ABSTRACT
Schistosomiasis is the second most prevalent tropical parasitic disease after malaria, and one of the leading cause of morbidity and mortality in developing countries especially in Africa. This study was carried out among students, farmers and fishermen/women from four villages in the Central River Region of The Gambia. The aim of this study was to show the prevalence value of schistosomiasis in Central River Region of The Gambia. Questionnaires were administered to acquire data such as age, gender, prior schistosomiasis infection and treatment. One hundred and ninety-five blood and 192 urine samples collected from 117 females and 78 males were examined. Microscopy, ELISA and Polymerase Chain Reaction (PCR) techniques were used to detect and characterize schistosome isolates from the biological samples. Prevalence of Schistosoma haematobium was 28.7 with 41.0% in males and 23.9% in females. The highest prevalence value among the villages was in Brikama Ba with 53.1% while the age group 6-15 years had the highest prevalence of 50.0%. Schistosoma mansoni was only detected in Jahally village (1.5%). Schistosomiasis detection was highest using ELISA (40%) and lowest using microscopy (24.5%). PCR gave a 28.7% prevalence value.
Key words: Prevalence, Schistosoma, diagnostic techniques, Central River Region.
Schistosomiasis is a chronic disease also well known as bilharzia or snail fever, parasitic flukes of the genus Schistosoma cause it. It remains extremely prevalent in many low-income and middle-income countries (Steinauer et al., 2008; Colley et al., 2014; Adenowo et al., 2015: Oboh et al., 2018; Li et al., 2019). The estimated global prevalence showed that at least 229 million people required preventive treatment in 2018 and more than 97.2 million people were reported to have been treated (GBD, 2016; WHO, 2017, 2020). The name bilharzia was coined from the name of Theodor Bilharz, a German surgeon. He was the first to recognize the etiological agent Schistosoma haematobium in 1851 while working in Cairo, Egypt (Nour, 2010).
Schistosomiasis is one of the neglected tropical diseases (NTDs) in Africa. NTDs are hidden epidemics of enormous health and economic significance for African countries (Adenowo et al., 2015; Li et al., 2019). They are hidden because many African countries are unable to establish and address the associated health issues due partly to inadequate resources for an appropriate understanding of the biological and social characteristic of these diseases and they have been mostly wiped out in the more developed parts of the World also (Adenowo et al., 2015). Schistosomiasis is the second most prevalent tropical parasitic disease after malaria, and is a leading cause of morbidity and mortality for developing countries especially in Africa (Adenowo et al., 2015). Adult schistosomes are white or greyish worms of 7-20 mm in length with a cylindrical body that features two terminal suckers, a complex tegument, a blind digestive tract, and reproductive organs (Gryseels et al., 2006; Adenowo et al., 2015). Infection is acquired by exposure to fresh water that contains cercariae (the parasitic form that is infective for final host) released by infected snails (the intermediate host) (Corachan, 2002; Ntounifor and Ajayi, 2007). The transmission cycle needs contamination of surface water by excreta, specific freshwater snails as intermediate hosts, and human water contact (Gryseels et al., 2006). Numerous species of Schistosoma are pathogenic parasites of humans: S. haematobium is responsible for urinary schistosomiasis. Schistosoma mansoni, Schistosoma japonicum, Schistosoma mekongi and Schistosoma malayi are responsible for gastrointestinal (GI) and hepatosplenic schistosomiasis. Schistosoma intercalatum affects the GI tract but are associated with a lower morbidity. Schistosoma matheei and Schistosoma bovis are occasional parasites of human and Schistosoma incognitum may also show infective strains to humans (Corachan, 2002; Oboh et al., 2018). The species differ in their final location in the human host, the species of the intermediate host(snail), the pathology they induce, and the number, size and shape of the eggs produced (IARC, 2012). Schistosoma transmission is extremely dependent on environmental conditions, mainly those affecting the snail host. Climate change modifies aquatic environments and subsequently the transmission and distribution of waterborne diseases (Mas-Coma et al., 2009). Schistosomiasis is typically found in poor rural communities, where fishing and agricultural activities are dominant. Domestic and recreational activities such as clothes washing, water fetching and swimming expose women and children to infection (WHO, 2017). The river Gambia is at the focus of activities for people living in the Central River Region (CRR). Folks living in these communities wash their clothes and take a bath in the river. This region has numerous fresh water bodies and the main irrigated rice fields of the country. The river is fresh water hence the high prevalence