ABSTRACT
Considering the potential benefit of meristem culture in the mass production of quality propagation materials, an experiment was conducted to investigate meristem culture in KUWE (G1) and 223098 (G2) genotypes of anchote. Concentration levels of 6-benzylamimopurine (BAP) with and without gibberellic acid (GA3) and naphthalene acetic acid (NAA) for shoot induction while BAP and kinetin (Kn) for shoot multiplication were evaluated. Full and half nutrient-strength of Murashige and Skoog medium (FMS and HMS) with sole application of indole butyric acid (IBA) and indole acetic acid (IAA) were tested for root induction. About 80% (G2) and 71% (G1) of meristems induced shoots at 1 and 0.5 mg/L BAP, respectively. The highest shoot number (6.52), in G2, was recorded at 0.5 mg/L BAP while 0.5 mg/L BAP + 0.5 mg/L Kn produced 6.32 shoots in G1. FMS + 0.5 mg/L IBA was effective in 100% root induction in both genotypes. Survival rates of 58% (G1) and 42% (G2) were obtained. Generally, 0.5 mg/L BAP (G1) and 1 mg/L BAP (G) are best for shoot induction while 0.5 mg/L BAP (G2) and 0.5 mg/L BAP+0.5 mg/L Kn (G1) for multiple shoot development with best root formation on FMS+0.5 mg/L IBA.
Key words: Direct regeneration, genotype, meristem explants, MS media, plant growth regulators (PGRs).
Anchote [Coccinia abyssinica (Lam.) Cong.] is an annual trailing vine belonging to the Cucurbitaceae family. It is an endemic species found both cultivated and in the wild in Ethiopia and best known and grown principally for its tuberous root even though its tender leaves and immature fruits are also widely used as food (Demel et al., 2010; Ermias et al., 2011). The tuber of anchote is the richest in protein, calcium and iron contents as compared to other common and widespread root tubers (Habtamu and Kelbessa, 1997; Habtamu et al., 2013). The low content of anti-nutritional factors also reflects the desirable quality of the tuber. Though not well investigated, the edible leaves and fruits of anchote have been indicated containing high nutritive composition,
even better than the commonly used tubers (Desta, 2011; Girma and Dereje, 2015). Anchote has been used as folklore medicine to heal the bone fracture, backache, displaced joints and other diseases such as gonorrhea, tuberculosis, and cancer (Dawit and Estifanos, 1991; Amare, 2003).
Anchote is traditionally cultivated in some districts of Western, Southwestern and Southern parts of Ethiopia, areas where anchote is widely domesticated, on altitude between 1300 and 2800 m.a.s.l with 762 to 1016 mm annual rainfall (Amare, 2003). The average yield at farmers level has been estimated from 10 to 20 t/ha (Amsalu et al., 2008) which is significantly low than the maximum tuber yield (76-80 t/ha) obtained under experimental condition (Desta, 2011; Daba et al., 2012). Since anchote is commonly propagated by seed, the high out-crossing nature of amchote flowers (Edwards et al., 1995) has been challenging the production sustainability and breeding of the crop. As a result, the scarcity of quality propagation material coupled with lack of improved variety is among the most important constraints in anchote production. In fact, such a bottleneck could be undoubtedly improved through applying plant cell, tissue and organ culture technique called “meristem culture”.
Culturing of an organized tissue in the form of very small shoots or meristems is the most valuable application of plant cell, tissue and organ culture in order to produce plenty of axenic and genetically stable propagation materials of elite varieties (Alam et al., 2004; Badoni and Chauhan, 2009; Rani and Raina, 2000; George and Debergh, 2008). However, meristem culture is a complex process that is affected by multiple plant endogenous factors including phytohormones and the culture environment.
Folla et al. (2013) conducted direct plantlet regeneration experiment from nodal and shoot tip explants of anchote using 6-benzylamimopurine (BAP) and kinetin for culture establishment, BAP and indole acetic acid (IAA) for shoot multiplication and half nutrient-strength MS medium supplemented with indole butyric acid (IBA), IAA and naphthalene acetic acid (NAA) for root induction. Similarly, Jane et al. (2016) conducted nodal culture experiment in anchote using BAP, kinetin and thidiazuron (TDZ) for micro-shoot formation, while IBA and NAA on half nutrient-strength of MS medium for root induction. Shekhawat et al. (2014), in Coccinia indica, have demonstrated the effect of BAP and kinetin on the nodal bud break and shoot multiplication while IAA and IBA on the root induction. However, a research report on meristem culture of anchote, broadly in Coccinia species, has not been found.
