Full Length Research Paper
ABSTRACT
An efficient callus-mediated regeneration system was developed for high-frequency production of planting material of sugarcane genotypes LSC and B36464. Spindle leaf segments cultured on MS basal medium supplemented with 2,4-D or picloram at 1, 2, 3 or 4 mg/L resulted in callus induction. Callus induction was higher on 2,4-D amended medium compared to picloram. Nevertheless, for both auxins, callus induction improved significantly (p ≤ 0.05) with increasing concentration; the highest (82 and 82.5% for B36464 and LSC respectively) was achieved at 4 mg/L. For shoot induction, calli were transferred to MS medium supplemented with BAP (0.1, 0.5, 1.0 or 1.5 mg/L). The highest number of shoots (18.13 and 16.75 for B36464 and LSC respectively) was achieved at 1.5 mg/L. Serial subculture at four-week intervals on a higher concentration of BAP (2.5 mg/L), in combination with NAA (0.5 mg/L) and GA3 (0.5 mg/L), resulted in a four-fold increase in shoot number within 16 weeks. On this medium, 40% of shoot clusters of B36464 formed well-defined shoots. On MS medium containing solely NAA (3 mg/L), 88 and 72% (B36464 and LSC respectively) formed roots. Post-flask acclimatisation of the plantlets led to 85 and 91% survival rates in LSC and B36464 respectively after which plantlets were successfully transferred to field conditions. The callus-mediated regeneration system reported in this study has the potential to sustainably provide sugarcane planting material for the emerging sugar industry in Ghana.
Key words: Sugarcane, spindle leaf explants, callus-mediated organogenesis, plantlet regeneration, 2,4-D, Picloram.
INTRODUCTION
Globally, sugarcane (Saccharum officinarum L.) is an industrial crop used mainly to produce sugar and bioethanol. In Ghana, its full potential is yet to be exploited as most of the country’s sugarcane is used either for the production of local alcoholic drink “akpeteshie” by small-scale distilleries or sold fresh as a snack (Ababio and Lovatt, 2015). Recently however, the crop is gaining more prominence in the country as an industrial crop particularly with the re-commissioning of the Komenda Sugar Factory in the Central Region of Ghana.
The sugar factory is expected to trigger large demand for planting materials for the establishment of plantations which in turn will serve as feedstock. Such plantations may be established conventionally through vegetative propagation (planting of setts). However, setts are usually unavailable in commercial quantities and require high labour input for preparation and planting (Bello-Bello et al., 2018). Even when available, setts are often contaminated by pests and diseases resulting in low yields (Jalaja et al., 2008).
An alternative method of generating large quantities of planting material is tissue culture. Since the 1960s, the technique has been shown to be highly efficient for rapid multiplication of sugarcane (Heinz and Mee, 1969; Ho and Vasil, 1983). Plantlets have been successfully regenerated from various types of explants including immature leaf rolls (Soares et al., 2014), apical meristems (Ramgareeb et al., 2010), young leaves (Chengalrayan and Gallo-Meagher, 2001)and axillary buds (Vazquez Molina et al., 2005). Additionally, tissue culture has been shown to be advantageous in that, plants are disease-free thereby exhibiting increased field productivity and improved sugar yields (Sawant, 2014; Bello-Bello et al., 2018).
Although worldwide, significant progress has been made in refining tissue culture protocols for sugarcane, the crop has been shown to be genotype-dependent/ specific in its response to culture conditions (Jadhav et al., 2001). For instance, sugarcane genotypes can vary highly in their response to exogenous growth regulators in culture media (Gandonou et al., 2005). Consequently, efficient regeneration of specific genotypes often requires optimization of in vitro protocols (Tesfa and Ftwi, 2018).
Thus, in this study, we tested the response of two important local sugarcane cultivars to a simple and efficient callus-mediated in vitro regeneration system. This method of organogenesis not only presents a good strategy for rapid multiplication of the crop but also allows for future improvement of these cultivars via genetic transformation and in vitro mutagenesis (Jamil et al., 2017). For example, callus-mediated regeneration has been used to improve sugarcane for resistance to pests and diseases as well as tolerance to drought and salinity (Matsuoka et al., 2001; Eldessoky et al., 2011). Given the expected demand for large quantities of sugarcane planting material in Ghana, we envisage that the application of this technique to locally adapted and economically important cultivars has great potential to support the emerging sugar industry in the country. Additionally, it will contribute to job creation throughout-grower schemes, and improve productivity and income of smallholder farmers in sugarcane-growing regions.
MATERIALS AND METHODS
Study location
The experiment was conducted at the Biotechnology Centre of Biotechnology and Nuclear Agriculture Research Institute (BNARI), Ghana Atomic Energy Commission (GAEC) from March 2019 to September 2020.
