ABSTRACT
Jatropha is a drought-tolerant plant producing seed oil for the biodiesel. Limitation to the development of jatropha is unavailability of high-yielding varieties and efficient in vitro regeneration system which is required for micropropagation. In this study, in vitro regeneration system from jatropha juvenile cotyledon was established. Firstly, concentrations of hormones in the MS medium were optimized and it was found that 1.5 mgL-1 benzyl adenine (BA) + 0.05 mgL-1 indole-3-butyric acid (IBA) + 0.5 mgL-1 thidiazuron (TDZ) turned out to be the best for shoot induction (3.82 ± 0.18 shoots/explant). Secondly, shoot induction medium was fortified with different concentrations of glutamine and adenine sulfate. It was found that 25 mgL-1 each of glutamine and adenine sulfate was the most effective, resulting to 9.09 ± 0.37 shoots/explant and 93.0% regeneration frequency. Regenerated shoots were cultured on medium containing 0.5 mgL-1 BA and different concentrations of gibberellic acid (GA3), 0.5 mgL-1 GA3 with 0.5 mgL-1 BA were found to be the best for shoot elongation (2.13 ± 0.18 cm). The highest frequency of root (40%) was observed on the medium with 0.5 mgL-1 IBA. The established procedures will be useful for the mass propagation and genetic transformation of elite jatropha genotypes.
Key words: Adenine sulfate (Ads), benzyl adenine (BA), indole-3-butyric acid (IBA), in vitro regeneration, glutamine (Gln).
Jatropha (Jatropha curcas) is a potent biofuel crop native to Mexico and Central America and now is distributed throughout tropical and subtropical regions (Kumar and Tewari, 2015). Its seed contains high amount of oil in the concentration of 30 – 60% (Openshaw, 2000; Kumar and Sharma, 2008), which is non-edible (Chhetri et al., 2008) and suitable for the biodiesel (Heikal et al., 2015). Due to the depletion of fossil fuel reserves, increasing petroleum prices and global climate changes, jatropha has received considerable attention as renewable energy sources (Pandey et al., 2012). However, the low productivity under certain conditions restricts fuel usage of jatropha, mainly because it has not been domesticated for large-scale production. Therefore, increasing oil yield, growing ability under abiotic stresses and improvement of agronomic traits must have a priority. Jatropha plant is propagated through asexual methods and by seeds, and thus seed yield and oil content varies significantly (Jha et al., 2007). Furthermore, seeds of J. curcas have a limited viability and can only be stored for 15 months after which their viabilities are reduced by 50% (Kochhar et al., 2005). Cuttings propagation of jatropha can be carried out for maintaining true to type genotypes, but the produced plants do not have deep roots and the quality is not sufficient to meet the growing demand of J. curcas (Heller, 1996; Openshaw, 2000). Additionally, propagated plants exhibited lower longevity and resistance to drought and diseases (Sujatha et al., 2005). Generally, vegetative propagation methods have drawbacks such as sources of disease transmission (Fufa et al., 2019). Therefore, an efficient in vitro regeneration for mass production of disease free and true to type jatropha genotypes is desirable. It can propagate superior genotypes and contributes to plant improvement through the application of biotechnological techniques.
Recently, several studies have been reported on the regeneration of jatropha by using various explants and different combinations of phytohormones and additives (Chiangmai et al., 2015; Gangwar et al., 2015; Jadon et al., 2015; Liu et al., 2015; Mishra, 2018; Fufa et al., 2019). BA and IBA were found to be effective growth regulators for the induction of callus and shoot regeneration from various explants of J. curcas plant. Previously in the laboratory, the suitable concentration of TDZ (0.5 mgL-1) for callus induction and combination of BA and IBA for shoot multiplication from juvenile of jatropha were established by Khemkladngoen et al. (2011). However, the low regeneration efficiency is a main obstacle to jatropha regeneration. Thus, this study aimed to investigate the effect of adding adenine sulfate and glutamine in shoot regeneration media on shoots multiplication. Here, we have developed an efficient in vitro regeneration protocol for shoots induction, multiplication and plant regeneration from juvenile cotyledons explants.