The exact condition required to initiate and sustain plant cells in culture or to regenerate intact plants from cultured cells has been found to depend on many factors, of which most important are: genotype, explants, composition of basic medium and plant growth regulators (Reed, 1999; Slater et al., 2003; Loyola-Vargas and Vazques-Flota, 2006; George and Debergh, 2008). Due to variation with such factors, there was found no method that can be universally recommended with a new species of interest. Therefore, this research work was initiated to investigate meristem culture in KUWE and 223098 genotypes of anchote through regulating plant growth regulators and nutrient-strength of the medium thereby to find out the best media composition for each developmental stage of meristem culture.
Plant and experimental site
Seeds of two anchote accessions, KUWE (G1) and accession number 223098 (G2), each from different genotype cluster (Desta, 2011) were collected from Debre Zeit Agricultural Research Centre (DZARC). Mother plants were established in a greenhouse using pots filled with 2:1:1 ratio of soil, sand and compost, respectively. The experiment was conducted at the Plant Tissue Culture Laboratory of National Agricultural Biotechnology Research Centre (NABRC), Ethiopian Institute of Agricultural Research (EIAR), which is located at about 44 km far to the west of Addis Ababa, the capital city of Ethiopia.
Media preparation
The Murashige and Skoog (MS, 1962) basal nutrients with 3% sucrose were used as the basic components of the media. The pH of the medium was adjusted to 5.8 upon addition of plant growth regulators. Then, agar type I was added at a rate of 4 g/L while boiling to solidify the media. The prepared media were autoclaved for 20 min at a temperature of 121°C and 15 psi pressure and left for three days in the culture room before use to check for any contamination.
Shoot bud surface sterilization
Apical shoot buds of about 1 to 2 cm were collected from greenhouse grown plants at their full vegetative stage. Washing twice with tap water and once with the addition of liquid detergent and 1 to 2 drops of Tween-20 was done to remove surface dust and reduce the level of surface contaminants. Further surface disinfection was done under the laminar airflow hood by treating shoot buds with 70% ethanol for 30 s followed by 1% Clorox bleach (NaOCl) for 10 min based on the results of the preliminary experiment conducted to optimize shoot bud surface sterilization of anchote. Upon the completion of each surface disinfection treatment, shoot buds were rinsed three to four times with sterilized double distilled water to remove the effect of surface sterilizing agents.
Meristem establishment
Meristems (≤ 1 mm size) consisting of the meristematic dome with one to two leaf primordia were isolated using sterile hypodermic needle and scalpel under a dissecting microscope (Olympus) as described by Alam et al. (2004). Isolated meristems were quickly transferred to culture jars containing 50 ml volume of sterilized MS media supplemented with BAP (0.5, 1, 2 and 4 mg/L) alone and in combination with 0.1 mg/L GA3 and 0.01 mg/L NAA. Data on the number of days to shoot response, total response of meristems, shoot induction frequency and callus induction frequency was collected to evaluate shoot induction response of meristems.
Shoot multiplication
Normal and healthy meristem derived shoots (≥ 1 cm length) were isolated and inoculated on to freshly prepared media containing different concentrations of BAP (0.25, 0.5 and 1 mg/L) and kinetin (0.25 and 0.5 mg/L) singly and in combination. Data on the number of shoot length, leaf number and number of nodes was collected at the fourth week of culturing on the multiplication media.
Root induction
Normal and healthy shoots of about 2 cm and more in length were selected and inoculated on full and half nutrient-strength of the MS medium (FMS and HMS) supplemented with IBA and IAA individually, each at the same concentration levels (0.5, 1, 2 and 4 mg/L). Data on the root induction frequency, number of roots per shoot and root length was recorded at the fourth week of culturing on the rooting media.