Planting materials
Two genotypes of sugarcane, LSC and B36464 were used in the study. B36464 also locally referred to as Alata, is one of the main cultivars grown by smallholder farmers in the Komenda-Edina-Eguaafo-Abrem (KEEA) District of the Central Region, Ghana. It is also grown on commercial scale by farmers at Abura-Asebu-Kwamankese (AAK), Shama and Mpohor Wassa East districts in the Central Region due to their proximity to the Komenda Sugar Factory (GSS 2014; Yawson et al., 2018). The second genotype LSC is a popular landrace grown by smallholder farmers in several parts of Ghana including the Eastern, Central, Volta and Greater Accra regions. This landrace is often sold as a snack in small, peeled segments and provides income for many women in both rural and urban areas.
Explant preparation and sterilisation
Apical sections of field-grown canes (6 to 8 months old) were soaked overnight in 1% (w/v) fungicide solution (Bendazim® 50WP, active ingredient, carbendazim). Spindle leaf explants were obtained by removal of outer leaf whorls and trimming of the apical sections. The explants were then washed in mild detergent (Morning Fresh Dishwashing Liquid, Cussons®) solution for five minutes followed by soaking in 10% bleach (Clorox®, active ingredient – 6.05% sodium hypochlorite) for 30 min. Final surface sterilisation consisted of soaking explants in 0.2% (w/v) mercuric chloride for 10 min followed by thorough rinsing with three changes of sterile distilled water.
Callus induction
Thin slices (approximately 5 mm) of spindle leaf explants were inoculated on callus induction medium (CIM) which comprised Murashige and Skoog medium (1962) supplemented either with picloram or 2,4– dichlorophenoxyacetic acid (2,4 -D) at varying concentrations (0, 1, 2, 3 or 4 mg/L). The callus induction medium (CIM) also contained sucrose at 30 g/L. The pH of the culture medium was adjusted to 5.8 followed by addition of phytagel at 3.5 g/L and autoclaving at 121°C for 15 min. The cultures were maintained in the dark at 25 ± 1°C for 28 days, after which the number of explants that formed calli was scored. Fifty explants were cultured per treatment, consisting of 10 replicates.
Shoot induction from callus
For shoot induction, embryogenic calli (cream-coloured and friable) were transferred to shoot induction medium (SIM) consisting of MS medium supplemented with 6-benzylamino purine (BAP) at 0.1, 0.5, 1.0 or 1.5 mg/L, referred to hereafter as SIM1, SIM2, SIM3 and SIM4, respectively. The cultures were maintained under cool fluorescent light (16/8-h light/darkness; 2500 lux) at 25±1°C for six weeks after which the number of germinated shoots per callus clump was scored for the various treatments. Sixteen calli were cultured per treatment with each callus considered as a replicate.
Shoot proliferation and root induction
To improve shoot multiplication, micro shoot clusters were transferred to shoot proliferation media (SPM) containing BAP at 2.5 mg/L in combination with NAA (0.5 mg/L) and GA3 (0.5 mg/L). The total number of well-differentiated shoots was scored at the end of each culture cycle (every four weeks). Multiplication rates were calculated as a ratio of the total number of shoots at each sub-culture divided by the number of shoots at the previous subculture stage. Unrooted shoots were transferred to MS medium containing NAA at 3 mg/L (RIM) for root induction.
Plantlet acclimatization
Two hundred plantlets (100 per genotype) were washed gently with tap water followed by a five-minute rinse in 0.1% fungicide solution (Bendazim® 50WP, active ingredient, carbendazim). The plantlets were then transferred to polythene bags filled with soil, cocopeat and sawdust mixture (2:1:1) with high humidity conditions (>85%) created by covering the plantlets with transparent plastic cups for three days. The plantlets were maintained in a greenhouse at 28 ± 2°C and were irrigated every other day for six weeks. The number of plants that survived was recorded. Following successful acclimatisation, well-hardened plantlets (approximately 9 weeks old) were transferred to field conditions. Prior to transplanting, older leaves were trimmed. During transplanting, plantlets were carefully removed from polythene bags to minimise damage to roots. Plantlets were then placed in furrows two feet apart and covered with adequate amounts of soil and irrigated immediately after transplanting. All recommended intercultural operations (weeding, irrigation and pest control) were performed.
Statistical analysis
All experiments were laid out in a completely randomized design (CRD). For callus induction, each treatment was replicated 10 times. For shoot induction, each treatment was replicated 16 times. Data were analysed using Microsoft Excel 2010 and GraphPad Prism version 7 (San Diego, United States). All data were subjected to analysis of variance (ANOVA) and statistically significant results at the 5% level were compared either with Tukey’s or Sidak’s multiple comparisons test.
RESULTS AND DISCUSSION
Callus induction
Spindle leaf explants (Figure 1A) of sugarcane cultivars LSC and B36464 formed callus on MS media containing varying concentrations of 2,4-D or picloram by the twelfth day of incubation (Figure 1B, C). For both cultivars, the absence of 2,4-D and picloram in the culture media resulted in no callus production, indicating an auxin requirement for callus formation in sugarcane, similar to other plant species (Ikeuchi et al., 2013; Osman et al., 2016; Zang et al., 2016). Similar observations were made by Ramgareeb et al. (2010) and Alcantara et al. (2014)where various types of sugarcane explants failed to form callus on auxin-free media. Exogenous auxin supplementation is required to reprogram somatic cells to acquire pluripotency as well as initiate cell division leading to the formation of callus (Skoog and Miller, 1957; George et al., 2008; Fehér, 2019). An increase in the number and size of calli was observed after 21 days of incubation on both 2,4-D (Figure 1D, E) and picloram-supplemented media (Figure 1F, G).
