)) and X is the germination sequence. Based on the parameter estimate in Table 3, the estimated odd ratio of getting a nucellar seedling is
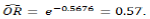
. This means that as the germination sequence increases by one unit, the odds of getting nucellar seedlings reduces by 57%.
Based on the fitted logistic regression, the predicted sequence to obtain 90% nucellar seedlings is 5.47. This means that, the germination sequence of less than6 has 90% chances of getting nucellar seedlings compared to zygotic seedlings. Figure 4 shows the logistic regression plot for the germination sequence vs. predicted probability of getting a nucellar seedling.
Using germination sequence as a reference in getting nucellar seedlings for source of cutting materials serve some challenges as the nurserymen have to closely monitor germination and tag the seedlings. Alternatively, morphological characteristics such as leaf number stem diameter and leaf area could also be used as reference with germination sequence. The results showed that there were significant (p<0.01) relationships between germination sequence and all the growth variables (Figure 5). All growth variables were negatively correlated with germination sequence, with the strongest relationship (r= -0.39; p ≤ 0.01) was recorded between germination sequence and stem diameter. This was followed by plant height (r= -0.38; p ≤ 0.01), leaf number (r = -0.29; p ≤ 0.01) and lastly leaf area (r = -0.27; p ≤ 0.01) (Figure 3). However, leaf number showed positive correlation with plant height (r = 0.49; p ≤ 0.01), stem diameter (r = 0.43; p ≤ 0.01) and leaf area (r = 0.43; p ≤ 0.01). These results showed that there was an increase in leaf number with the increase of plant height, stem diameter and leaf area. The same result was obtained by Shaban (2010) who found that leaf number was correlated positively with leaf area and plant height and leaf area for Zebda mango seedlings from Egypt. In term of plant height, there was a strong positive correlation with stem diameter (r = 0.76; p ≤ 0.01) and leaf area (r = 0.64; p ≤ 0.01). The stem diameter also had strong positive correlation with leaf area (r = 0.65; p ≤ 0.01). Zakaria et al. (2002) found that the seedlings from different variety of Mangifera species seeds differ in terms of vigour, plant size or height depending on whether they are nucellar or zygotic in origin. Zakaria et al. (2002) and Muralidhara et al. (2015) suggested that the removal of seed coat might had given a superior response in all initiation of plant height, stem diameter, number of leaves per plant and leaf area. At the same time, the different response growth of seedlings produced after germination and emergence that may be caused by competition between seedlings for nutrient uptake, light and space.
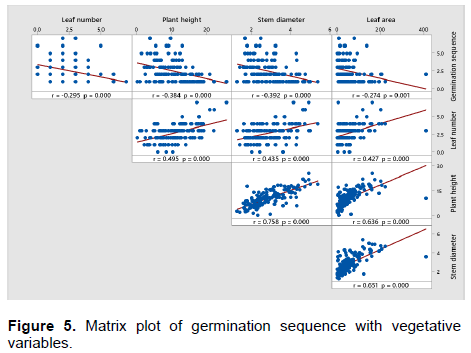
This suggested that in order to have 90% chances of getting nucellar seedling (germination sequence below 6), the seedling needs to exhibit several morphological characteristics; big stem girth, tall plant, high leaf number and large leaf area. These are morphological characteristics of vigor seedlings. Generally, the most vigorous seedling from each seed are used by the nurserymen for the production of rootstock however the nucellar seedling is not always the most vigorous, which results in uneven orchards (Simon et al., 2010). In ‘Uba’ mangos, 60% of the seedlings tested were discovered as zygotic, and not correlated with the vigorous character tested (Aline et al., 2014). In addition, 90% of the most vigorous seedlings from seeds of ‘Rosinha’ mangos collected in 2002 and 2003 were of zygotic origin, while seedlings from seeds harvested in 2004 were mostly identified as nucellar, indicating no relationship between the type of embryo and seedling size (Cordeiro et al., 2005).
Harumanis is a polyembryonic mango with average 3 seedlings per seed. Based on SSR molecular markers, zygotic seedlings were found towards the end of germination sequence with 8.9% of total seeds evaluated. DNA marker system was proven to be the ideal approach in identifying zygotic and nucellar seedlings as the identification is not influenced by environmental factor and agronomic practices. Choosing vigour seedling will increase the chances of getting nucellar seedlings, which can be used as cutting source for true-to-type planting material or for breeding purposes.