of Schistosoma in the region (Gambia NTD Mapping Report, 2015; Sanneh et al., 2017). The Gambian Government depends on the region to reach its goal of rice self-sufficient production among other agricultural development objectives. The high prevalence of schistosomiasis hamper the attainment of these goals. Most of the children in these regions help their parents in farming, when they are sick, their parents will have to stop farming and look after these children which lead to a double losses for them. Laboratories in The Gambia use stool to detect S. mansoni. This specie is sometimes challenging to detect as most people are unwilling to take their stool to the laboratories for testing causing late detection and subsequently leads to severe schistosomiasis. This can give rise to bladder cancer, damaged of organs and development issues in children (WHO, 2017). Earlier studies on the prevalence of schistosomiasis in humans were done using only microscopy in The Gambia (Gambia NTD Mapping Report, 2015). The prevalence of schistosomiasis in The Gambia is put at 4.3%, with a higher prevalence in the Central River Region (CRR) at 14.2% followed by Upper River Region at 9.4% (Gambia NTD Mapping Report, 2015). The River Gambia remains fresh throughout the year in these two regions. The two regions also have several fresh water bodies and are the major irrigated rice fields in the country. However, all other regions apart from North Bank East Region (NBER) are also endemic for schistosomiasis (Gambia NTD Mapping Report, 2015; Sanneh et al., 2017). The Government of The Gambia needs to embark on a nationwide sensitization work since, the awareness level about schistosomiasis continues to be very low, and this may imply that a great deal of the Government’s pledge to schistosomiasis control programs is extremely needed (Barrow et al., 2020). Microscopy as a detection tool for Schistosoma is not only associated with sensitivity limitation but it is also highly unspecific as it cannot characterize the parasite into species and sub-species level. To the best of our knowledge, no investigation/research has been conducted using molecular techniques or immunological tests in The Gambia. Molecular tools which are the most sensitive and specific tools will be used to detect S. mansoni in blood and urine. However, effective and efficient surveillance systems need to be put in place to curb the spread of this vector borne diseases across The Gambia (Kargbo and Kuye, 2020). The study aims of this study were to show the prevalence of schistosomiasis in the Central River Region of The Gambia.
Study area
This study was done in the Central River Region of The Gambia from March to April 2017 (Figures 1 and 2). This region is divided into two locations, north and south. It is separated by the river Gambia, four villages were selected at random from both locations of the region; Jahally (13°33` 40.09``N 14°58’19.53``W), Wali Kunda (13°34` 0``N 14°53` 0``W), Brikama Ba (13°32` 11.96``N 14°55 53.78`W), Kuntaur (13°40` 14.74``N 14°53` 23.90``W). Children (both sex) from the ages of 6-15 were randomly selected. Children less than 6 years old were not included in the study. Farmers and Fishermen/women were also selected using simple random sampling.
Sampling
The sample size was calculated using the formula described by Thrusfield (2007). The prevalence of Schistosoma used in CRR was 14.2% (Gambia NTD Mapping Report, 2015). A total of 188 samples were collected.
Ethical clearance
Ethical clearance was attained from the Scientific Coordinating Committee of The Gambia government and Medical Research Council Joint Committee. Before sample collection, permission was obtained from the Ministry of Basic and Secondary Education as well. The study was described to each participant for their understanding and cooperation. Moreover, an informed written consent form was signed by each study participant, and for the children, consent was obtained from their parents/guardians.
Microscopy of urine samples
Urine samples were collected in 50 ml conical tubes between the hours of 10.00 and 14.00 when schistosome eggs excretion is known to be highest (Obeng et al., 2008; Orsot et al., 2018). All the urine samples were examined and recorded as either clear amber, clear and cloudy or bloody before centrifugation for microscopy. These samples were examined by centrifugation technique (Ukaga et al., 2002). Ten ml of each urine sample was centrifuged at 1,500 rpm) for five minutes. The supernatant was decanted and the sediment examined under the microscope. Pasture pipette was used to add two drops of the sediment to a frosted microscope slide and covered with a cover slip slightly without the formation of air bubbles. The slide was examined under the microscope at low magnification (x10 and x40 objective lenses) for the presence of eggs of S. haematobium and S. mansoni. For quality control, duplicate slides were prepared for all samples. An experienced microscopist read all the positive slides and 10% of the negative (Sousa et al., 2019).
