ABSTRACT
Diabetes type 2, cancers, hypertension and obesity have become problematic diseases worldwide calling for more studies on their prevention and management. Antioxidant phytochemicals have shown potential to reduce cases of such chronic diseases therefore the present study quantified antioxidant phytochemicals in traditional dishes consumed in Zimbabwe. Traditional dishes are readily available therefore are cheap and accessible sources of food. Antioxidant phytochemicals were extracted using matrix solid phase dispersion and thin layer chromatography coupled to 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay and then quantified using high performance liquid chromatography. The levels of antioxidant phytochemicals ranged from 2 ± 0.5 to 620 ± 3 mg/g for the ethanol extract and 20 ± 3 to 377 ± 5 mg/g for the ethyl acetate extract. Type and amounts of antioxidant phytochemical present in a dish depended upon the thick porridge and relish combination selected. Combinations involving wild vegetables as relish consisted of better levels of antioxidant phytochemicals than meat combinations. No dish consisted of all the types of antioxidant phytochemicals therefore it is necessary to eat a variety.
Key words: Diabetes type 2, cancers, hypertension, obesity, phytocompounds.
Chronic diseases such as diabetes type 2, obesity, hypertension, and cancers have become a burden to the general public and governments worldwide. Specific lifestyle and dietary practices have shown either to decrease or increase the risk of chronic diseases (WHO, 2005). People have undergone significant changes in lifestyle and dietary practices whereby a decline in physical activity and consumption of foods consisting of high levels of carbohydrates and fats has taken a center stage (AFN, 2007). Increased consumption of trans-fatty acids, carbohydrates and sodium increases the risk of obesity and cardiovascular diseases (Soriano Garcia, 2013). Diabetes type 2 is increased by taking saturated fats and carbohydrates (Stadler, 2012). Therefore efforts to mitigate chronic diseases should mainly target the diet.
Although sticking to a healthy diet is an important aspect in prevention of chronic diseases however it requires its availability and affordability. Poverty results in people taking foods that promote chronic diseases. Families with small incomes tend to buy more processed and unhealthy foods in large retail grocery shops because the prizes are affordable (Gordon and Oddo, 2012; Mullany et al., 2013). Therefore interventions to be made should consider availability and affordability of healthy foods in the community. Traditional foods offer the best option since they are locally available. Traditional diets previously played an important role in food security and prevention of chronic diseases which were very rare in most African societies but now are on an increasing trend. It is believed that the increase in cases of chronic diseases is as a result of a decrease in the consumption of traditional foods (Jones Smith et al., 2013). Previous studies show a possibility of mitigating chronic diseases that directly affect human kind by improving dietary intake of antioxidant phytochemicals (Wang et al., 2013; Zhang et al., 2016).
The term antioxidant phytochemicals refers to chemical compounds which occur naturally in plants. They are responsible for their attractive colours such as, purple and red colours and typical smells such as those of herbs and fruits. Antioxidant phytochemicals that have been reported previously are mainly, phenolic acids and polyphenols (Andersen and Markham, 2006; Oh et al., 2011). Examples include sinapic acid, caffeic acid, chlorogenic acid, gallic acid, quercetin, kaempferol, rutin and catechin (Shetty et al., 2013; Naczk and Shahidi, 2004).
Antioxidant phytochemicals reduce the dangerous effects of reactive oxygen species by neutralizing them. Reactive oxygen species, produced by normal metabolic reactions or from the environment have been shown to be the major causes of cancers (Lushchak, 2011; Kyro et al., 2013), diabetes type 2 (Pitocco et al., 2013; Mursu et al., 2014), hypertension (Banday et al., 2007) and cardiovascular diseases (Yamada et al., 2011). Past studies have shown that raw traditional fruits and vegetables consist of significant amounts of antioxidant phytochemicals (Muchuweti et al., 2009; Chipurura et al., 2013; Carlsen et al., 2010; Mandindi, 2015), however little is known about the complete profile of antioxidant phytochemicals in traditional dishes especially those consumed in Africa (Mavengahama et al., 2013). In Zimbabwe and most African countries traditional dishes mainly consist of small grains or maize mealie meal thickened porridge (sadza, tsima, itschwala, phutu, or ugali) which is eaten in combination with a variety of boiled wild vegetables and seeds or meat as relish. This study therefore was designed to profile the different traditional dishes and quantify their antioxidant phytochemicals.
Chemicals
Chemical standards, salicylic acid, gallic acid, chlorogenic acid, caffeic acid, vanillic acid, p-coumaric acid, ferulic acid, quercetin, kaempferol, rutin, HPLC grade solvent (methanol), DPPH (2, 2-Diphenyl-1-picrylhydrazyl), hydrophilic lipophilic balance (HLB) water wettable sorbent and TLC plates, silica gel 60F254 (20 × 20 cm) and silica gel on aluminium strips (1.5 × 10 cm) were purchased from Sigma chemical company (St Louis MO, USA). All other chemicals were of analytical grade and were purchased from SkyLabs, Springfield, Johannesburg, South Africa.