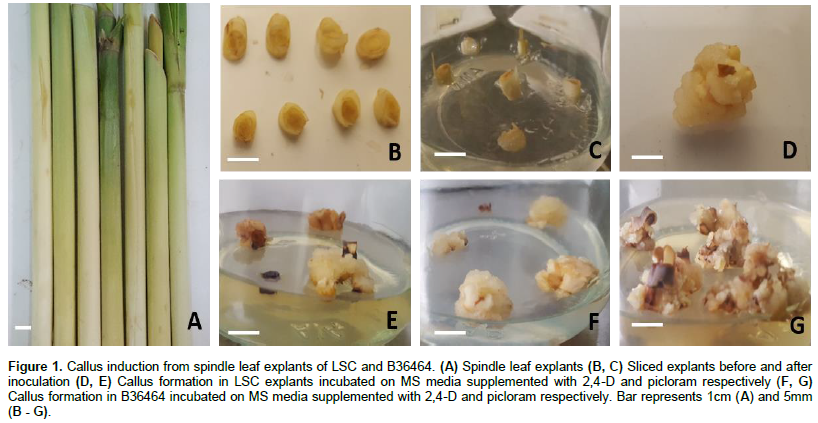
Callus induction on 2,4-D supplemented media was significantly higher (p ≤ 0.05) compared to picloram at the same concentrations (Table 1; supplementary Table 1) suggesting that 2,4-D is an effective auxin for callus induction in sugarcane. This is similar to earlier reports (Ho and Vasil, 1983; Gill et al., 2004; Dibax et al., 2011; Alcantara et al., 2014; Arjun Srinath, 2015). However, our findings contradict those of Gallo-Meagher et al. (2012)who reported higher callus induction on picloram- supplemented medium rather than 2,4-D in sugarcane. This may be due to differences in genotypic response to auxins as reported by Gandonou et al. (2005) and Mekonnen et al. (2014).
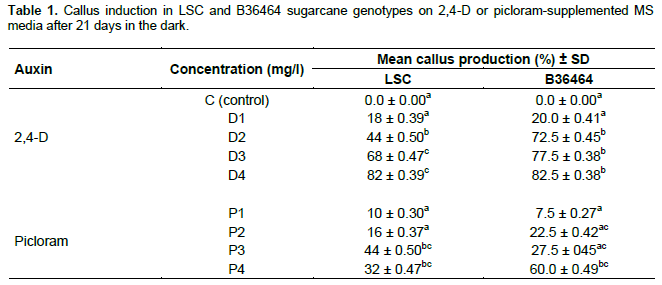
In general, induction of embryogenic calli improved with increasing auxin concentration (Table 1). For LSC, callus induction was best achieved at the highest concentrations of 3 and 4 mg/L for 2,4-D. Similar observations have been reported by several authors including Badawy et al. (2008), Jahangir et al. (2010) and Arjun Srinath (2015). A similar trend was observed for picloram. However, as picloram concentration increased from 3 to 4 mg/L callus induction rate declined (from 44 to 34%) in LSC. The reduction in callus formation may be attributed to the presence of endogenous hormones in the explant tissues prior to culture initiation (Can et al., 2008). Wernicke et al. (1986), explain that high auxin concentrations may prevent meristematic cell divisions, resulting in decreased reactions such as callus induction. In the genotype B36464, the best concentration for callus formation is between 2- 4 mg/L 2,4-D or 4 mg/L picloram. Nonetheless, the response of the two genotypes in this study to callus induction is beneficial in that, it is a first and crucial step in in vitro mutagenesis as well as genetic transformation protocols for improvement of local sugarcane genotypes. Calli are ideal propagules for reception of genes since they are mostly of single cell origin (Nagmani et al., 1987)and can also be reached by either physical or chemical mutagens in mutagenesis (ref).
Mean production of embryogenic callus is the ratio of callus formed to the number of explants cultured expressed as a percentage. Data represent mean ± SD. Values in a column followed by the same letters are not significantly different from each other at p ≤ 0.05 (Tukey’s pairwise comparison test).