ELISA test of blood samples
Blood samples were obtained by the finger prick method. The finger was cleaned with alcohol wipes and left for about three seconds to dry. Then using a pricker, the finger was pricked and gently squeezed to drop blood on the spots created on the filter paper. The spotted papers were left to dry away from direct sunlight packed in a sealable bag with desiccants and kept at ambient temperature for transportation to the Department of Biochemistry at Ahmadu Bello University Zaria in Nigeria. A regular paper puncher was used to cut 6 mm size paper discs from each filter paper and put in a well of the ELISA plate. To each sample in a well, 300 ml of phosphate buffer saline (PBS) containing 0.05% Tween 20 was added and incubated overnight in a refrigerator at 4°C (Gruner et al., 2015). Fifty microliters of the elute was used to test for IgG antibody response directed against schistosome egg antigens using Schistosoma IgG ELISA Kit. The ELISA plates are pre-coated with Schistosoma antigens. On adding of the elutes to the plate, antibodies in the samples were bonded to the antigens in the test well during the first incubation. After washing, enzyme conjugate was added and incubated at room temperature to allow the enzyme complex to bind to the antigen-antibody complex. After a few washings to remove unbound enzymes, a substrate was added that developed a blue coloration in the presence of the enzyme complex and peroxide. The plate was read on an ELISA plate reader at 450 nm with a reference filter at 620 nm (AccuDiag™ Schistosoma IgG ELISA Kit pamphlet).
DNA extraction from dried blood samples
A regular paper puncher was used to cut 6 mm discs from each blood soaked filter paper and put in a labelled 1.5 ml Eppendorf tube. Between the cuttings, the paper puncher was cleaned with 70% ethanol and allowed to dry to avoid contamination. 500 µl of phosphate buffer saline was added to the tubes and incubated (Lodh et al., 2013). The DNA was extracted using Quick-DNA Miniprep Plus Kit (ZYMO RESEARCH) according to the manufacturer’s protocol. The DNA concentration was measured using the NanoDrop spectrophotometer. Extracted DNA samples were stored at −20°C until required.
Molecular polymerase chain reaction (PCR) identification
Nested schistosome-specific PCR was performed using the DNA extracted from each blood sample. Each of the samples was tested for both S. mansoni and S. haematobium using specifically designed primers to amplify variable regions 600 and 770 bp within cox1mitochondrial DNA (mtDNA) of S. mansoni and S. haematobium respectively. For the S. haematobium, the schistosome cox1 mitochondrial DNA (mtDNA) region was amplified using an outer primer for S. haeamtobium as follows Sh1 (5′-CGTATTTTAGGTTTATGG-3′) Sh2 (5′-CGAACTACACTTCCTAAGCA-3′) and inner primer Sh3 (5′- CGTGGTTTCATTAGATGTTTA-3′) with inner reverse primer Sh4 (5′- CGACAAATCAATCCATAATAC-3′). For S. manson, the PCR was carried out by using outer forward primer Sm1 (5`- CGTTGATTAAGAAGATTATGA-`3) with outer reverse primer Sm2 (5`-CGTGAAATTGACAGATCCA-`3), and inner forward primer Sm3 (5`-ATGTTACGATGTCTGTTCGGT-3`) with inner reverse primer Sm4 (5`- CGATAAAGGAGGATATAGAGTTC-3`). The cox1 mitochondria DNA gene was used as a DNA barcode to detect animal species because its mutation rate is often fast enough to distinguish between closely related species. It is highly abundant in the cell and has highly conserved regions and structures among con-specifics (Sady et al., 2015).
PCR amplification was performed in 25 μl reaction mixture and consisted of 14.75 μl of nuclease free water, 2.5 μl of PCR buffer containing 25 mM MgCl2, 2.5 μl of dNTPs, and 0.5 μl of each of the primers, 0.25 μl of Taq polymerase and 4 μl of DNA. The outer primers were used for the first round of the nested PCR with the following PCR cycling conditions for the first reaction was: an initial denaturing step of 95°C for 5 min, followed by 30 cycles of 95°C for 60 s, the annealing temperature of 60°C for 30 s, elongation at 72°C for 45 s and final extension at 72°C for 4 min. The second round in which 4 μl of the products of the first round was used as DNA, with the inner primers and the same volume of the other components (nuclease free water, PCR buffer dNTPs and Taq polymerase with the following PCR conditions: an initial denaturing step of 95°C for 5 min followed by 30 cycles of 95°C for 60 s, the annealing temperature of 55°C for 30 s, elongation at 72°C for 45 s and final extension at 72°C for 4 min. The alteration in annealing temperature was due to the expected amplicon size; for the outer primers it is expected to be around 770 bp whiles for the inner, it is around 600 bp. Amplicons were visualized on a 1% agarose gel stained with Ethidium bromide using 100 bp ladder to estimate band sizes.
Statistical analysis
The prevalence of schistosomiasis in the area was calculated. The Chi-squared test was used to determine the association between factors and prevalence. Odds ratio (OR) was determined to show the association between the factor and infection rate/prevalence. Overall, age and sex specific prevalence of the disease was calculated and expressed as a percentage (%).