All experiments were conducted at Plant Bioengineering for Bioenergy Laboratory, Department of Biotechnology, Osaka University. Suita City, Osaka, Japan.
Plant materials and preparation of explants
Mature decorated seeds of jatropha (Thai line) were surface-sterilized with 70% (v/v) ethanol for 2 min followed by 40% (v/v) sodium hypochlorite and 0.01% Triton X-100 for 10 min, and then washed five times with sterile distilled water. After washing, surface-sterilized seeds were soaked overnight. The sterilized seeds were germinated in vitro on half strength hormone-free Murashige and Skoog (MS) medium (Murashige and Skoog, 1962) containing 3% (w/v) sucrose, 10 mgL-1 thiamine, 100 mgL-1myo-inositol (pH 5.8) and 0.8% agar at 25°C for one week. The juvenile cotyledons (Figure 1a) were used to prepare explants as described in Khemkladngoen et al. (2011). They were cut into pieces (3 mm x 3 mm) and used as explants (Figure 1b).
Callus induction
Explants were cultured on a callus induction medium consisting of MS medium supplemented with different concentration sets of benzyl adenine (BA: 1.0, 1.5 and 3.0 mgL-1) and indole-3-butyric acid (IBA: 0.5 and 0.05 mgL-1), as well as 0.5 mgL-1 thidiazuron (TDZ) and 0.8% (w/v) agar. Cultures were incubated at 25 ± 2°C under 16-h lights (31-35 µmol photon m-2 s-1)/ 8 h dark photoperiod for two weeks. The experiment was replicated twice.
Shoot regeneration
Two approaches were used in this study. Firstly, calli induced from cotyledon explants in the callus induction media were subcultured on MS medium supplemented with the same concentration sets of BA and IBA as the callus induction media. Secondly, calli induced from cotyledon explants in the callus induction medium containing 1.5 mgL-1 BA, 0.05 mgL-1 IBA and 0.5 mgL-1 TDZ were subcultured on MS medium supplemented with combination of 1.5 mgL-1 BA and 0.05 mgL-1 IBA in the presence of different concentrations of adenine sulfate and glutamine mixture (0, 5, 10, 15, 20 and 25 mgL-1 each). Cultures were incubated under the same condition as described above for 2 weeks and subcultured twice. The frequency of shooting response and shoots formed per explant were recorded. Each experiment was conducted twice.
Shoot elongation
The regenerated shoots were cultured on MS medium supplemented with 0.5 mgL-1 BA in combination with different concentrations (0.1 – 1.0 mgL-1) of gibberellic acid (GA3) for shoot elongation. Cultures were incubated under the same condition as described above for 4 weeks. The experiment was replicated twice.
Rooting and acclimatization
The elongated shoots were excised individually and transferred to rooting medium, which consisted of a half-strength B5 medium supplemented with 2% (w/v) sucrose, 10 mgL-1 thiamine, 100 mgL-1 myo-inositol, four concentrations of glutamine (0, 5, 10 and 15 mgL-1) in combination with 0.5 mgL-1 IBA and 0.7% agar (pH 5.8). Cultures were incubated under the same condition as described above for 6 weeks. Plantlets with rooted shoots were transplanted into autoclaved soil in small pots covered with transparent plastic lids and maintained under high humidity for 7 days, and thereafter gradually exposed to the growth chamber condition. Established plantlets were then transferred to plastic pots containing soil and cultivated in a growth chamber. Each experiment was replicated twice.
Statistical analysis
Data of shoot number per explant, percentage of explant response, shoot length, root number per explant and percentage of root shoot were collected. Recorded data were analyzed using one-way ANOVA and the mean separations were carried out using Tukey’s HSD test at P≤0.05. All statistical analysis was performed using SPSS 22.0 (SPSS Inc. USA).