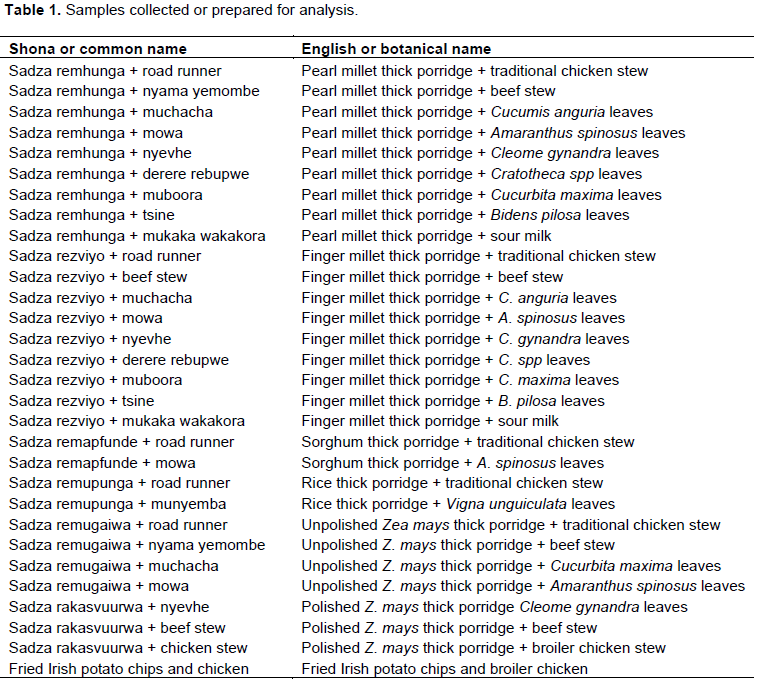
Sample collection
Traditional dishes were purchased in triplicate from food outlets around the city of Harare and from rural areas in Mashonaland East and Central. They were directly transported in a cooler box with ice to the laboratory. Some were prepared by the authors under the supervision of an experienced elderly person using raw materials bought from Mbare musika, a national market place in Harare, Zimbabwe. Samples were kept under refrigeration in the dark until required for analysis. Samples were selected basing on availability and affordability. Description of samples is shown in Table 1. Two “affluent” dish types, polished maize seed thick porridge and beef stew and fresh chips and broiler chicken from popular fast food outlets were also purchased in triplicate for comparison with the traditional dishes.
Sample preparation
Matrix solid phase dispersion was used as an initial method for extracting phytochemicals from the blended samples (Barker, 2007). HLB (0.04 g) was thoroughly mixed with 2 g of the blended and homogenized sample using a mortar and pestle. The resultant mixture was then loaded into 10 mL fritted propylene syringe barrels. Packed barrels were washed with ultrapure water prepared internally using Millipore water purification unit and vacuum dried for 2 h. Elution of phytochemicals was achieved using 12 mL of ethanol under vacuum to speed up the process. The eluent was then vacuum dried on a rotary evaporator at 60°C. The contents were weighed and redissolved in 1 mL of ethanol and stored in a refrigerator until required for thin liquid chromatographic (TLC) analysis. Elution was repeated twice to ensure that all the compounds of interest were extracted from the matrix and sorbent. The process was repeated for the ethyl acetate extract.
Analytical TLC analysis
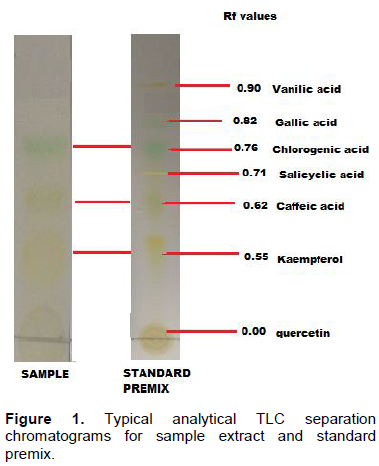
Aluminium silica gel coated strips (1.5 × 10 cm) were activated by heating gentle in an oven and then each spotted with standard premix, ethanol and ethyl acetate extracts. Spots were visualized by spraying revealing agents and observing under UV lamp at 254 and 375 nm using a Spectroline UV viewing cabinet. Several solvent mixtures were tested in the separation of the standard premix consisting of caffeic, chlorogenic, gallic, salicylic and vanillic acid, quercetin and kaempferol in order to determine the best solvent mixture that would efficiently separate the phenolics. The best solvent mixture was found to be ethanol/acetic acid/water in the ratio 6:11:3 (v/v) (Figure 1) and this was used throughout the
study in separating sample extracts. Elution was allowed through a distance of 8 cm in an air tight development chamber. The developed plates were air dried and then kept in a cool and dry place until required for phytochemical screening.
Analysis of phenolic compounds
The developed chromatographic strips were dried briefly over a hot plate and then sprayed with Folin-Ciocalteu reagent and ethanolic aluminium chloride (Singh et al., 2012).
Preparative TLC analysis
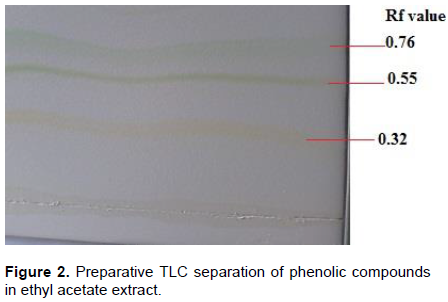
Silica gel TLC plates (60F254, 20 × 20 cm) were activated and marked at each edge and then the extracts were applied in the form of bands of 2 mm in height. The plates were developed over a distance of 12 cm using a solvent mixture consisting of ethanol, acetic acid and water in the ratio 6:11:3 (v/v). The plates were then air dried at room temperature. The bands were scratched into test tubes and dissolved in 2 mL of original solvent. The contents were vacuum filtered using Whatman no. 1 filter paper followed by 0.45 µm glass sample filters. The contents were placed in amber glass vails until required for antioxidant, UV and HPLC analysis.