Analysis of demographic distribution of participants
Age distribution of the study participant (Figure 3) shows that adults of the age bracket 16-30 years constituted almost half of the participants from which blood and urine sample were obtained. Children of school age were about one-quarter while those above 30 years represented the remaining. Analysis of the occupational status of the participants also showed that farmers formed approximately half of the participants, with students and fishers each accounting for about one quarter (Figure 4). A large proportion of the population from Jahally village had not received any drug treatment for schistosomiasis.
Macroscopic results
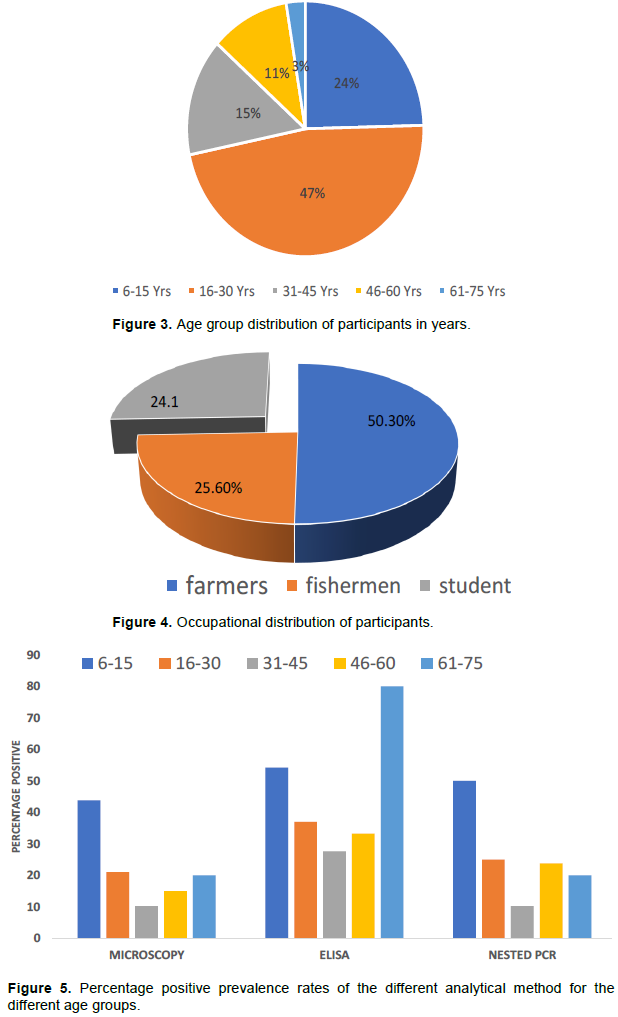
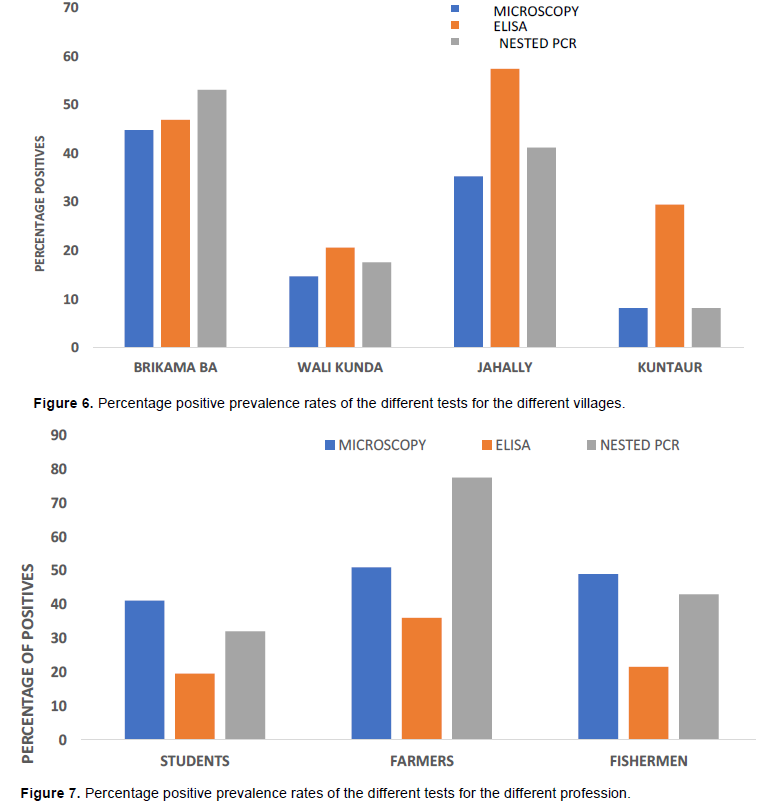
Urine examination showed that Amber clear urine (44.8%) was more frequent than cloudy urine (39.9%) or bloody (10.9%) urine with clear urine (4.7%) being less frequent. Microscopy revealed an overall prevalence of 24.5% (47 of 192) for S. haematobium infection in the urine samples examined in this study (Figure 5). Figure 6 shows that, Brikama Ba recorded the highest prevalence among the four villages with 44.8% (13 of 29) whiles Kuntaur had the lowest prevalence with 8.2% (5/61). Jahally had a prevalence of 35.3% (24 of 68) and Wali Kunda 14.7% (5 of 34). The prevalence was higher in males (33.3%) than in females (18.8%). The infection distribution by age shows that school children from the ages of 6 to 15 years have the highest prevalence as shown in Figure 7 with 43.8% (21 of 48) followed by age group 16-30 years with 21.1% (19 of 90). The oldest age groups 61-75 years has a prevalence of 20% (1 of 5) and 46-60 years recorded 15% (3 of 20). The lowest prevalence occurred in the age group 31-45 years with 10.3% (3 of 29) (Figure 3). In terms of occupation, fisherwoman have the lowest prevalence with 14.8% (4 of 27) whiles fishermen recorded 23.5% (3 of 18). Students had the highest prevalence with 41.2% (21 of 51) followed by farmers with 19.6% (19 of 97) as indicated in figure 4. 28.8% (44 of 153) of those that were positive had never received drug (praziquantel) for treatment of schistosomiasis. In comparison 7.7% (3 of 49) had recently been treated with praziquantel. Statistical analysis (Table 2) showed that there was a statistically significant difference in prevalence between males and females and between all age groups (P<0.05).
Nested PCR