Shoot induction from calli
The addition of cytokinins, particularly BAP, to MS medium has been shown to be effective for inducing shoots from sugarcane callus cultures (Ather et al.. 2009; Dibax et al.. 2011; Arjun Srinath. 2015). Thus, to determine the optimum concentration of BAP for shoot induction from callus for the two cultivars, we transferred friable calli to MS medium containing BAP ranging from 0.1 to 1.5 mg/L BAP. By the second week, green microstructures were observed on the surfaces of calli of both B36464 (Figure 2A, B) and LSC (Figure 2C, D), suggesting the formation of totipotent shoot primordia. The presence of high concentration of cytokinin in the shoot induction medium induces shoot stem cell regulators from callus (Gordon et al., 2007). According to Dibax et al. (2013), the formation of such shoot primordia is triggered from both nodular and friable calli due to the presence of cytokinins which initiate a pathway redirection. The formation of shoot primordia on callus of sugarcane resulted in the development of shoots within 28 days of culture. The mean number of shoots formed per callus explants ranged from 9.69 ± 2.55 to 18.13 ± 3.07 in B36464 genotype and 8.44 ± 4.19 to 16.75 ± 3.55 in LSC genotype (Figure 3; Supplementary Table 2). The shoot induction rates obtained from callus cultures in this study are higher than those obtained from shoot tip or lateral bud culture (Danso et al., 2011; Mekonnen et al., 2014)making this protocol efficient for commercial production of sugarcane planting materials. Callus develops more shoot primordium, hence more shoots than from direct organogenesis in shoot tips. Shoot regeneration from callus is a result of localised cell division leading to differentiation of globular meristemoids which can develop into shoots (Ove?ka et al., 1997). For example, in Nicotiana tabacum, the palisade cells divide to form callus which gives rise to shoot primordia and finally regeneration into shoot (Gupta et al., 1966; Zhi-hong and Gui-Yun, 1980).
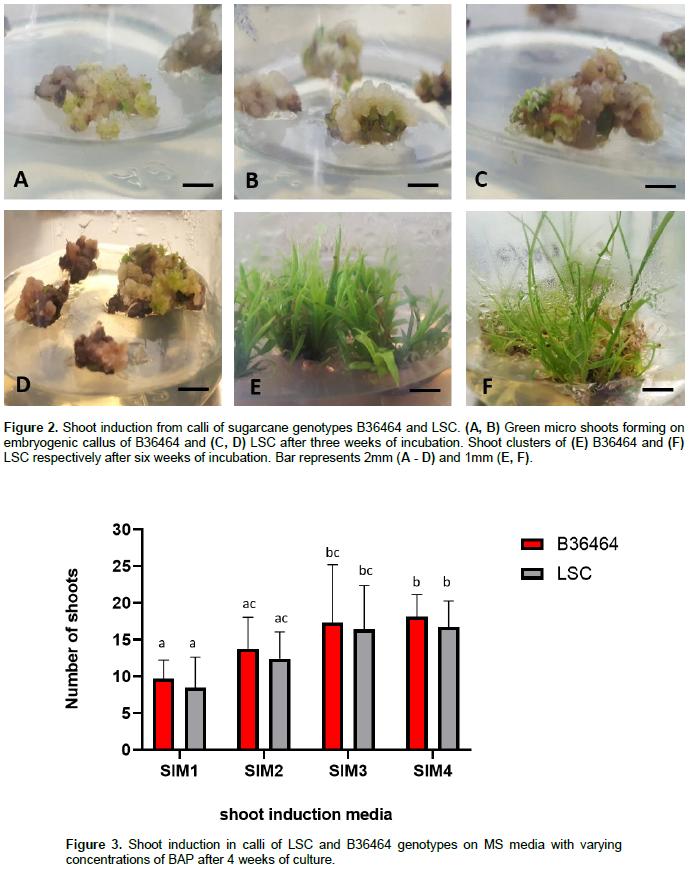
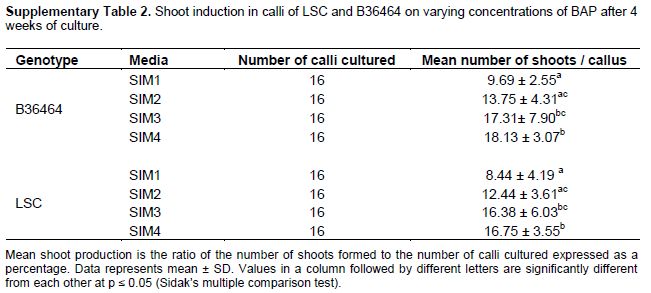
The lowest concentrations of BAP (0.1 and 0.5 mg/L) induced the least number of shoots from callus in both genotypes. However, at higher BAP concentrations of 1.0 and 1.5 mg/L, we recorded a significant increase (p ≤ 0.05) in shoot induction in both genotypes (Supplementary Table 2). An increase in shoot production from sugarcane callus cultures with increasing BAP concentration has been reported by Behera and Sahoo (2009) and Hapsoro (2017). A comparison between the two genotypes showed higher shoot induction from callus of variety B36464 compared with LSC, suggesting genotype effect on shoot induction. Similarly, genotypic differences have been observed in shoot regeneration from callus cultures of different sugarcane cultivars (Gandonou et al., 2005; Kaur and Kapoor, 2016; Di Pauli et al., 2021).
Shoot proliferation and root induction
Given the increasing frequency of shoot induction (from callus) with increasing BAP concentration in the previous experiment, we evaluated the proliferation of shoots on MS medium supplemented with a higher concentration of BAP (2.5 mg/L) in combination with low concentrations of NAA (0.1 mg/L) and GA3 (0.1 mg/L). In sugarcane, as in many plant species, a high cytokinin to auxin ratio in the culture medium has been shown to effectively stimulate shoot regeneration and differentiation (Ikeuchi et al., 2013; Fehér, 2019). In order to increase plantlet production and maximise economic gains, four serial subcultures were used to multiply the shoots in culture.