Determination of antioxidant activity
All the solutions from the scratched bands were analyzed for antioxidant activity. This was performed by adding 5 drops of extract into 1 mL of 0.01 M ethanolic solution of DPPH. The time the colour changed from purple to yellow and colour intensities were noted (Figure 3). The bands which showed very strong antioxidant activity were further analyzed using HPLC to quantify the phytochemicals.
HPLC analysis
Analysis of standards and samples were performed on a Varian HPLC coupled to a prostar 325 variable UV detector. The detector was controlled remotely by the Varian star or Galaxie workstation version 6 software. Samples were separated on a Varian Microsorb MV 1005 packed C18 column, 250 × 4.6 mm id and 5 µm particle size. An isocratic separation mode was used. Different mobile phases were tested in order to determine the mobile phase that would best separate the standards efficiently. The most efficient mobile phase consisted of HPLC grade methanol and 1% aqueous acetic acid prepared in the ratio 25:75 (v/v). The mobile phase was prepared every day and degassed in a sonicator at 30°C for 5 min. Analysis conditions were room temperature, flow rate 1 mL/min and injection volume, 20 µL. The wavelength of analysis was 310 nm (Gird et al., 2014) which was also confirmed by scanning samples and standard premixes over the range of 200 to 1000 nm on a UV spectrophotometer. Standard premix was prepared by mixing 1000 mg/mL of each standard, caffeic, chlorogenic, gallic, salicylic and vanillic acid (phenolic acids) and quercetin and kaempferol (flavonoids). The calibration curve was determined over a concentration range of 1 to1000 mg/mL and was used for quantification. Peak identification was performed by comparing pick retention times and by spiking individual standards into selected samples. Limit of detection and quantification was found to be 0.01 and 0.08 ng/mL. The percentage recovery obtained by spiking 2 mg/mL of selected standards in sample matrix ranged from 89.3 ± 0.6 to 92.5 ± 0.6%.