There was a prevalence of 28.7% (56 of 195) based on the PCR results for S. haematobium. Brikama Ba had the highest prevalence among the four villages with 53.1% (17 of 32) while Kuntaur had the lowest prevalence 8.2% (5 of 61). Jahally has a prevalence of 41.2% (28/68) whiles Wali Kunda had a prevalence of 17.6% (6 of 34). Plate 1 shows some of the bands at about 700 bp for S. haematobium for blood samples while Plate 2 shows the bands for S. mansoni in urine samples corresponding to band 600 bp. The infection distribution by age shows that school children from the ages of 6-15 had the highest prevalence of 50.0% (24 of 48) followed by age group 16-30 years with 25.0% (23 of 92). Age group 46-60 years had a prevalence of 23.8% (5 of 21). The oldest age group 61- 75 years had a prevalence of 20% (1 of 5) while the age group 31-45 years had the lowest prevalence with 10.3% (3 of 29) as shown in Table 1. In terms of occupation, fisherwomen have the lowest prevalence with 19.2% (5 of 26) whiles fisherman had a prevalence of 23.8% (5 of 21). Students had the highest prevalence with 49.0% (25 of 51) and farmers with a prevalence of 21.6 % (21 of 97). 34.0% (53 of 156) of those that are positive have never received any drug (praziquantel) for treatment of schistosomiasis whilst 7.7% (3 of 39) have recently received praziquantel. Generally, a higher infection was recorded among males 41.0% (32/78) than in females 23.9% (28 of 117). Statistical analysis showed that there was a statistically significant difference in incidence between males and females (P<0.05) as well as in all other risk factors (age, the drug received and occupation). Table 2 shows the distribution of the schistosoma species detected in the urine and blood sample. While S. mansoni was detected only in urine samples from Jahally, S. haematobium was identified in the blood samples from four villages. The highest percentage of S. haematobium was in Brikama while the lowest was in Kuntaur.
ELISA
ELISA revealed an overall prevalence of 40.5% (79 of 195) for Schistosoma and this method was the most sensitivity method when compared to microscopic and molecular detection methods used in this study Figure 5. Jahally had the highest prevalence with 57.4% (39 of 68) and Wali Kunda with the lowest prevalence with 20.6% (7 of 34). Brikama Ba has an incidence of 46.9% (15 of 32) as against 29.5% for Kuntaur. Statistical analysis on Table 3 shows that there was a statistically significant prevalence difference between male and female (P<0.05). Chi-square test showed that there was no significant difference between the prevalence rates of the different age groups (P>0.05).