Shoot induction and multiplication
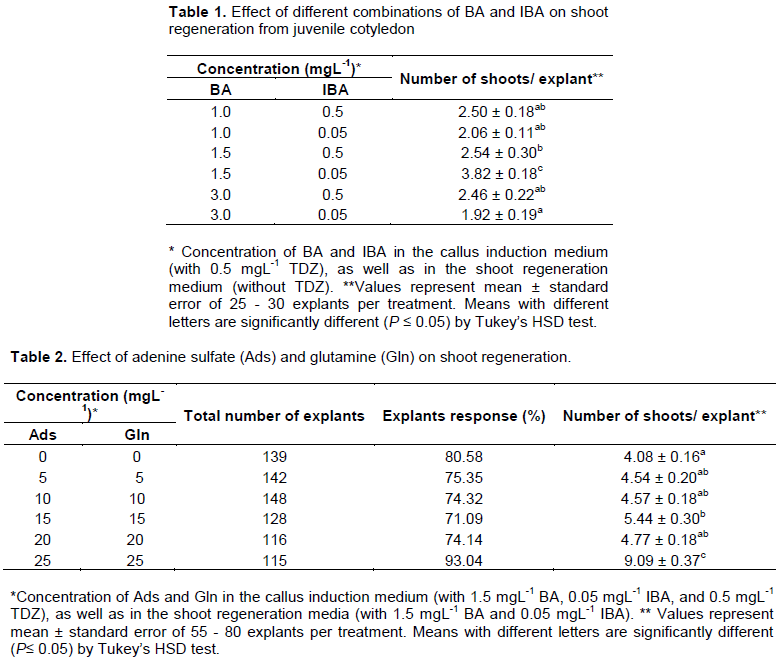
The results showed that BA and IBA affected the shoot regeneration with 0.5 mgL-1 TDZ, and the combination of 1.5 mgL-1 BA and 0.05 mgL-1 IBA was the most effective in regenerating shoots from calli (3.82 ± 0.18 shoots/explant) (Table 1 and Figure 1c, d). This finding was similar to previous results reported by Chiangmai et al. (2015), Nanasato et al. (2015) and Fufa et al. (2019). The study was divergent from previous study in our lab by Khemkladngoen et al. (2011) which showed that the combination of 3 mgL-1 BA and 0.1 mgL-1 IBA produced the highest regeneration frequency from calli due to genotype difference.
Low shoot multiplication rate is a major constraint facing in vitro regeneration protocol of jatropha plant. Evaluation on the synergistic effect of glutamine and adenine sulfate on shoot regeneration and multiplication was further studied. The effectiveness of organic nitrogen source particularly glutamine for multiplication and maintenance of healthy in vitro tissue for long time periods have been reported in other plant species (Green et al., 1990; Ogita et al., 2001; Vasudevan et al., 2004; Sanjaya et al., 2005). The synergistic effects of adenine sulphate and cytokinin on stimulating cell growth and enhancing shoot formation of Holarrhena antidysenterica were observed by Raha and Roy (2001). The simulative role of adenine sulfate in shoot multiplication was emphasized in different woody species such as Melia azedarach (Husain and Anis, 2004), Bauhinia vahlii (Dhar and Upreti, 1999), and Petrocarpus marsupium (Husain et al., 2008). Several studies showed the enhancement of shoot multiplication of jatropha in MS medium containing BA and IBA fortified by adenine sulfate and glutamine (Maharana et al., 2012; Samson et al., 2013; Mishra, 2018; Hegazi et al., 2020). The inclusion of glutamine and adenine sulfate exhibited significant effect on shoot multiplication. Among the different concentrations evaluated, 25 mgL-1 each of glutamine and adenine sulfate was the most effective for shoot regeneration. When comparing with the media without glutamine and adenine sulphate (4.08 ± 0.16 shoots/explant), the addition of 25 mgL-1 each of glutamine and adenine sulfate resulted in more than two-fold increase in the shoot number (9.09 ± 0.37 shoots/explant) (Table 2 and Figure 1e, f). The enhancement of shoot multiplication might be due to synergistic effect of glutamine and adenine sulfate. Thus, the results were similar with previous reports by Maharan et al. (2012), Samson et al. (2013), Mishra (2018) and Hegazi et al. (2020) in jaropha plant which revealed that the addition of glutamine and/or adenine sulphate significantly enhanced shoot multiplication.