Traditional dish samples
A total of 27 different traditional dishes (Table 1) were encountered during sampling. Most of them consisted of small grains thick porridge and wild vegetables leaves or meat as relish. The relish were prepared by either boiling or frying in cooking oil without addition of spices. Three other dishes consisting of polished maize meal thick porridge and fried chips were collected for comparison sake. These dishes are considered to be modern and are consumed more often by most affluent families in Zimbabwe.
Analytical TLC
Rf values for the analytical TLC analysis of the several dishes are shown in Table 2. The Rf values ranged from 0.05 to 0.96. The ethyl acetate extract produced more spots than the ethanol extract.
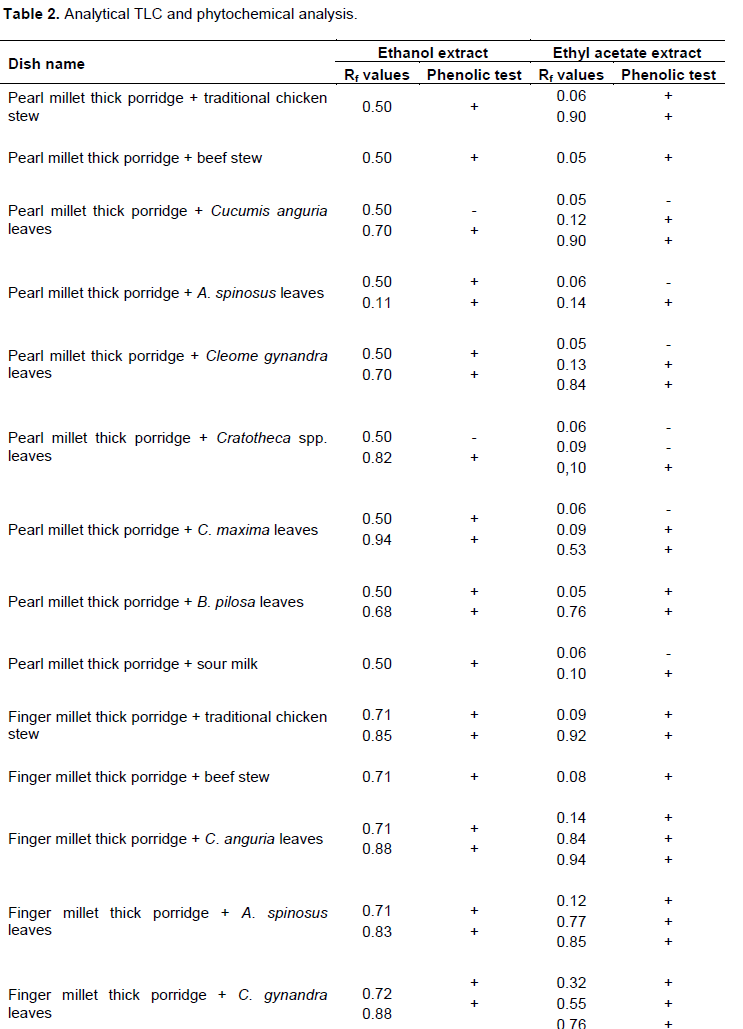
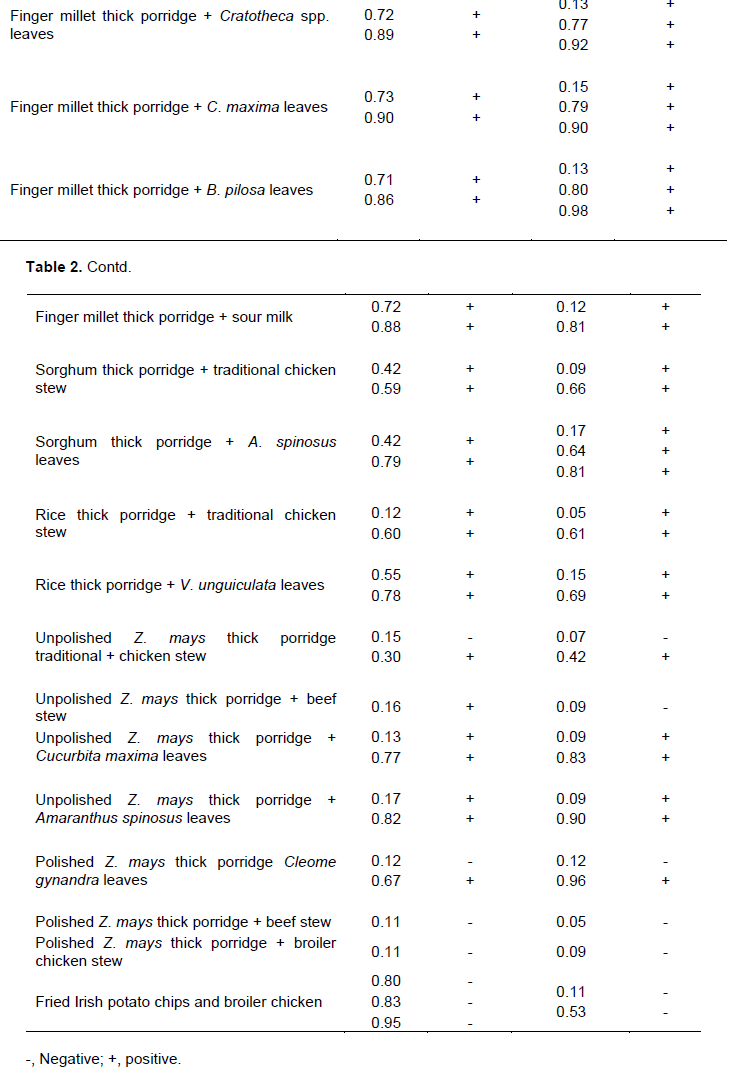
Analysis of phenolic compounds
Analyses to test for presence of phenolic compounds showed that most spots for traditional dishes tested positive for presence of phenolic compounds while all spots for polished Zea mays seeds and meat tested negative. Unpolished Zea mays seeds and meat dishes tested positive for phenolic compounds. Comparing Rf values for samples and those for standard premix (Figure 1) shows that the traditional dishes consist of phytochemicals with the same Rf values as the standards, caffeic, chlorogenic, gallic, salicylic and vanillic acid and quercetin and kaempferol. Therefore these standards were used for HPLC quantitative analysis of antioxidant phytochemicals exhibiting very strong antioxidant activity.
Antioxidant activity analysis
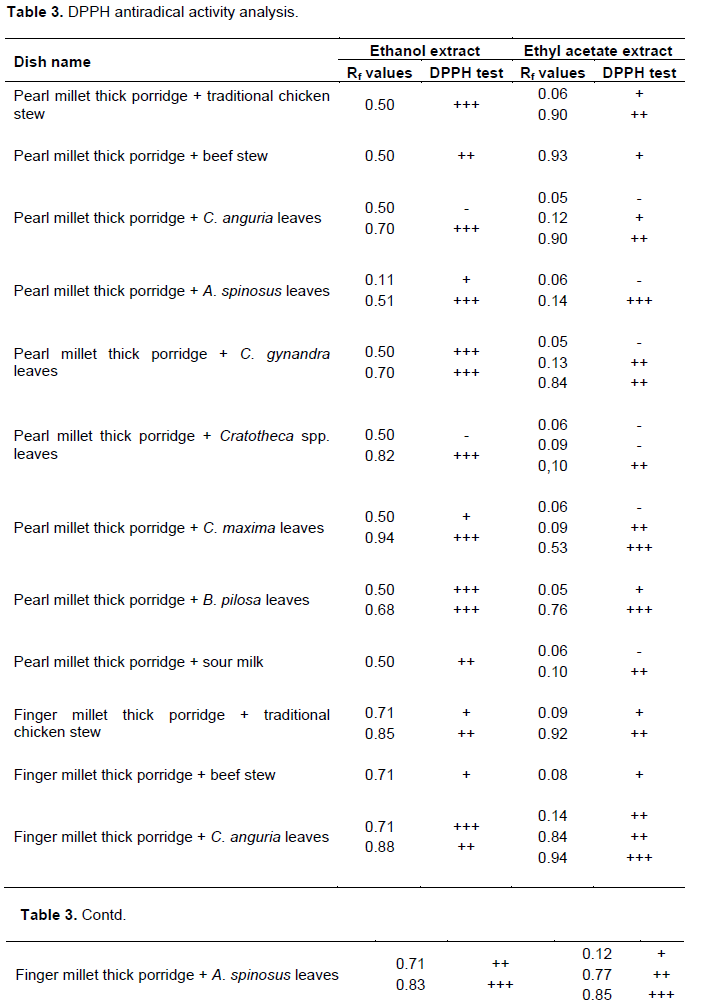
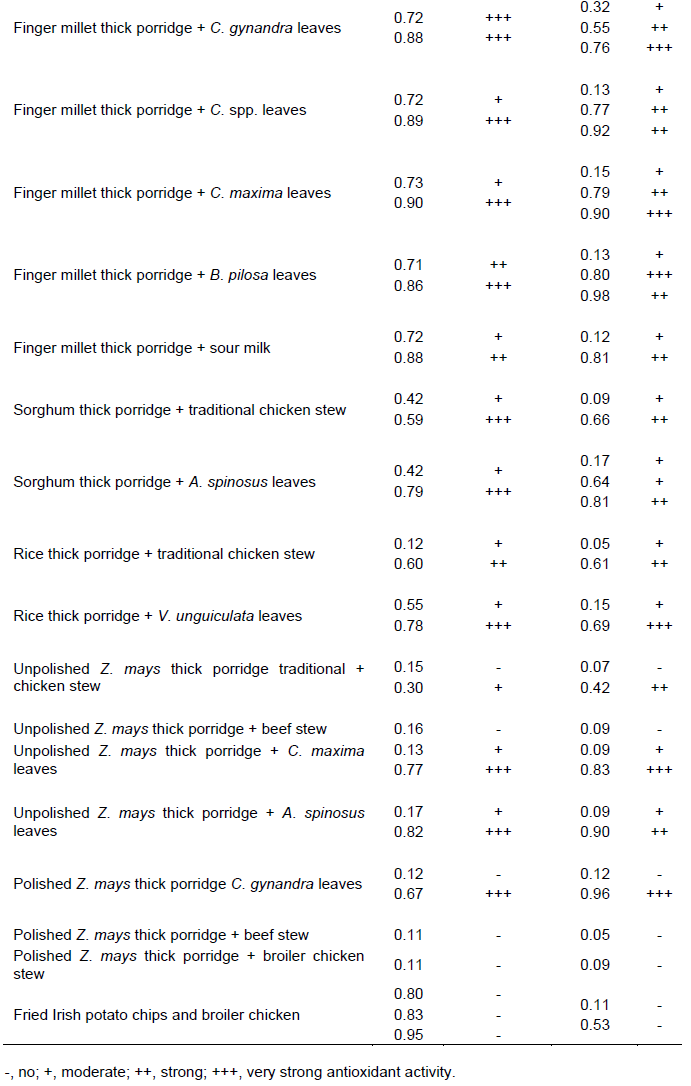