Culture condition
Cultures were treated with hormone-free medium for a week whenever transferring them to a different type and combinations of plant growth regulators (PGRs) to avoid their carry-over effect. Hormone-free medium was used as a control treatment for all the developmental stages of meristem culture investigated in this experiment. All culturing processes were undertaken in the aseptic condition of the laminar air flow hood. Unnecessary callus developed at the base of shoots was trimmed with the help of surgical blade before shoots subjected to multiplication and root induction treatments. All cultures were kept in a growth room under 16 h light (2700 lux light intensity) and 8 h dark cycle at a temperature of 25 ± 2°C. To facilitate growth, the cultures were transferred to fresh media of the same combination at every four weeks interval.
Acclimatization procedures
Acclimatization was done by transplanting healthy root-formed shoots in a pot filled with sterilized sand, soil and compost in a ratio of 2:1:1, respectively. Roots were rinsed several times with tap water to remove the attached agar before planting them onto the prepared soil medium. Pots with plantlets were placed in a greenhouse by covering them with a plastic sheet. The cover was gradually removed and the survived plantlets were counted after four weeks of acclimatization.
Design and data analysis
All the factorially combined treatments were arranged in Completely Randomized Design (CRD). Each data was collected from a total of 20 sample units, five observations per culture jar replicated four times. Data of all the quantitative parameters were subjected to statistical analysis with SAS computer statistical tool (version 9.1). Least Significant Difference (LSD) test was conducted to compare treatment means, which revealed a significant difference of F-test in the analysis of variance (ANOVA), at p ≤ 0.05 probability level.
Effect of plant growth regulators on meristem culture establishment
Meristems showed their first growth response by increasing in size with greenish white color and became greener as they continued their growth and development of shoots (Figure 1A to C). Meristems of both genotypes established with lower concentrations of BAP alone and/or in combination with GA3 responded to shoot within less than 10 days of incubation. An increase in the number of days to shoot induction response with a decrease in shooting frequency was observed at the higher levels of BAP as well as in all treatments containing NAA. This is mainly due to unnecessary callus development at the base of meristems (Figure 1D), which suppressed the development of shoots. Though the callusing potential of BAP and NAA was investigated earlier in nodal culture of Coccinia grandis (Thiripurasundari and Rao, 2012), such a phenomenon was investigated in different plant species: Telfairia occidentalis (Adesoye et al., 2012), potato (Yasmin et al., 2011), sweet potato (Alam et al., 2010), and tomato (Ishag et al., 2009). Geleta and Tileye (2011), on the other hand, obtained maximum shoot induction frequency in sweet potato meristem culture with the combination of BAP, NAA and GA3 which argue with the present findings in anchote.
Genotypes varied in their maximum shoot induction potential and in their requirement for optimum BAP concentration. The highest shoot induction frequency was obtained in G2 at 1 mg/L BAP while maximum shoot induction frequency in G1 was obtained at 0.5 mg/L BAP (Data not shown). The effectiveness of BAP alone at a lower concentration in the initiation medium of eggplant meristem culture (Sharmin et al., 2008) and shoot culture in other genotype of anchote (Folla et al., 2013) was reported, which are in agreement with the current finding. The variation between genotypes may be due to their totipotentiality and endogenous level of PGRs as stated by Piqueras and Debergh (1999). Keeping the variation between genotypes, the current flinging is even better than the maximum shoot induction frequency obtained by Folla et al. (2013) in shoot culture of anchote.