Shoot elongation
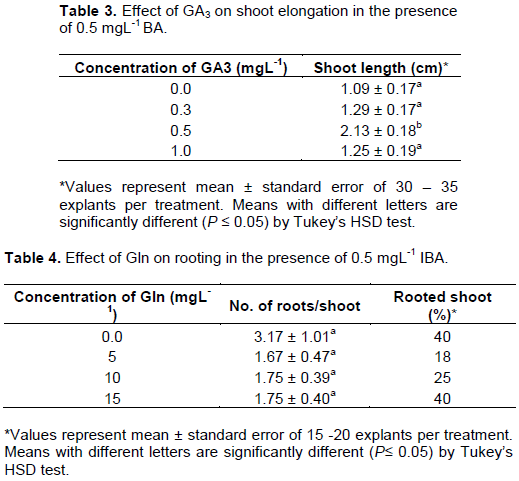
The physiological role of GA3 is well known on shoot elongation and widely used for in vitro regeneration of different plants (Machado et al., 2011; Gonbad et al., 2014; Padrón et al., 2020). The results showed that GA3 at the concentration of 0.5 mgL-1 significantly improved shoot elongation of jatropha (2.13 ± 0.18 cm) (Table 3 and Figure 1g). The result was consistent with the results reported recently by Amiri and Mohammadi (2021) when they used the combination of BA and GA3 for in vitro regeneration of Sumac plant. The established regeneration protocol of jatropha in our lab by Khemkladngoen et al. (2011) did not examine the effects of GA3 shoot elongation. The results of this study indicated that GA3 remarkably improved shoot elongation of regenerated shoots.
Rooting and acclimation
The promontory effect of IBA on in vitro rooting of jatropha shoots was reported previously (Deore and Johnson, 2008; Singh et al., 2010; Sharma et al., 2011). Jatropha is recalcitrant for in vitro regeneration at exactly rooting and acclimatization (Pankaj and Divay, 2011). Recently, Liu et al. (2015) found that the addition of glutamine to the medium in the presence of IBA effectively stimulated the initiation and growth of roots in jatropha and 16 mgL-1 of glutamine exhibited the best rooting rate (51.72%). The highest rooting induction efficiency (40%) was observed in medium containing 15 mgL-1 of glutamine, as well as that without glutamine (Table 4; Figure 1h, i). This result showed that the addition of glutamine did not significantly affect rooting efficiency, which might be due to in vitro elongated shoots that were regenerated in medium containing glutamine. Rooted plantlets were transplanted and acclimatized successfully to the soil for 3 weeks. The acclimatized plants exhibited normal morphological growth (Figure 1j, k).
The study described the enhanced in vitro regeneration protocol of jatropha from juvenile cotyledon. Explants were cultured on shoot induction medium fortified with different concentrations of adenine sulfate and glutamine. The highest number of shoots per explant with high regeneration frequency was achieved in shoot induction medium fortified by 25 mgL-1 each of adenine sulfate and glutamine. The results also showed the highest shoot multiplication rate in jatropha plant that has been never done before. Enhanced in vitro regeneration protocol would be useful for large-scale production and Agrobacterium-mediated transformation of elite jatropha genotypes.
The authors have not declared any conflict of interests.
REFERENCES
Amiri S, Mohammadi R (2021). Establishment of an efficient in vitro propagation protocol for Sumac (Rhus coriaria L.) and confirmation of the genetic homogeneity. Scientific Reports 11(1):173.
Crossref
|
|
|
Chhetri AB, Tango MS, Budge SM, Watts KC, Islam MR (2008). Non-edible plant oils as new sources for biodiesel production. International Journal of Molecular Sciences 9(2):169-180.
Crossref
|
|
|
|
Chiangmai PN, Pootaeng OY, Meetum P, Kamkajon K, YuiamW, Rungphan N, Ninkaew P (2015). Regeneration of adventitious shoots from callus and leaf explants in Jatropha curcas L. 'Phetchaburi'. Silpakorn University Science and Technology Journal 9(1):28-39.
|
|
|
Deore AC, Johnson TS (2008). High-frequency plant regeneration from leaf-disc cultures of Jatropha curcas L. an important biodiesel plant. Plant Biotechnology Reports 2(1):10-15.
Crossref
|
|
|
Dhar U, Upreti J (1999). In vitro regeneration of a mature leguminous liana (Bauhinia vahlii) (Wight and Arnott). Plant Cell Reports 18(7):664-669.