Separation of the phenolics in the samples was achieved using preparative TLC plates visualized by spraying revealing agents and a UV lamp at 254 and 375 nm. Extracts were applied on the TLC plates in the form of bands (Figure 2). Results for antioxidant analysis using DPPH a widely used radical for testing antiradical activity are shown in Table 3. Antioxidant activity was ascertained by a change of colour of the DPPH solution from purple to yellow (Figure 3). The results show that most traditional dishes exhibited antioxidant activity. Antioxidant activity ranged from moderate to very strong. Bands exhibiting very strong antioxidant activity were scratched and amounts of antioxidant phytochemicals quantified using HPLC. All bands for unpolished Zea mays seeds and meat dishes did not show antioxidant activity.

HPLC analysis
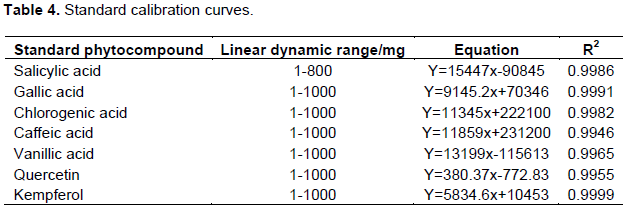
Standard calibration curves were generated by plotting peak area against concentration. Standard calibration curves for all other standards except salicylic acid showed good linearity between 1 and 1000 mg. Salicylic acid showed good linearity between 1 and 800 mg (Table 4). The calibration curves were used for quantification of antioxidant phytochemicals in traditional dishes. Typical chromatograms observed for HPLC analysis of standard premix and extracts are shown in Figure 4 The concentration of antioxidant phytochemicals are shown in Tables 5 and 6. Ethanol extract showed more bands with very strong antioxidant activity than ethyl acetate extracts however ethyl acetate extract had more bands than the ethanol extract. Concentration of antioxidant phytochemicals in the ethanolic extract ranged from 2 ± 0.5 to 620 ± 3 while that for ethyl acetate ranged from 20 ± 3 to 377 ± 5 mg/g sample. The phytochemical with the highest concentration was salicylic acid in finger millet thick porridge and C. anguria boiled leaves at Rf value of 0.71 for ethanolic acid and chlorogenic acid in pearl millet thick porridge and B. pilosa leaves at Rf value of 0.76 in ethyl acetate extract.