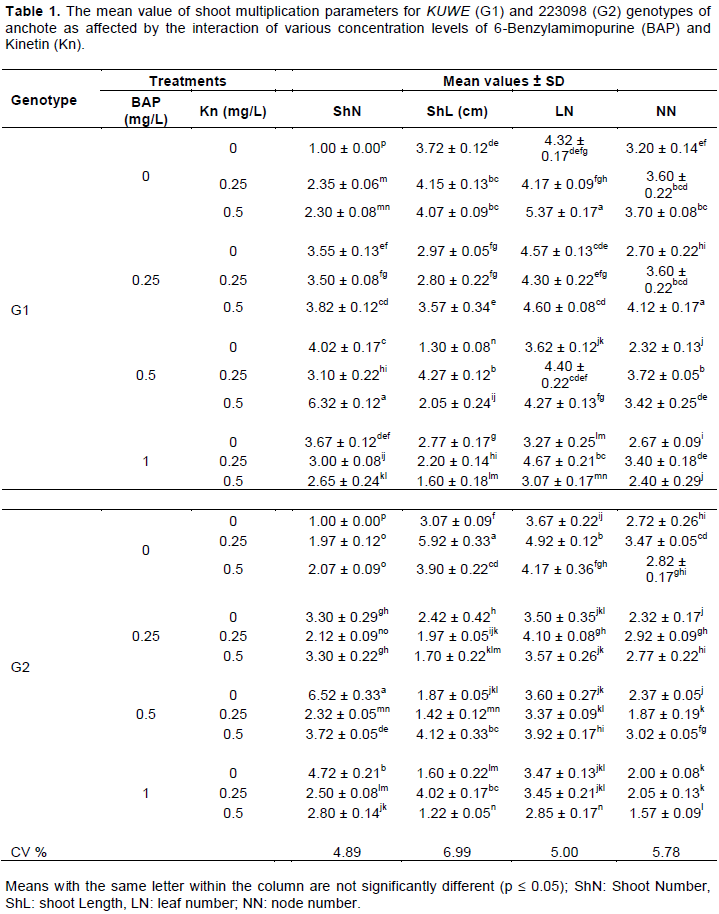
Effect of genotype and cytokinins on shoot multiplication
Interaction of genotype, BAP and kinetin resulted in a significant (p ≤ 0.05) variation on the multiplication and development of meristem derived shoots (Table 1). Genotypes were not significantly different in the production of maximum shoot number. However, maximum shoot number in G1 was obtained in a medium supplemented with 0.5 mg/L BAP + 0.5 mg/L kinetin, while in G2, 0.5 mg/L BAP alone was effective in the production of multiple shoots. Application of BAP alone at a concentration of 1 mg/L in G2 and 0.5 mg/L in G1 was also satisfactory in the production of multiple shoots as compared to the control treatments, which produced only a single shoot. This result indicates the effectiveness of BAP than kinetin for multiple shoot production from meristem-derived shoots of anchote. Supportive results were reported by Bhujyan (2013) in potato varieties. Though, genotypic variation in cassava (Mapayi et al., 2013), eggplant (Sharmin et al., 2008) and sweet potato (Geleta and Tileye, 2011) shoot multiplication, in response to a given cytokinins, has been well demonstrated. Folla et al. (2013) obtained a maximum of about four shoots at fourth week on multiplication medium; where the number of shoots per explant increased to 10 as the culture time increases. Similarly, Shekhawat et al. (2014) in nodal culture of C. indica obtained an increased number of shoots through subsequent sub-culture. In the present experiment, however, a maximum of about six shoots were obtained within four weeks of culture duration. This clearly shows the culture time duration factor, apart from explant variation which was responsible for an increased rate of shoot multiplication.
Most of the media supplemented with kinetin produced longer shoots and higher numbers of leaf and node regardless of the genotype difference (Table 1). The positive effect of kinetin on shoot elongation in Stevia rebaudiana has been reported recently by Sridhar and Aswath (2014). Moreover, the negative effect of BAP on plantlet height was demonstrated by Sanavy and Moeini (2003) in potato cultivars. G1 was found superior to G2 in leaf and node number regardless of the type and concentration of cytokinins used. A genotypic difference in potato meristem culture has been reported by Al-Taleb et al. (2011) in terms of shoot numbers, shoot length and number of leaves of meristem-derived shoots.