Crossref
|
|
|
Fufa H, Tesema M, Daksa J (2019). In vitro regeneration protocol through direct organogenesis for Jatropha curcas L. (Euphorbiaceae) accessions in Ethiopia. African Journal of Biotechnology 18(31):991-1003.
Crossref
|
|
|
|
Gangwar M, Sharma S, Chauhan RS, Sood H (2015). Indirect shoot organogenesis in Jatropha curcas (L.) for in vitro propagation. Indian Journal of Research 4(12):56-58.
|
|
|
Gonbad RA, Sinniah UR, Abdul Aziz M, Mohamad R (2014). Influence of Cytokinins in Combination with GA3 on Shoot Multiplication and Elongation of Tea Clone Iran 100 (Camellia sinensis (L.) O. Kuntze). The Scientific World Journal 2014:9.
Crossref
|
|
|
Green B, Tabone T, Felker P (1990). A comparison of amide and ureide nitrogen sources in tissue culture of tree legume Prosopis alba clone B2 V50. Plant Cell, Tissue and Organ Culture 21:83-86.
Crossref
|
|
|
|
Hegazi GA, El-Hanafy NA, Mohamed AMA, Abu-Elkheir ZA (2020). In vitro regeneration of the biofuel crop Jatropha curcas. Plant Archives 20(2):2122-2127.
|
|
|
Heikal E, Khalil S, Abdou I (2015). Biodiesel from Jatropha Oil. In: Sayigh A. (eds) Renewable Energy in the Service of Mankind Vol I. Springer, Cham.
Crossref
|
|
|
|
Heller J (1996), Physic nut. Jatropha curcas L. Promoting the conservation and use of underutilized and neglected crops. 1. Institute of Plant Genetics and Crop Plant Research, Gatersleben/ International Plant Genetic Research's Institute, Rome.
|
|
|
|
Husain MK, Anis M (2004). In vitro proliferation of shoots of Melia azedarach L. from mature trees. In: D'Souza L, Anuradha M, Nivas S, Hegde S, Rajendera K eds- Biotechnology for a better future. SAC, Mangalore pp. 294-301.
|
|
|
Husain MK, Anis M, Shahzad A (2008). In vitro propagation of a multipurpose leguminous tree (Pterocarpus marsupium Roxb.) using nodal explants. Acta Physiologiae Plantarum 30(3):353-359.
Crossref
|
|
|
|
Jadon S, Singh V, Shrivastava N, Wahi N, Bhadauria A (2015). Micropropagation of Jatrophacurcas L. with different hormonal treatmentsIndian Research Journal of Genetics and Biotechnology 7(1):35-40.
|
|
|
Jha BT, Mukherjee P, Datta MM (2007). Somatic embryogenesis in Jatropha curcas Linn. an important biofuel plant. Plant Biotechnology Reports 1(3):135-140.
Crossref
|
|
|
Khemkladngoen N, Cartagena J, Shibagaki N, Fukui K (2011). Adventitious shoot regeneration from juvenile cotyledons of a biodiesel producing plant Jatropha curcas L. Journal of Bioscience and Bioengineering 111(1):67-70.
Crossref
|
|
|
Kochhar S, Singh SP, Kochhar VK (2005). Effect of auxins and associated biochemical changes during clonal propagation of the biofuel plant Jatropha curcas. Biomass and Bioenergy 32(12):1136-1143.
Crossref
|
|
|
Kumar A, Tewari SK (2015). Origin, Distribution, Ethnobotany and Pharmacology of Jatropha curcas. Research Journal of Medicinal Plant 9(2):48-59.
Crossref
|
|
|
Kumar A, Sharma S (2008). An evaluation of multipurpose oil seed crop for industrial uses (Jatropha curcas L.): a review. Industrial Crops and Products 28(1):1-10.
Crossref
|
|
|
Liu Y, Tong X, Hui W, Liu T, Chen X, Li J, Zhuang C, Yang Y, Liu Z (2015). Efficient culture protocol for plant regeneration from petiole explants of physiologically mature trees of Jatropha curcas L. Biotechnology & Biotechnological Equipment 29(3):479-488.