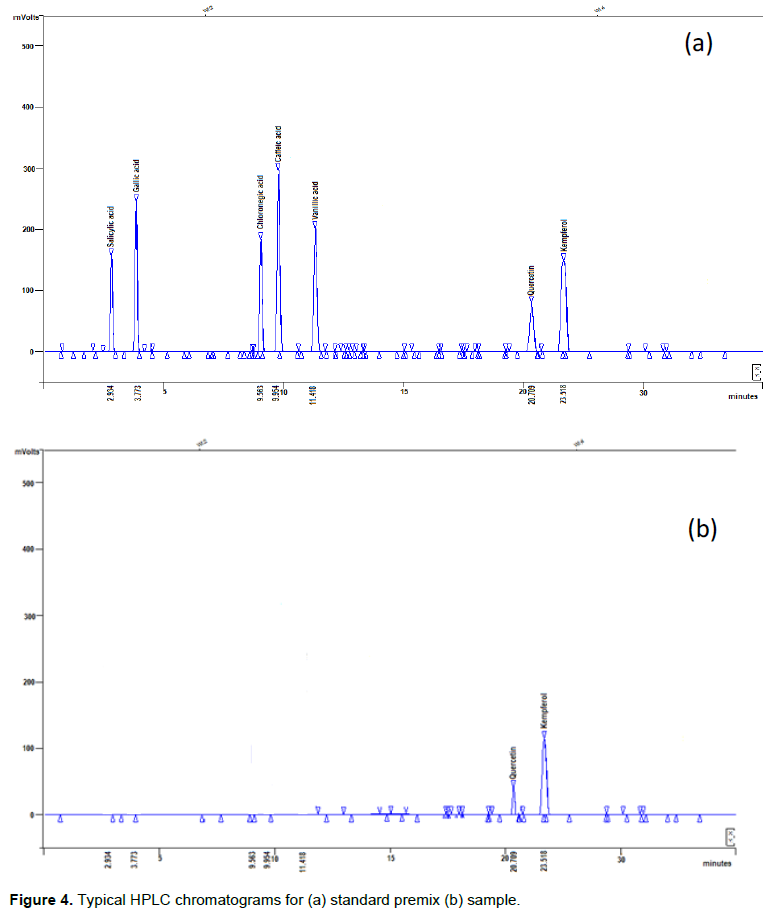
Ethanol, acetic acid and water in the ratio 6:11:3 (v/v) was found to be the best mobile phase that could separate the phytochemicals efficiently. Ethanol extracts gave 1 to 2 spots while ethyl acetate extract gave 1 to 3 spots depending on the type of the dish. The polarity of solvent has been previously observed to contribute to the type of phenolic compound that can be extracted. Flavones and less polar phenolic acids have been previously extracted using ethyl acetate and chloroform (Naczk and Shahidi, 2004). Alcohols or alcohol-water mixtures were found to be the best solvents for extracting flavonoid glycosides and more polar phenolic acids (Naczk and Shahidi, 2004).
Plant phenolics are responsible for antioxidant activity of plant extracts (Adedapo et al., 2011). Most traditional dishes studied in the present study consist of phenolics and antioxidant activity however in different amounts. Polished maize thick porridge and meat as relish tested negative for both phenolics and antioxidant tests (Tables 2 and 3). This shows that phenolic compounds in the traditional dishes may be responsible for the observed antioxidant activity. This was further substantiated by the results observed for analytical TLC analysis of extracts and standard premix (Figure 1). Based on the present results, each traditional dish at least consists of either a phenolic acid or a flavonoid exhibiting a very strong antioxidant activity. The contents of phenolics portraying a very strong antioxidant activity varied depending on dish combinations. For instants, pearl millet thick porridge and traditional chicken stew (Rf value 0.50) consist of quercetin and kaempferol only while pearl millet thick porridge and C. anguria leaves (Rf value 0.70) consist of salicylic acid and caffeic acid (Table 5). It is important to note that combining thick porridge with vegetables offer better results than meat products. Polished Z. mays thick porridge with boiled Cleome gynandra leaves as relish portrayed strong antioxidant activity while polished Z. mays thick porridge with either broiler chicken or beef stew exhibited no antioxidant activity. Pearl millet thick porridge and traditional chicken stew exhibited very strong antioxidant activity than pearl millet thick porridge and beef meat. The antioxidant activity of the former may be attributed to the traditional chicken which is raised using small grains and wild vegetables as its food. Antioxidant activity depends on the number of hydroxyl groups present in the molecule. It is believed that the the number of hydroxyl groups that can donate hydrogen to reduce free radicals. Thus results of the present results are important as previous studies have reported that polyphenols from traditional foods may not be beneficial due to complexation to mineral elements (Gupta et al., 2006).