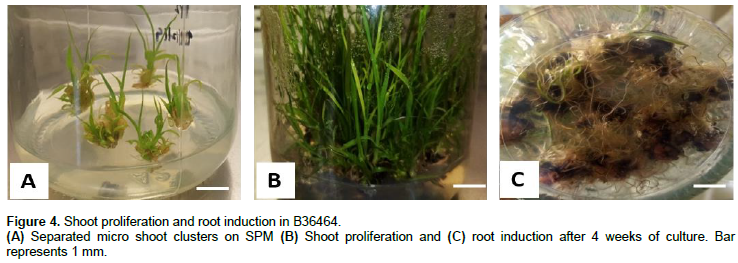
After subculture cycle 1, the total number of shoots produced in LSC genotype was higher (2902) compared to B36464 (2797) (Table 2 and Figure 4A, B). However, after 8 weeks of culture on SPM (cycle 2), shoot production was higher (7209) in B36464 compared to LSC (6927) and this trend was maintained for the next two cycles (till 16 weeks of culture). The total number of shoots formed in both genotypes increased more than two-fold by the second subculture (at 8 weeks) and four-fold by the fourth subculture (at 16 weeks) (Table 2). This high frequency of plantlet production indicates the efficiency of the callus-mediated technique for sugarcane micro-propagation.
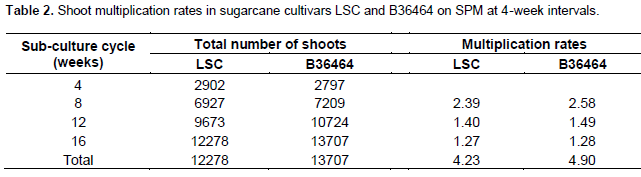
Interestingly, shoot multiplication rates decreased with successive subcultures (second subculture onwards) likely due to the cumulative effects of BAP in succeeding subcultures which might have led to high endogenous concentration of BAP, hence the phytotoxic effect. In general, decline in shoot multiplication rates as a result of repeated in vitro subculture has been reported in several plant species (Norton and Norton, 1986; Hussain et al., 2007; Vujovi? et al., 2012). Although reducing the concentration of hormones in the culture medium can delay the decline in shoot multiplication rates (Vujovi? et al., 2012), regular initiation of new cultures from spindle leaf explants is necessary to maintain high shoot multi-plication rates. The initiation of new cultures is important to reduce the risk of somaclonal variation which is quite common in sugarcane (Khan, 1999; Khan et al., 2008). It also ensures genetic homogeneity of plantlets and the production of true-to-type planting materials for the establishment of commercial plantations (Petolino et al., 2003). However, in this work using LSC and B36464 genotypes, we did not observe any genetic or somaclonal variation in plants transferred to the field at six months old.
With respect to root induction, 25% of shoot clusters of LSC formed well-defined roots spontaneously on BAP-containing medium (SPM) after 4 weeks of culture com-pared to 57% in B36464 shoot clusters (Figure 4C). This phenomenon of spontaneous root formation at the shoot maturation stage on media containing only cytokinins has been reported by Zamir et al. (2012). Nonetheless, to improve root formation from in vitro shoots, we supplemented MS media with 3 mg/L NAA which resulted in marked improvement in root development in both B36464 (88%) and LSC (72%) after 8 weeks of culture.
Plantlet acclimatization
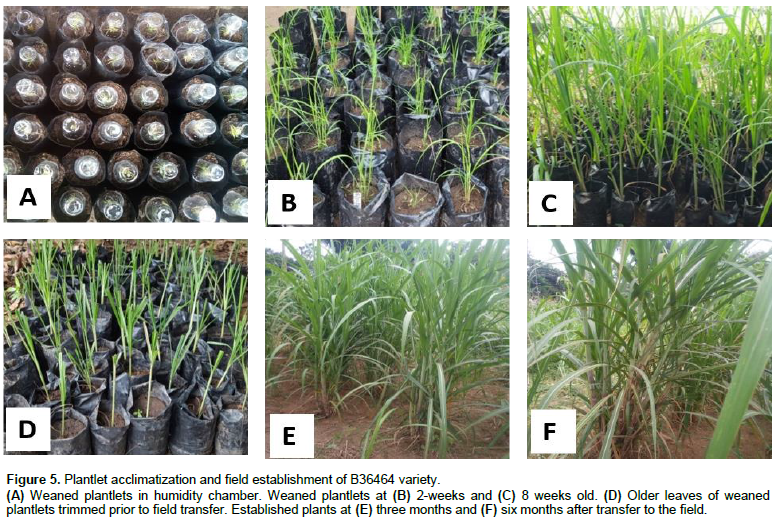
Efficient weaning and hardening of in vitro-generated plantlets are critical for successful transfer of plantlets to field conditions. To determine the efficiency of weaning and hardening, a total of 200 plantlets (100 per genotype) were transferred to the greenhouse for weaning. Survival rates of 85 and 91% were achieved for LSC and B36464, respectively (Figure 5). The rates of acclimatization obtained for plantlets of both sugarcane genotypes in this study are similar to survival rates obtained for other commercial Indian varieties (Kaur and Kapoor, 2016)but higher compared to other reports where up to 87% survival rates were obtained (Mekonnen et al., 2014). Well-developed roots in in vitro plantlets prior to weaning have been shown to enhance survival of plants at the weaning stage (Danso et al., 2011). Other factors such as genotype and the medium used for weaning also play significant role in post-flask acclimatisation: cocopeat: soil: sawdust mixture (2:1:1) could explain the differences in plantlet acclimatization compared with other studies. The weaned plantlets were successfully transferred to field after 8 weeks of hardening for further development.