Effect of genotype, media nutrient-strength and rooting hormones on root induction
Interaction of genotype, MS media nutrient-strength, rooting hormone and their concentrations resulted in a significant (p ≤ 0.05) variation on the root induction and development parameters (Table 2). Full nutrient-strength of MS medium with a lower concentration of IBA was found effective in terms of root formation in both genotypes; 0.5 mg/L is the best, which resulted in 100% root formation. Al-Taleb et al. (2011) also reported related findings in different genotypes of potato. In contrary, Jane et al. (2016) and Folla et al. (2013) obtained maximum root induction frequency, in other genotypes of anchote, on half nutrient-strength of MS medium supplemented with lower concentration of IBA. Full nutrient-strength of MS medium supplemented with 1 and 2 mg/L IAA as well as half nutrient-strength of MS medium supplemented with 0.5 mg/L IAA also resulted in 100% root induction frequency in G2. However, roots formed in these media were found morphologically abnormal (Figure 2A and B) as compared to the normal morphological appearance roots (Figure 2C and D) obtained from full nutrient- strength of MS medium supplemented with 0.5 mg/L IBA. This might be associated with the development of unnecessary callus. Unnecessary callus formation was observed at an elevated concentration of IBA and almost at all concentrations of IAA, regardless of the variation in nutrient-strength of the MS medium. Such a phenomenon was also observed by Shekhawat et al. (2014) in C. indica and Folla et al. (2013) in other genotypes of anchote.
There has been observed, generally, an increase in the number of root per shoot with an increase in concen-trations of rooting hormones regardless of the variation between genotypes and media nutrient-strength (Table 2). However, a reduction in root length was observed as the number of roots per shoot increases. A higher number of roots at an elevated concentration of rooting hormones may be associated with the formation of callus at the base of cuttings which is responsible for adventitious root formation as described earlier by Jutta et al. (2005). A general reduction in number of roots due to callus development at a lower concentration of rooting hormones was reported by Folla et al. (2013) in other genotypes of anchote, which is in contrary with the current findings.
Acclimatization of plantlets
Meristem-derived plantlets were grown normal and healthy upon acclimatization (Figure 2E). Survival rates obtained in both genotypes indicated low performance with respect to the given soil medium composition (2:1:1 ratio of sand, soil and compost, respectively). However, plantlets of G1 survived better (58%) than plantlets of G2 (42%), after four weeks of acclimatization. Regardless of the genotype difference, Yoseph and Tileye (2013) obtained about 60% survival rate of in vitro produced plantlets of anchote, acclimatized on the same potting mixture used in the present study. The low survival rates might be associated mainly with the composition of potting substrates as indicated by Folla et al. (2013) and Jane et al. (2016).
Meristem culture in both KUWE (G1) and 223098 (G2) genotypes of anchote has been investigated in detail in this study. The optimum media composition has also been determined for each developmental stage of meristem culture. Maximum shoot induction frequency was achieved using 0.5 and 1 mg/L BAP in G1 and G2, respectively. Multiple shoot production was achieved by applying 0.5 mg/L BAP alone and combined application of BAP and kinetin each at 0.5 mg/L, in G2 and G1, respectively. Whereas, full nutrient-strength MS medium supplemented with 0.5 mg/L IBA was found effective in the in vitro production of roots, in both genotypes. Plantlets survival rate of 58% in G1 and 42% in G2 were found after a month of acclimatization on 2:1:1 ratio of soil, sand and compost, respectively. The findings of this study can be applied for mass production of quality propagation materials of anchote as well as for any investigation following the technique of meristem culture. However, further experiment might be conducted to improve the survival rate of plantlets during acclimatization.
The authors have not declared any conflict of interests.
The author expresses a deep gratitude to the Ministry of Education (MoE) for their financial support and National Agricultural Biotechnology Research Center, Ethiopian Institute of Agricultural Research, for providing laboratory facilities with all the necessary chemicals and reagents.
REFERENCES
|
Adesoye AI, Okooboh GO, Akande SR, Balogun MO, Odu BO (2012). Effect of phytohormones and genotype on meristem and shoot tip culture of Telfairia occidentalis (Hook F.). Journal of Applied Biosciences 49:3415-3424.
|
|
|
|
Alam I, Sharmin SA, Naher MK, Alam MJ, Anisuzzaman M, Alam MF (2010). Effect of growth regulators on meristem culture and plantlet establishment in sweet potato [Ipomoea batatas (L.) Lam.]. Plant Omics Journal 3(2):35-39.
|
|
|
|
|
Alam MF, Banu MLA, Swaraz AM, Parvez S, Hossain M, Khalekuzzaman M, Ahsan N (2004). Production of virus-free seeds using meristem culture in tomato plant under tropical conditions. Journal of Plant Biotechnology 6:221-227.