Crossref
|
|
|
Machado MP, da Silva ALL, Biasi LA (2011). Effect of plant growth regulators on in vitro regeneration of Lavandula dentata L. shoot tips. Journal of Biotechnology and Biodiversity 2(3):28-31.
Crossref
|
|
|
|
Maharana S, Vivekananda M, Motilal B, Ramya M, Jogeswar P (2012). In vitro regeneration from node and leaf explants of Jatropha curcas L. and evaluation of genetic fidelity through RAPD markers. Indian Journal of Biotechnology 11(3):280-287.
|
|
|
|
Mishra S (2018). In-vitro direct shoot organogenesis in Jatropha curcas L. Journal of Pharmacognosy and Phytochemistry 7(2):1777-1780.
|
|
|
Murashige T, Skoog F (1962). A revised medium for rapid growthand bioassays with tobacco tissue cultures. Physiologia Plantarum 15(3):473-497.
Crossref
|
|
|
Nanasato Y, Kido M, Kato A, Ueda T, Suharsono S, Widyastuti U, Tsujimoto H, Akash K (2015). Efficient genetic transformation of Jatropha curcas L. by means of vacuum infiltration combined with filter-paper wicks. In Vitro Cellular & Developmental Biology-Plant 51(4):399-406.
Crossref
|
|
|
Ogita S, Sasamoto H, Yeung EC, Thorpe TA (2001). The effects of glutamine on the maintenance of embryogenic cultures of Cryptomeria japonica. In Vitro Cellular & Developmental Biology-Plant 37(2):268-273.
Crossref
|
|
|
Openshaw K (2000). A review of Jatropha curcas: an oil plant unfulfilled promise. Biomass and Bioenergy 19(1):1-15.
Crossref
|
|
|
Padrón IES, Meza PMP, Díaz CML (2020). Evaluation of sucrose and GA3 in an in vitro shoot culture of Alpinia purpurata (Zingiberaceae). Ciencia y Tecnología Agropecuaria 21(2):e1193.
Crossref
|
|
|
Pandey VC, Singh K, Singh JS, Kumar A, Singh B, Singh RP (2012). Jatropha curcas: a potential biofuel plant for sustainable environmental development. Renewable and Sustainable Energy Reviews 16(5):2870-2883.
Crossref
|
|
|
|
Pankaj K, Divay G (2011). Plant tissue culture of Jatropha curcas L.: a review. Imperial Journal of Pharmacognosy & Natural Products 1(1):2248-9754.
|
|
|
Raha S, Roy SC (2001). In vitro plant regeneration in Holarrhena antidysenterica Wall. through high frequency axillary shoot proliferation. In Vitro Cellular & Developmental Biology-Plant 37(2):232-236.
Crossref
|
|
|
|
Samson DM, Guy M, Philippe D, Jean PB, André T (2013). In Vitro Micropropagation of Jatropha curcas L. from Bud Aggregates. Journal of Technology Innovations in Renewable Energy 2:145-154.
|
|
|
Sanjaya T, Rathore S, Ravishanker Rai V (2005). Micropropagation of Pseudoxytenanthera stocksii Munro. In Vitro Cell. In Vitro Cellular & Developmental Biology-Plant 41(3):333-337.
Crossref
|
|
|
Sharma S, Kumar N, Reddy MP (2011). Regeneration in Jatropha curcas: factors affecting the efficiency of in vitro regeneration. Industrial Crops and Products 34(1):943-951.
Crossref
|
|
|
Singh A, Reddy MP, Chikara J, Singh S (2010). A simple regeneration protocol from stem explants of Jatropha curcas- a biodiesel plant. Industrial Crops and Products 31(2):209-213.
Crossref
|
|
|
Sujatha M, Makkar H, Becker K (2005). Shoot bud proliferation from axillary nodes and leaf sections of non-toxic Jatropha curcas L. Plant Growth Regulation 47(1)83-90.
Crossref
|
|
|
Vasudevan A, Selvaraj N, Ganapathi A, Kasthurirengan S, Ramesh Anbazhagan V, Manickavasagam M (2004). Glutamine: a suitable nitrogen source for enhanced shoot multiplication in Cucumis sativus L. Biologia Plantarum 48(1):125-128.
Crossref
|