CONCLUSION
The emerging sugar industry in Ghana and across Africa requires the availability of large quantities of sugarcane planting materials to ensure sustainability of the industry. As a strategy for meeting this demand, the response of two important locally adapted cultivars to micro-propagation via somatic embryogenesis was tested. The successful induction of embryogenic calli from spindle leaf explants in the two cultivars followed by efficient plantlet regeneration on cytokinin-auxin amended MS medium augur well for adequate and sustainable supply of planting materials for the sugarcane industry. A four-fold increase in shoot production in 16 weeks demonstrates the efficiency of our protocol for large scale production of sugarcane planting material. Thus, this study has demonstrated that somatic embryogenesis using spindle leaf explants offers an efficient protocol for large scale production of disease-free sugarcane plantlets for the emerging sugar industries across Africa.
ABBREVIATIONS
2,4-D, 2,4-dichlorophenoxyacetic acid; BAP, 6 – benzylamino purine; BNARI, Biotechnology and Nuclear Agriculture Research Institute; GAEC, Ghana Atomic Energy Commission; MS, Murashige and Skoog Basal medium; NAA, 1-naphthaleneacetic acid; Picloram, 4-amino-3,5,6-trichloropicolinic acid.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
ACKNOWLEDGEMENTS
The authors are grateful to the management of BNARI (GAEC) for the use of laboratory space and provision of resources necessary for this experiment and wish to express gratitude to the entire staff of the Biotechnology Centre for their diverse contributions to this work. The authors appreciate Mr. Nehemiah Naanie and Mr. Owula for their enormous assistance in the maintenance of the sugarcane germplasm on station. This study was funded by the government of Ghana through the Biotechnology and Nuclear Agriculture Research Institute (BNARI), Ghana Atomic Energy Commission.
REFERENCES
|
Ababio P, Lovatt P (2015). A review on food safety and food hygiene studies in Ghana. Food Control 47:92-97. |
|
|
Alcantara GBd, Dibax R, Oliveira RAd, Bespalhok Filho JC, Daros E (2014). Plant regeneration and histological study of the somatic embryogenesis of sugarcane (Saccharum spp.) cultivars RB855156 and RB72454. Acta Scientiarum. Agronomy 36(1):63-72. |
|
|
Arjun Srinath R (2015). Callus Induction and Organogenesis in Sugarcane (Saccharum officinarum L.) var 93v297. International Letters of Natural Sciences 48:14-22. |
|
|
Ather A, Khan S, Rehman A, Nazir M (2009). Optimization of the protocols for callus induction, regeneration and acclimatization of sugarcane cv. Thatta-10. Pakistan Journal of Botany 41(2):815-820. |
|
|
Badawy O, Nasr M, Alhendawi R (2008). Response of sugarcane (Saccharum species hybrid) genotypes to embryogenic callus induction and in vitro salt stress. Sugar Tech 10(3):243-247. |
|
|
Behera K, Sahoo S (2009). Rapid In vitro Micro propagation of Sugarcane (Saccharum officinarum L. cv- Nayana) Through Callus Culture. Nature and Science 7(4):1-10. |
|
|
Bello-Bello J, Mendoza-Mexicano M, Perez Sato JA (2018). In Vitro Propagation of Sugarcane for Certified Seed Production. In Sugarcane-Technology and Research. IntechOpen. |
|
|
Can E, Çelikta? N, Hatipo?lu R (2008). Effect of Auxin Type and Concentrations in Different Media on the Callus Induction and Shoot Formation of Crested Wheatgrass (Agropyron Cristatum (L.) Gaertn). Biotechnology & Biotechnological Equipment 22(3):782-786. |
|
|
Chengalrayan K, Gallo-Meagher M (2001). Effect of various growth regulators on shoot regeneration of sugarcane. In Vitro Cellular and Developmental Biology-Plant 37(4):434-439. |
|
|
Danso KE, Azu E, Elegba W, Asumeng A, Amoatey HM, Klu G (2011). Effective decontamination and subsequent plantlet regeneration of sugarcane (Saccharum officinarum L.) in vitro. International Journal of Integrative Biology 11(2):90-96. |
|
|
Di Pauli V, Fontana PD, Lewi DM, Arturo F, Erazzu LE (2021). Optimized somatic embryogenesis and plant regeneration in elite Argentinian sugarcane (Saccharum spp.) cultivars. Journal of Genetic Engineering and Biotechnology 19(1):1-8. |