|
|
|
|
|
Al-Taleb MM, Hassawi DS, Abu-Romman SM (2011). Production of virus-free potato plants using meristem culture from cultivars grown under Jordanian environment. American-Eurasian Journal of Agricultural and Environmental Sciences 11(4):467-472.
|
|
|
|
|
Amare G (2003). Some Common Medicinal and Poisonous Plants used in Ethiopian Folk Medicine. Addis Ababa University Press, Addis Ababa, Ethiopia.
|
|
|
|
|
Amsalu N, Weyessa G, Assefa T, Wubishet A, Asfaw K, Edossa E (2008). Variety development of taro, cassava, yam, and indigenous root and tuber crops of Ethiopia. In Gebremedhin WG, Endale G, Berga L (eds) Root and tuber crops: The untapped resources. EIAR, Addis Ababa, Ethiopia pp. 303-315.
|
|
|
|
|
Badoni A, Chauhan JS (2009). Effect of Growth Regulators on Meristem-tip Development and in vitro Multiplication of Potato Cultivar 'Kufri Himalini. Nature Science 7(9):31-34.
|
|
|
|
|
Bhujyan FR (2013). In vitro meristem culture and regeneration of three potato varieties of Bangladesh. Research in Biotechnology 4(3):29-37.
|
|
|
|
|
Daba M, Derbew B, Wosene GS, Waktole S (2012). Growth and yield performance of anchote [Coccinia abyssinica (Lam.) Cogn.] in response to contrasting environment. Asian Journal of Plant Sciences 4:172-181.
|
|
|
|
|
Dawit A, Estifanos H (1991). Plants as a primary source of drugs in the traditional health practices of Ethiopia. In Engels JMM, Hawkes JG and Melaku W (eds) Plant Genetic Resources of Ethiopia. Cambridge University Press, Cambridge pp. 101-113.
Crossref
|
|
|
|
|
Demel T, Feyera S, Mark M, Million B, Pia B (2010). Edible wild plants in Ethiopia. Addis Ababa University Press, Addis Ababa, Ethiopia.
|
|
|
|
|
Desta F (2011). Phenotypic and nutritional characterization of anchote [Coccinia abyssinica (Lam.) Cogn] accessions of Ethiopia. M.Sc.Thesis, Jimma Univeristy, Jimma, Ethiopia.
|
|
|
|
|
Edwards S, Mesfin T, Hedberg I (1995). Flora of Ethiopia and Eritrea. National Herbarium, Addis Ababa University and Uppsala University, Sweden 2(2):456.
|
|
|
|
|
Ermias L, Zemede A, Ensermu K, Van Damme P (2011). Wild edible plants in Ethiopia: A review on their potential to combat food insecurity. Africa Focus 24(2):71-121.
Crossref
|
|
|
|
|
Folla B, Balcha A, Mezgebe G (2013). In vitro propagation of anchote (Coccinia abyssinica) (Lam.) Cogn.]. African Journal of Plant Science 7(6):253-264.
Crossref
|
|
|
|
|
Geleta D, Tileye F (2011). In vitro production of virus-free sweet potato [Ipomoea batatas (L.) Lam] by meristem culture and thermotherapy. Ethiopian Journal of Science 34(1):17-28.
|
|
|
|
|
George EF, Debergh PC (2008). Micropropagation: Uses and methods. In: George EF, Hall AM,De Klerk GJ (eds) Plant propagation by tissue culture, Third Edition, Vol. I. Published by Springer, Dordrecht, The Netherlands pp. 29-65.
Crossref
|
|
|
|
|
Girma A, Dereje H (2015). Yield and nutrient concentration of anchote [Coccinia abyssinica (Lam.) Cogn.] affected by harvesting dates and in-situ storage. African Journal of Crop Science 3(5):156-161.
|
|
|
|
|
Habtamu F, Fekadu B, Gullelat D (2013). Effect of traditional processing methods on nutritional composition and antinutritional factors of anchote [Coccinia abyssinica (Lam.) Cogn.] tubers grown in Western Ethiopia. Journal of Food Processing and Technology 4:1-8.