This study revealed that there is endemicity of S. haematobium in CRR of The Gambia with a prevalence of 28.7% among school children, farmers and fishermen/women. This is in agreement with findings from the previous study that revealed high transmission rate of 14.2% for S. haematobium infection in CRR (Gambia NTD Mapping Report, 2015). The higher prevalence obtained in this study could be due to the fact the PCR used in this study is more sensitive and can detect the Schistosoma in all stages of infection (Corcoran and da Silva, 2014) compared with microscopy that was employed in the diagnosis and characterization of the Schistosoma species in previous studies. The lowest prevalence of schistosomiasis recorded in Kuntaur may be attributed to mass administration of anti-schistosomal drug carried out in early 2017, few weeks before the start of sample collection in this village. Questionnaire survey attested to the fact that, 60.7% of people in this village benefited from the mass drug administration held in early 2017. The other villages were not included in the mass drug administration. The study also revealed a higher prevalence in males compared with their female counterpart. There was a significant association between the disease and sex. This result is similar to the NTD mapping study done in The Gambia which showed a higher prevalence in male than in female (Gambia NTD Mapping Report, 2015). A similar survey in Senegal also showed a higher prevalence in boys than in girls (Senghor et al., 2014). This could be because males (especially the students) have more frequent contacts with water than females because in traditional African settings, young females are more associated with indoor activities than their male counterpart. In the study population, 73.9% of the positive students (6-15) are boys. Boys are fond of going to the streams and ponds to swim, wash domestic animals, bath etc. Generally, prolonged and more frequent contact with water causes more exposure to the snail intermediate hosts in the water bodies. Since the villages have pipe-borne water, the girls usually stay at home and generally use tap water for housework, thus reducing their contacts with other water sources. It is for this difference in their exposure to water bodies where the snail intermediate vectors habit that may explain the differences in the infection rates rather than their gender differences. The prevalence of infection in age groups 6-15 and 16-30 shows that there is a significant association between the age and prevalence of the disease. The higher prevalence among the age group 6- 15 years is most likely because they spend more time in the water swimming, bathing, washing, fishing and other water activities. On the other hand, there was no association in the age group of 61-75 years. The association between praziquantel administration and prevalence of schistosomal infection is aptly demonstrated in this study. Communities which had received mass drug administration against schistosomal disease recorded much lower incidence as compared with those had did not. This shows that praziquantel protects against Schistosoma in the region although there is still a considerable level of infection persistence. This find was similar to that of Woldegerima et al. (2019), who showed that the used 40 mg/kg of Praziquantel against S. mansoni was highly efficient in the elimination and control of Schistosomiasis. The cure rate was not associated with age, occupation or gender. Students had the highest prevalence this correlates with the age group 6-15 years as all the students are within this age group. The more prolonged exposure of this age group to the water bodies has been adduced for this observation. This study is in an agreement with the findings of Sacolo-Gwebu et al. (2019) and; Exum et al. (2019), who also showed that, age group of students was highly correlated to infection rate. While the higher prevalence in fishermen/women (though not significant) compared to farmers may be attributed to longer contacts with waters, the higher prevalence recorded among fishermen compared with the figure in fisherwomen is unexplainable.
The higher detection rate with ELISA compared with PCR and Microscopy (Figure 5) might be since ELISA detects antibodies to Schistosoma and cannot distinguish between active and past infection, with parasite-specific antibodies remaining in the system long after the disease has been cured (Doenhoff et al., 2004; Sousa et al., 2019). ELISA cannot distinguish between the different species of Schistosoma prevalence recorded in this study is for both S. mansoni and S. haematobium and possible other species, even though there is no report of any other parasitic species in the region or within the country. The manual washing carried out during the ELISA test because of the lack of automated ELISA plate washer, may also have contribute to the observed higher prevalence. The low prevalence of S. mansoni reported for CRR in this study agrees with previously reported low prevalence for S. mansoni in the country NTD Mapping Report, 2015). The NTD mapping study reported a prevalence of 0.4% for S. mansoni in CRR. The detection of S. mansoni in blood and urine using nested PCR with appropriate primers is revealing as this organism (S. mansoni) is usually detected in stool samples only of infected patients. This may be attributed to the higher sensitivity of PCR compared to ELISA and microscopy.
The study showed that prevalence of schistosomiasis infection varied among different age groups and occupation in the central River Region of the Gambia and was largely dictated by level of contact with water bodies and previous drug treatment with anti-schistosomal drug and previous drug of people living in the Central River Region of The Gambia. Schistosomiasis is still a serious public health problem in this region. The molecular technique showed that S. haematobium is the dominant causative nematode of Bilharzias in the Gambia. The study also indicated that S. mansoni can be detected in blood and urine. To reduce the prevalence of schistosomiasis in The Gambia, mass drug administration using praziquantel should be carried out in the villages that have not benefited from it yet. A community health eradication campaign and adequate health education should be promoted on the control of the disease. Since the infection of children can be evaded, the community should be educated on the mode of transmission of the disease and the pathology of the disease and therefore encourage them to adopt control measures. Since there is the availability of running water, children should be discouraged from swimming and washing in the river.