|
|
Dibax R, Alcantara G, Machado M, Filho JCB, oliveira RA (2013). Protocol optimization and histological analysis of in vitro plant regeneration of 'RB92579' and 'RB93509' sugarcane cultivars. Ciência Rural 43:49-54. |
|
|
Dibax R, Alcantara G, Bespalhok J, Machado M, Oliveira Y, Lopes da Silva AL (2011). Plant regeneration of sugarcane cv. RB931003 and RB98710 from somatic embryos and acclimatization. Journal of Biotechnology and Biodiversity 2(3):32-37. |
|
|
Eldessoky DS, Ismail RM, Abdel-Hadi AH, Abdallah NA (2011). Establishment of regeneration and transformation system of sugarcane cultivar GT54-9 (C9). GM Crops 2(2):126-134. |
|
|
Fehér A (2019). Callus, Dedifferentiation, Totipotency, Somatic Embryogenesis: What These Terms Mean in the Era of Molecular Plant Biology? Frontiers in plant science 10:536. |
|
|
Ghana Statistial Service (GSS) (2014). 2010 Population and Housing Census. District Analytical Report Ghana Statistial Service. |
|
|
Gallo-Meagher M, English R, Abouzid A (2012). Thidiazuron stimulates shoot regeneration of sugarcane embryogenic callus. In Vitro Cellular and Developmental Biology-Plant 36(1):37-40. |
|
|
Gandonou C, Errabii T, Abrini J, Idaomar M, Chibi F, Skali-Senhaji N (2005). Effect of genotype on callus induction and plant regeneration from leaf explants of sugarcane (Saccharum sp.). African Journal of Biotechnology 4(11):1250-1255. |
|
|
George EF, Hall MA, De Glerk G (2008). Plant propagation by tissue culture volume 1. Somatic embryogenesis Springer, New York, USA. |
|
|
Gill N, Gill R, Gosal S (2004). Factors enhancing somatic embryogenesis and plant regeneration in sugarcane (Saccharum officinarum L.). Indian Journal of Biotechnology 3:119-123. |
|
|
Gordon SP, Heisler MG, Reddy GV, Ohno C, Das P, Meyerowitz EM (2007). Pattern formation during de novo assembly of the Arabidopsis shoot meristem. Development 134(19):3539-3548. |
|
|
Gupta G, Guha S, SC M (1966). Differentiation of buds from leaves of Nicotiana tabacum Linn. in sterile culture. Phytomorphology 16:175-182. |
|
|
Hapsoro D (2017). In Vitro Shoot Formation on Sugarcane (Saccharum officinarum L.) Callus as Affected by Benzyladenine Concentrations. Indonesian Journal of Agronomy 40(1):56-61. |
|
|
Heinz D, Mee G (1969). Plant Differentiation from Callus Tissue of Saccharum Species1. Crop Science 9(3):346-348. |
|
|
Ho WJ, Vasil IK (1983). Somatic embryogenesis in sugarcane (Saccharum officinarum L.) I. The morphology and physiology of callus formation and the ontogeny of somatic embryos. Protoplasma 118(3):169-180. |
|
|
Hussain TM, Chandrasekhar T, Gopal GR (2007). High frequency shoot regeneration of Sterculia urens Roxb. an endangered tree species through cotyledonary node cultures. African Journal of Biotechnology 6(4):1643-1649. |
|
|
Ikeuchi M, Sugimoto K, Iwase A (2013). Plant callus: mechanisms of induction and repression. Plant Cell 25(9):3159-3173. |
|
|
Jadhav AB, Vaidya E, Aher VB, Pawar AM (2001). In-vitro multiplication of 'Co-86032' sugarcane (Saccharum officinarum) hybrid. The Indian Journal of Agricultural Sciences 71(2):113-115. |
|
|
Jahangir G, Nasir I, Sial R, Javid M, Husnain T (2010). Various Hormonal Supplementations Activate Sugarcane Regeneration In-vitro. Journal of Agricultural Science 2(4):231. |
|
|
Jalaja N, Neelamathi D, Sreenivasan T (2008). Micropropagation for Quality Seed Production in Sugarcane in Asia and the Pacific. Vol. 1. Food and Agriculture Organization of the United Nations. |
|
|
Jamil S, Shahzad R, Talha GM, Sakhawat G, Sajid ur R, Sultana R, Iqbal MZ (2017). Optimization of Protocols for In Vitro Regeneration of Sugarcane (Saccharum officinarum). International Journal of Agronomy 2017:2089381. |
|
|
Kaur R, Kapoor MP (2016). Plant Regeneration Through Somatic Embryogenesis in Sugarcane. Sugar Tech 18(1):93-99. |
|
|
Khan HU (1999). Somaclonal variation in sugarcane through tissue culture and subsequent screening for salt tolerance. Department of Plant Breeding and Genetics, Faculty of Agriculture, Gomal University, Dera Ismil Khan. |
|
|