Crossref
|
|
|
|
|
Habtamu F, Kelbessa U (1997). Nutritional and antinutritional characteristics of anchote (Coccinia abyssinica). Ethiopian Journal of Health Development 11(2):163-168.
|
|
|
|
|
Ishag S, Osman MG, Khalafalla MM (2009). Effects of growth regulators, explant and genotype on shoot regeneration in tomato (Lycopersicon esculentum c.v. Omdurman). International Journal of Sustainable Crop Production 4(6):7-13.
|
|
|
|
|
Jane K, Peter N, Margaret K (2016). Improved Micropropagation of plantlets from nodal explants of anchote (Coccinia abyssinica) - A Calcium and protein-rich tuber. Hortscience 51(7):905-909.
Crossref
|
|
|
|
|
Jutta LM, Amy V, Christopher DT (2005). Analysis of indole-3-butyric acid-induced adventitious root formation on Arabidopsis stem segments. Journal of Experimental Botany 56(418):2095-2105.
Crossref
|
|
|
|
|
Loyola-Vargas VM, Vázques-Flota F (2006). Plant cell culture protocols. In: Loyola-Vargas VM, Vázquez-Flota F (eds) Methods in Molecular Biology. Second Edition. Humana Press Inc., New Jersey pp. 3-8.
Crossref
|
|
|
|
|
Mapayi EF, Ojo DK, Oduwaye OA, Porbeni JBO (2013). Optimization of in vitro propagation of Cassava (Manihot esculenta) genotypes. The Journal of Agricultural Science 5(3):261-269.
|
|
|
|
|
Murashige T, Skoog F (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Plant Physiology 15:473-497
Crossref
|
|
|
|
|
Piqueras A, Debergh PC (1999). Morphogenesis in micropropagation, morphogenesis in plant tissue cultures. Dordrecht. The Netherlands: Kluwer Academic Publisher.
Crossref
|
|
|
|
|
Rani V, Raina SN (2000). Genetic fidelity of organized meristem-derived micropropagated plants: A critical reappraisal. In Vitro Cellular & Developmental Biology 36(5):319-330.
Crossref
|
|
|
|
|
Reed BM (1999). Design a micropropagation system: Workshop presentation from the 1998 SIVB Congress on in vitro Biology. In Vitro Cellular and Developmental Biology Plant 35:275-284.
Crossref
|
|
|
|
|
Sanavy S, Moieni MJ (2003). Effects of different hormone combinations and planting beds on growth of single nodes and plantlets resulted from potato meristem culture. Plant Tissue Culture 13(2):145-150.
|
|
|
|
|
Sharmin SA, Kabir AH, Mandal A, Sarker KK, Alam MF (2008). In vitro propagation of eggplant through meristem culture. Agriculturae Conspectus Scientificus 73(3):149-155.
|
|
|
|
|
Shekhawat MS, Ravindran, CP, Manokari M (2014). Developmental and hormonal regulation of direct shoots and roots regeneration in Coccinia indica L. International Journal of Natural Sciences Research 2(7):103-112.
|
|
|
|
|
Slater A, Scott N, Fowler M (2003). Plant Biotechnology: The Genetic Manipulation of Plants. Oxford University Press Inc, New York. pp. 42.
|
|
|
|
|
Sridhar TM, Aswath CR (2014). Influence of additives on enhanced in vitro shoot multiplication of Stevia rebaudiana (Bert.): An important antidiabetic medicinal plant. American Journal of Plant Sciences 5:192-199.
Crossref
|
|
|
|
|
Thiripurasundari U, Rao MV (2012). Indirect organogenesis from nodal explants of Cocinia grandis (L.) Voigt. Indian Journal of Biotechnology 11:352-354.
|
|
|
|
|
Yasmin A, Jalbani A, Raza S (2011). Effect of growth regulators on meristem tip culture of local potato cvs Desiree and Patrones. Pakistan Journal of Agriculture, Agricultural Engineering and Veterinary Sciences 27(2):143149.
|
|
|
|
|
Yoseph Y, Tileye F (2013). Micropropagation of anchote [Coccinia abyssinica (Lam.) Cogn.]: High calcium content tuber crop of Ethiopia. African Journal of Agricultural Research 8(46):5915-5922.
|
|