The authors have not declared any conflict of interests.
YKE and TTB conceived and designed the experiments; AM, AK and MEE collected the data, performed the experiments, analysed the data and as well wrote the paper.
This work was funded by the Africa Center of Excellence for Neglected Tropical disease and forensic Biotechnology, Ahmadu Bello University, Zaria, Nigeria and The Africa Center of Excellence Project Team, The Gambia. This work is dedicated to the late Dr. TT Bem for his invaluable contribution and guidance throughout this work.
REFERENCES
|
Adenowo AF, Oyinloye BE, Ogunyinka BI, Kappo AP (2015). Impact of human schistosomiasis in sub-Saharan Africa. Brazilian Journal of Infectious Diseases 19(2).
Crossref
|
|
|
|
Barrow A, Badjie M, Touray J, Kinteh B, Nget M, Touray E, Kinteh SLS, Sillah SO, Darboe L, Jallow Y, Badjan M, Gaye M, Solomon P, Jatta S (2020). Knowledge, Attitude, and Practice of Provincial Dwellers on Prevention and Control of Schistosomiasis: Evidence from a Community-Based Cross-Sectional Study in the Gambia. Journal of Tropical Medicine 2020-9. Article ID 2653096.
Crossref
|
|
|
|
|
Colley DG, Bustinduy AL, Secor WE, King CH (2014). Human schistosomiasis. Lancet 383:2253-2264.
Crossref
|
|
|
|
|
Corachan M (2002). Schistosomiasis and International Travel. Clinical Infectious Disease 35(4):446-450.
Crossref
|
|
|
|
|
Corcoran C, da Silva M (2014). Diagnosing Schistosomiasis. Pathchat Edition No. 11.
|
|
|
|
|
Doenhoff MJ, Chiodini PL, Hamilton J (2004). Specific and sensitive diagnosis of schistosome infection: can it be done with antibodies? Trends in Parasitology 20:35-39.
Crossref
|
|
|
|
|
Exum NG, Kibira SPS, Ssenyonga R, Nobili J, Shannon AK, Ssempebwa JC, Tukahebwa EM, Radloff S, Schwab KJ, Makumbi FE (2019). The prevalence of schistosomiasis in Uganda: A nationally representative population estimate to inform control programs and water and sanitation interventions. PLoS Neglected Tropical Disease 13(8):e0007617.
Crossref
|
|
|
|
|
Gambia NTD Mapping Report (2015). The Gambia Neglected Tropical Disease (Schistosomiasis and Soil Transmitted Helminths) Mapping Report, Ministry of Health, The Gambia 2015. (Unpublished report).
|
|
|
|
|
Global Burden Disease (GBD) (2016). Global, Regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: A systemic analysis for the Global Burden of Disease Study 2016. Lancet 390:1211-1259.
|
|
|
|
|
Gruner N, Stambouli O, Ross RS (2015). Dried Blood Spots - Preparing and processing for use in Immunoassays and in Molecular Techniques. Journal of Visualized Experiments 97:e52619.
Crossref
|
|
|
|
|
Gryseels B, Polman K, Clerinx J, Kestens L (2006). Human schistosomiasis. Lancet 368:1106-1118.
Crossref
|
|
|
|
|
International Agency for Research on Cancer (IARC) (2012). Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 100B. Schistosoma haematobium. Available from:
View
|
|
|
|
|
Kargbo A, Kuye RA (2020). Epidemiology of tsetse flies in the transmission of trypanosomiasis: Technical review of The Gambia experience. International Journal of Biological Science and Chemical Science 14(3):1093-1102.
Crossref
|
|
|
|
|
Li EY, G D, Lo NC, Zhu X, King CH (2019). Improving public health control of schistomiasis with a modified WHO strategy: a model-based comparison study. The Lancet Gobal Health 7(10).
Crossref
|
|
|
|
|
Lodh N, Mwansa JCL, Mutengo MM, Shiff CJ (2013). Diagnosis of Schistosoma mansoni without the Stool: Comparison of Three Diagnostic Tests to Detect Schiostosoma mansoni Infection from Filtered Urine in Zambia. America Journal of Tropical Medicine and Hygiene 89(1):46-50.
Crossref
|
|
|
|
|
Mas-Coma S, Valero MA, Bargues MD (2009). Climate change effects on trematodiases, with emphasis on zoonotic fascioliasis and schistosomiasis. Veterinary Parasitology 163:264-280.
Crossref
|
|
|
|
|
Nour NM (2010). Schistosomiasis: Health Effects on Women. Reviews in Obstetrics and Gynecology 3(1):28-32.
|
|
|
|
|
Ntounifor HN, Ajayi JJ (2007). Studies on the ecology and distribution of some medically important freshwater snail species in Bauchi Sate Nigeria. International Journal of Biological Science and Chemical Science 1(2):121-127.