For the ethanolic extract, finger millet thick porridge combined with boiled C. anguria leaves as relish (Rf value 0.71) consist of high levels of salicylic acid portraying high antioxidant activity, 620 ± 3 mg/g of sample followed by pearl millet thick porridge and C. gynandra leaves at Rf value of 0.70, 507 ± 3 mg/g. Pearl millet thick porridge and C. anguria leaves (Rf value 0.70), pearl millet thick porridge and B. pilosa leaves (Rf value 0.68), finger millet thick porridge and C. gynandra leaves (Rf value 0.72) also consisted of salicylic acid as the phenolic acid exhibiting high antioxidant activity. This is in contrast with ethyl acetate extract. Only one dish, rice thick porridge and V. unguiculata boiled leaves (Rf value 0.69) consisted of salicylic acid. The trend was similar for the other phenolics. Thus ethanol is a better solvent for extracting antioxidant phytochemicals than ethyl acetate. This is because ethanol tends to interact more with non-complexed hydroxyl groups through hydrogen bonding. Gallic acid was also found in large quantities in pearl millet thick porridge combined with Cratotheca spp. leaves and in unpolished Z. mays thick porridge with A. spinosus leaves at a concentration of 411 ± 1 and 612 ± 5 mg/g respectively. Generally for both ethyl acetate and ethanolic extracts the traditional dishes studied in the present study consist of more phenolic acids than polyphenols. It is important to note that polished maize thick porridge and meat and fried boiler chicken and Irish potato chips exhibited no antioxidant activity. These dishes are considered to be the food of the affluent while traditional dishes as food of the poor people living in rural areas (Mavengahama et al., 2013). High temperatures involved in frying results in formation of free radicals which may diminish natural antioxidants in foods as they are involved in removing them (Castro et al., 2011; Mariutti et al., 2008). Previous studies have shown that addition of vegetables improved the quality of meat based foods (Mariutti et al., 2011) Presence of significant amounts of antioxidant phytochemicals in traditional dishes means that they are good candidates for fighting chronic diseases. The importance of traditional dishes has remained until recently largely unacknowledged and unrecognized by the common man, policy makers and nutritionist. This is because there is a general lack of studies discussing the importance of traditional dishes to human health (Flyman and Afolayan, 2006). Although many websites discuss the importance of eating traditional foods scientific information to back the claims are still limited. Results of the present study reveal that there is a need to promote consumption of traditional foods and their incorporation in national food baskets. Instead of them being taken as supplements or alternatives in case of unavailability of ‘exotic dishes’ (Kepe, 2008) they should be considered as an integral part of the main meal. Traditional dishes carry food for the poor or famine food tags hence they are unpopular to majority of people especially the young ones. Some people would not eat traditional dishes when exotic foods are available. The current philosophy about traditional dishes prohibits their cultivation. They are only harvested from the forest. This is exploitative and unsustainable and may results in decline of the species. It could lead to genetic erosion and possible loss of biodiversity (Bharucha and Pretty, 2010). There is a need therefore for more studies in order to encourage cultivation and utilization of traditional foods.
Traditional dishes studied in the present study consist of higher amounts of antioxidant phytochemicals as compared to spiced fresh chips and broiler chicken meat. Levels of antioxidant phytochemicals depend on the thick porridge and relish combination chosen. Wild vegetables offer better results as compared to meat as relish only. Polished maize thick porridge and meat consist of low amounts of antioxidant phytochemicals. No dish consisted of all antioxidant phytochemicals therefore eating a variety of them will offer better results. Presence of antioxidant phytochemicals shows that eating traditional dishes frequently may result in reduced cases of free radicals induced chronic diseases such as cancers, diabetes type 2, hypertension and obesity.
The authors have not declared any conflict of interests.
REFERENCES
|
Adedapo A, Jimo F, Afolayan (2011). Comparison of the nutritive value and biological activities of the acetone, methanol and water extracts of the leaves of Bidens pilosa and Chenopodium album. Acta Pol. Pharm. 68:83-92.
|
|
|
|
AFN (2007). Traditional foods. Are they safe for first nation consumption? Assembles of First nations Environmental Stewardship Unit. Ottawa. Ontario.
View
|
|
|
|
|
Andersen ØM, Markham KR (2006). Flavonoids Chemistry, biochemistry and applications. London: CRC press Taylor and Francis Group. Pp. 1-10.
|
|
|
|
|
Banday AA, Muhammad AB, Fazili FR, Lokhandwala M (2007). Mechanisms of oxidative stress-induced increase in salt sensitivity and development of hypertension in Spraque-Dawley rats. Hypertension 49:664-671.
Crossref
|
|
|
|
|
Barker SA (2007). Matrix solid phase dispersion (MSPD). J. Biochem. Biophys. Methods 70:151-162
Crossref
|
|
|
|
|
Bharucha Z, Pretty J (2010). The roles and values of wild foods in agricultural systems. Philos. Trans. R. Soc. Lond. B Biol. Sci. 365:2913-2926.
Crossref
|
|
|
|
|
Carlsen MH, Halvorsen BL, Holte K, Bohn S.K, Dragland S, Sampson L, Willey C, Senoo H, Umezono Y, Sanada C (2010). The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutr. J. 3:1-11.
Crossref
|
|
|
|
|
Castro WF, Mariutti LRB, Bragagnolo N. (2011). The effects of colorifico on lipid oxidation color and vitamin E in raw and grilled chicken patties during frozen storage. Food Chem. 124:126-131.
Crossref
|
|
|
|
|
Chipurura B, Muchuweti M, Kasiyamhuru A (2013) Wild vegetables consumed in Buhera district of Zimbabwe and their phenolic compound content. Ecol. Food Nutr. 52:178-189.
Crossref
|
|
|
|
|
Flyman MV, Afolayan AJ (2006). A survey of plants used as wild vegetables in four districts of Botswana. Ecol. Food Nutr. 45:405-415.
Crossref
|
|
|
|
|
Gird CE, Nenai I, Costea T, Dutu LE, Poesiu ML, Ciupitu N (2014). Quantitative analysis of phenolic compounds from Salvia officinalis L. leaves. Farmacia 62:649-657.
|
|
|
|
|
Gordon A, Oddo V (2012). Addressing child hunger and obesity in Indian country Report to congress office of research and evaluation. Food and Nutrition service. Alexanda, VA. www.fns.usda.gov/addesing child-hunger-and obesity-Indian-country-report-congress. Pp. 1-15.
|
|
|
|
|
Gupta S, Lakshimi AJ, Prakash J (2006). In vitro bioavailability of calcium and iron from selected green leafy vegetables. J. Sci. Food Agric. 86:2147-215.