The authors have not declared any conflict of interests.
This research work was supported by MARDI and Malaysian Government under 11th Development Fund Project (P-RH405). The authors also thank Mr. Muhammad Faris Ali for assisting in samples preparation and data collection.
REFERENCES
|
Aron Y, Czosnek H, Gazit S, Degani C (1998). Polyembryony in mango (Mangifera indica L.) is controlled by a single dominant gene. Horticultural Science 33:1241-1242.
Crossref
|
|
|
|
Arif IA, Khan HA, Shobrak M, Al Homaidan AA, Al Sadoon M, Al Farhan AH, Bahkali AH (2010). Interpretation of electrophoretograms of seven microsatellite loci to determine the genetic diversity of the Arabian Oryx. Genetics and Molecular Research 9(1):259-265.
Crossref
|
|
|
|
|
Aline R, Fernandes S, TâniaM, Lopes SD, Damião CC, Chamhum SC (2014). Identification of 'Ubá' mango tree zygotic and nucellar seedlings using ISSR markers. Revista Ceres 61(5):597-604.
Crossref
|
|
|
|
|
Ahmad Hafiz B, Mahmad Noor J, Mohd Asrul S, Hartinee A (2020). Growth performance of different mango (Mangifera indica L.) varieties as rootstock for Harumanis planting material production. Journal of Tropical Plant Physiology 12(1):49-56.
|
|
|
|
|
Cordeiro MCR, Ramos VHV, Pinto ACQ, Fraga LMS, Dias JN, Lopes GKB (2005). Molecular identification of the zygotic or nucellar origin of polyembryonic mango seed 'Rosinha' seedlings based on RAPD. In: 3rd Brazilian Congress of Plant Breeding, Gramado. Anais, Embrapa Wheat. CD-ROM.
|
|
|
|
|
Degani C, Cohen M, Reuveni O, El-Bastri R, Gazit S (1993). Frequency and characteristics of zygotic seedlings from polyembryonic mango cultivars, determined using isozymes as genetic markers. Acta Horticulturae 341:78-85.
Crossref
|
|
|
|
|
Desai BB (2004). Seeds handbook: biology, production, processing and storage. 2nded. New York, CRC Press, p. 787.
Crossref
|
|
|
|
|
Yadav D, Pal AK, Singh SP (2018). Vegetative methods of plant propagation: II- grafting, cutting, layering and budding in mango. International Journal of Pure and Applied Bioscience 6(3): 575-586.
Crossref
|
|
|
|
|
Department of Agriculture (DOA) (2019). Crops Statistic (Sub-Sector Food Crops) 2019. Putrajaya, Malaysia.
View Accessed August 13, 2020.
|
|
|
|
|
Eiadthong W, Yonemori K, Kanzaki S, Sugiura A, Utsunomiya N, Sabhadrabandhu S (2000). Amplified fragment length polymorphism analysis for studying genetic relationships among Mangifera species in Thailand. Journal of the American Society of Horticultural Science 125(2):160-164.
Crossref
|
|
|
|
|
Elisa Del CMO, Andrade‑Rodríguez M, Mario RR, Angel VM (2012). Identification of zygotic and nucellar seedling in polyembryonic mango cultivars. Pesquisa Agropecuária Brasileira 47(11):1629-1636.
Crossref
|
|
|
|
|
Farook RSM, Ali H, Harun A, Ndzi DL, Shakaff AYM, Jaafar MN, Husin Z, Aziz AHA (2013). Harumanis mango flowering stem prediction using machine learning techniques. Research Notes in Information Science 13:46-51.
|
|
|
|
|
Khalid NS, Abdullah AH, Shukor SAA, Fathinulsyahir AS, Chau SC, Nor Dalila ND, Mansor H (2017). Image processing techniques for Harumanis disease severity and weighting estimation for automatic grading system application. Journal of Telecommunication, Electronic and Computer Engineering 10:1-15.
|
|
|
|
|
Liu K, Muse SV (2005). Power Marker: an integrated analysis environment for genetic marker analysis. Bioinformatics 21(9):2128-2129.