Khan I, Dahot M, Seema N, Sajida B, Khatri A (2008). Genetic variability in plantlets derived from callus culture in sugarcane. Pakistan Journal of Botany 40(2):547-564. |
|
|
Matsuoka M, Ideta O, Tanio M, Hayakawa A, Miwa H (2001). Agrobacterium tumefaciens-mediated transformation of sugarcane using cell suspension culture with a novel method. Paper presented at the International Society of Sugar Cane Technologists. Proceedings of the XXIV Congress, Brisbane, Australia pp. 17-21 September 2001. Australian Society of Sugar Cane Technologists. 2:660-662. |
|
|
Mekonnen T, Diro M, Manju S, Tadesse N (2014). Protocol optimization for in vitro mass propagation of two sugarcane (Saccharum officinarum L.) clones grown in Ethiopia. African Journal of Biotechnology 13(12):1358-1368. |
|
|
Nagmani R, Becwar M, Wann S (1987). Single-cell origin and development of somatic embryos in Picea abies (L.) Karst. (Norway spruce) and P. glauca (Moench) Voss (white spruce). Plant Cell Reports 6(2):157-159. |
|
|
Norton ME, Norton CR (1986). Change in shoot proliferation with repeated in vitro subculture of shoots of woody species of Rosaceae. Plant Cell, Tissue and Organ Culture 5(3):187-197. |
|
|
Osman NI, Jaafar Sidik N, Awal A (2016). Effects of variations in culture media and hormonal treatments upon callus induction potential in endosperm explant of Barringtonia racemosa L. Asian Pacific Journal of Tropical Biomedicine 6(2):143-147. |
|
|
Ove?ka M, Bobák M, Šamaj J (1997). Development of shoot primordia in tissue culture of Papaver somniferum L. Biologia Plantarum 39(4):499-506. |
|
|
Petolino JF, Roberts JL, Jayakumar P (2003). Plant cell culture. In: Handbook of Industrial Cell Culture. Springer pp. 243-258. |
|
|
Ramgareeb S, Snyman S, Van Antwerpen T, Rutherford R (2010). Elimination of virus and rapid propagation of disease-free sugarcane (Saccharum spp. cultivar NCo376) using apical meristem culture. Plant Cell, Tissue and Organ Culture 100(2):175-181. |
|
|
Sawant R (2014). Sawant RA,Tawar PN, Meti NT, Ranjekar PK (2014). Role of sugarcane micropropagation for production of quality seed. International Journal of Recent Biotechnology 2(4):34-41. |
|
|
Skoog F, Miller CO (1957). Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symposia of the Society for Experimental Biology 11:118-130. |
|
|
Soares RR, Ferreira ME, Gamarano MC, Ribeiro RC, Sabino VM, Pereira BMH (2014). The use of histological analysis for the detection of somatic embryos in sugarcane. African Journal of Biotechnology 13(6):762-767. |
|
|
Tesfa M, Ftwi M (2018). In Vitro Plant Regeneration of Sugarcane (Saccharum spp.) Variety Inoculated Under Different Levels of Plant Growth Regulators. Journal of Plant Biochemistry and Physiology 6(4):1000227. |
|
|
Vazquez Molina DE, De Los Santos A, Lecona Guzman KA, Sumano Muniz O, Velazquez Mendez M, Rincon Rosales R, Llaven O, Dendooven L, Gutierrez-Miceli FA (2005). Sugar cane buds as an efficient explant for plantlet regeneration. Biologia Plantarum 49(4)481-485. |
|
|
Vujovi? T, Ruzic D, Cerovi? R (2012). In vitro shoot multiplication as influenced by repeated subculturing of shoots of contemporary fruit rootstocks. Horticultural Science 39(3):101-107. |
|
|
Wernicke W, Grost J, Molkovits L (1986). The ambiguous role of 2,4-dichlorophenoxyacetic acid in wheat tissue culture. Physiologia Plantarum 68(4):597-602. |
|
|
Yawson DO, Adu MO, Osei KN (2018). Spatial assessment of sugarcane (Saccharurn spp. L.) production to feed the Komenda Sugar Factory, Ghana. Heliyon 4(11):e00903. |
|
|
Zamir R, Khalil, Shah, Khan M, Khan NA, Shahenshah (2012). Efficient In Vitro Regeneration of Sugarcane (Saccharum Officinarum L.) from Bud Explants. Biotechnology and Biotechnological Equipment 26(4):3094-3099. |
|
|
Zang Q, Zhou L, Zhuge F, Yang H, Wang X, Lin X (2016). Callus induction and regeneration via shoot tips of Dendrocalamus hamiltonii. SpringerPlus 5(1):1-7. |
|
|
Zhi-hong X, Gui-Yun L (1980). Histo-Cytological Observations on Callus and Bud Formation in Cultures of Nicotiana tabacum L. Journal of Integrative Plant Biology 22(1). |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0