Crossref
|
|
|
|
|
Obeng BB, Aryeetey YA, de Dood CJ, Amoah AS, Larbi IA, Deelder AM, Yazdanbakhsh M, Hartgers FC, Boakye DA, Verweij JJ, Van dam GJ, Van lieshout L (2008). Application of a circulating-cathodic-antigen (CCA) strip test and real-time PCR, in comparison with microscopy, for the detection of Schistosoma haematobiumin urine samples from Ghana. Annals of Tropical Medical Parasitology 102:625-633.
Crossref
|
|
|
|
|
Oboh MA, Idowu TE, Mafe MA, Otunbanjo OA (2018). Post-treatment assessment of praziquantel rfficacy among school-age children infected with schistosomiasis in Ipogun area of Ondo State, Nigeria. International Journal of Biological Science and Chemical Science 12(6):2464-2473.
Crossref
|
|
|
|
|
Orsot MN, Meite A, N'guessan NA, Djie NN, Ouattara M, Coulibaly JT, Assare RK, Diakite NR, Mama AD, Djie LMA, N'goran EK (2018). Detailed distribution of schistosomiasis and soil-transmitted helminthiasis among schoolchildren in Belier and Marahoue region, Central Cote d'Ivoire: a tool for efficient control. International Journal of Biological Science and Chemical Science 12(4):1532-1542.
Crossref
|
|
|
|
|
Sacolo-Gwebu H, Chimbari M, Kalinda C (2019). Prevalence and risk factors of schistosomiasis and soil-transmitted helminthiases among preschool aged children (1-5 years) in rural KwaZulu-Natal, South Africa: A cross-sectional study. Infectious Disease of Poverty 8:47.
Crossref
|
|
|
|
|
Sady H, Al-Mekhlafi HM, Ngui R, Atroosh WM, Al-Delaimy AK, Nasr NA, Dawaki S (2015). Detection of Schistosoma mansoni and Schistosoma haematobium by Real-Time PCR with High Resolution Melting Analysis. International Journal of Molecular Science 16:16085-16103.
Crossref
|
|
|
|
|
Sanneh B, Joof E, Sanyang AM, Renneker K, Camara Y, Sey AP, Jagne S, Baldeh I, Ceesay SJ, Sambou SN, Ogoussan K (2017). Field evaluation of a schistosome circulating cathodic antigen rapid test kit at point-of-care for mapping of schistosomiasis endemic districts in The Gambia. PLoS ONE 12(8):e0182003.
Crossref
|
|
|
|
|
Senghor B, Diallo A, Sylla SN, Doucouré S, Ndiath MO, Gaayeb L, Félicité F, Djuikwo- Teukeng FF, Ba C, Sokhna C (2014). Prevalence and intensity of urinary schistosomiasis among school children in the district of Niakhar, region of Fatick, Senegal. Parasites and Vectors 7:5.
Crossref
|
|
|
|
|
Sousa SRM, Dias IHL, Fonseca ALS, Contente BR, Nogueira JFC, Oliveira TNC, Geiger SM, Enk MJ (2019). Concordance of the point-of-care circulating cathodic antigen test for the diagnosis of intestinal schistosomiasis in a low endemicity area. Infectious Diseases of Poverty 8:37.
Crossref
|
|
|
|
|
Steinauer ML, Agola LE, Mwangi NI, Mkoji GM, Loker ES (2008). Molecular Epidemiology of Schistosomamansoni. Infectious Genetic Evolution 8(1):68-73.
Crossref
|
|
|
|
|
Thrusfield M (2007). Veterinary epidemiology. 3rd Edition, Blackwell Science Ltd., Oxford.
View
|
|
|
|
|
Ukaga FN, Onyeka PIK, Nwoke BEB (2002). Practical medical parasitology for biological and medical students. Avan Global Publication. Owerri, Nigeria, 341pp.
|
|
|
|
|
WHO (2020). World Health Organization Schistosomiasis fact sheet.
|
|
|
|
|
Woldegerima E, Bayih AG, Tegegne Y, Aemero M, Zeleke AJ (2019). Prevalence and Reinfection Rates of Schistosoma mansoni and Praziquantel Efficacy against the Parasite among Primary School Children in Sanja Town, Northwest Ethiopia. Journal of Parasitology Research 8:Article ID 3697216.
Crossref
|
|
|
|
|
World Health Organization (WHO) (2017). Schistosomiasis, Fact Sheet, No. 115. Available at:
View. Assessed on 22/02/18.
|
|