Crossref
|
|
|
|
|
Mace ES, Buhariwalla KK, Buhariwalla HK, Crouch JH (2003). A high-throughput DNA extraction protocol for tropical molecular breeding programs. Plant Molecular Biology Report 21(4):459-460.
Crossref
|
|
|
|
|
Meland M, Frøynes O, Fotiric Akšić M, Pojskić N, Kalamujić Stroil BK, Lasic L, Gasi F (2020). Identifying pollen donors and success rate of individual pollinizers in European plum (Prunus domestica L.) using microsatellite markers. Agronomy 10(2):1-16.
Crossref
|
|
|
|
|
Miah G, Rafii MY, Ismail MR, Puteh AB, Rahim HA, Islam K, Latif MA (2013). A review of microsatellite markers and their applications in rice breeding programs to improve blast disease resistance. International journal of molecular sciences 14(11):22499-22528.
Crossref
|
|
|
|
|
Muralidhara BM, Reddy YTN, Srilatha V, Akshitha HJ (2015). Effect of seed coat removal treatments on seed germination and seedling attributes in mango varieties. International Journal of Fruit Science 16:1-9.
Crossref
|
|
|
|
|
Mohd Asrul S, Hartinee A, Mahmad Nor J, Mohamad Bahagia AG (2018). Morphological characterisation of Harumanis mango (Mangifera indica Linn.) in Malaysia. International Journal of Environmental and Agriculture Research 4(1):36-42.
|
|
|
|
|
Muhamad Hafiz MH, Hartinee A, Nor Dalila ND, Zul Helmey MS, Razali M, Ab Kahar S, Siti Aisyah A, Wan Mohd Reza IWH, Shaidatul Azdawiyah AT (2019). Effect of multilocation production on the reproductive growth, yield and quality of Harumanis mango. International Journal of Current Advanced Research 08(04):18175-18180.
|
|
|
|
|
Ravishankar KV, Lalitha A, Dinesh MR (2000). Assessment of genetic relatedness among mango cultivars of India using RAPD markers. The Journal of Horticultural Science and Biotechnology 75(2):198-201.
Crossref
|
|
|
|
|
Ravishankar KV, Chandrashekara P, Sreedhara SA, Dinesh MR, Lalitha A, Saiprasad GVS (2004). Diverse genetic bases of Indian polyembryonic and monoembryonic mango (Mangifera indica L) cultivars. Current Science 87:870-871.
|
|
|
|
|
Rosidah M, Faridah H, Jamaliah MY, Norzaidi MD (2010). Examining market accessibility of Malaysia's Harumanis mango in Japan: challenges and potentials. Business Strategy Series 11(1):3-12.
Crossref
|
|
|
|
|
Ravishankar KV, Mani BH, Anand L, Dinesh MR (2011). Development of new microsatellite markers from Mango (Mangifera indica) and cross-species amplification. American Journal of Botany 98(4):96-99.
Crossref
|
|
|
|
|
Schuelke M (2000). An economic method for the fluorescent labeling of PCR fragments. Nature Biotechnology 18(2):233-234.
Crossref
|
|
|
|
|
Simon AM, Festus KA, Gudeta S, Oluyede CA (2010). Rootstock growth and development for increased graft success of mango (Mangifera indica) in the nursery. African Journal of Biotechnology 9(9):1317-1324.
Crossref
|
|
|
|
|
Shaban AEA (2010). Comparative study on some polyembryonic mango rootstocks. American-Eurasian Journal Agriculture and Environmental Science 7(5):527-534.
|
|
|
|
|
Vasanthaiah HKN, Ravishankar KV, Mukunda GK (2007). Mango. In: Kole. C. (ed.) Genome mapping and molecular breeding in plants. Springer-Verlag, Berlin Heidelberg 4:303-323.
Crossref
|
|
|
|
|
Zakaria W, Tengku Ab. Malik TM, Masri M (2002). Germination pattern of three Mangifera species. Journal of Tropical Agriculture and Food Science 30(2):163-171.
|
|
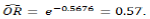 . This means that as the germination sequence increases by one unit, the odds of getting nucellar seedlings reduces by 57%.
. This means that as the germination sequence increases by one unit, the odds of getting nucellar seedlings reduces by 57%.