Crossref
|
|
|
|
|
Jones-Smith JC, Karter AJ, Warton EM, Kelly M, Kersten E, Moffet HH, Ader N, Schillinger D, Laraia BA. (2013). Obesity and the food environment: Income and ethnicity difference among people with diabetes. Diabet. Care 36:2697-2705.
Crossref
|
|
|
|
|
Kepe T (2008). Social dynamics of the value of wild vegetables (imfino) in South Africa rural area. Ecol. Food Nutr. 4:530-536.
|
|
|
|
|
Kyro C, Skele G, Loft S, Landberg R, Christensen J, Lund E, Nilsson LM, Palmqvist R, Tjonneland A, Olsen A. (2013). Intake of whole grains from different cereal and food sources and incidence of colorectal cancer in the Scandinavian. HELGA cohort. Cancer Causes Control 24:1368-1374.
Crossref
|
|
|
|
|
Lushchak VI (2011). Environmental induced oxidative stress in aquatic animals. Aquat. Toxicol. 101:13-30.
Crossref
|
|
|
|
|
Mandindi TK (2015). Phytochemical and antioxidant composition of selected local wild plants in South Africa. Consideration of alternative nutrients for health promotion. IPCBEE 81:87
|
|
|
|
|
Mariutti LRB, Nogueira GC, Bragagnolo N (2011). Lipid and cholesterol oxidation in chicken meat are inhibited by sage but not by garlic. J. Food Sci. 6:909-915.
Crossref
|
|
|
|
|
Mariutti, LRB, Orlien V, Bragagnolo N, Skibstead LH (2008). Effect of sage and garlic on lipid oxidation on high pressure processed chicken meat. Eur. Food Res. Technol. 227:337-344
Crossref
|
|
|
|
|
Mavengahama S, Mclachlan M, de Clercq W (2013). The role of wild vegetable species in household food security in maize based subsistence cropping systems. Food Chem. 5:227-233.
Crossref
|
|
|
|
|
Muchuweti M, Kasiamhuru A, Benhura MMM, Chipurura B, Amuna P, Zotor F, Parawira W (2009). Assessment of the nutritional value of wild leaf vegetables consumed in the Buhera district of Zimbabwe: A preliminary study. Acta Horticul. 806:323-330.
Crossref
|
|
|
|
|
Mullany B, Neault N, Tsingine D, Powers J, Lovato V, Clitso L, Massey S, Talgo A, Speakman K, Barlow A (2013). Food insecurity and household eating patterns among vulnerable American-Indian families associations with caregiver and food consumption characteristics. Public Health Nutr. 16:752-760.
Crossref
|
|
|
|
|
Mursu J, Virtanen K.J, Tuomainen TP, Nurmi T, Voutilainen S (2014). Intake of fruit berries and vegetables and risk of type 2 diabetes in Finnish men: the kuopiolschaemic heart diseases risk factor study. Am. J. Clin. Nutr. 99:328-333.
Crossref
|
|
|
|
|
Naczk M, Shahidi F (2004). Extraction and analysis of phenolics in food. J. Chromatogr. A 1054:95-111.
Crossref
|
|
|
|
|
Oh MM, Cary EE, Rajashekar CB (2011). Antioxidant phytochemicals in Lettice grown in high tunnels and open field. Hortic. Environ. Biotechnol. 52:133-139.
Crossref
|
|
|
|
|
Pitocco D, Tesauro M, Alessando R, Ghirlanda G, Cardillo C (2013). Oxidative stress in diabetes: Implication for vascular and other complications. Int. J. Mol. Sci. 14:21525-21550.
Crossref
|
|
|
|
|
Podsedek A (2007). Natural antioxidant and antioxidant capacity of Brassica vegetables. A review. LWT Food Sci. Technol. 40:1-11.
Crossref
|
|
|
|
|
Shetty AA, Magadum S. Managanvi K (2013). Vegetables as Sources of Antioxidants. J. Food Nutr. Disor. 21:564-576.
Crossref
|
|
|
|
|
Singh D, Singh P, Gupta A, Solanki S, Sharma E, Nema R (2012). Qualitative Estimation of the presence of Bioactive Compound in Centella asiatica: An Important Medicinal Plant. Int. J. Life Sci. Med. Sci. 2:1456-1459.
|
|
|
|
|
Soriano Garcia MG (2013). Emerging Role of Natural Antioxidants in Chronic Disease Prevention with an Emphasis on Vitamin E and Selenium. In. Oxidative Stress and Chronic Degenerative Diseases - A Role for Antioxidants, Dr. Jose Antonio Morales-Gonzalez (Ed.), InTech. Pp. 420-448.
Crossref
|
|
|
|
|
Stadler K (2012). Oxidative stress in diabetes. Adv. Exp. Med. Biol. 771: 272-282.
|
|
|
|
|
Wang L, Chen J, Xie H, Ju X, Liu RH (2013). Phytochemical profiles and antioxidant activity of adlay varieties. J. Agric. Food Chem. 61:5103-5113.
Crossref
|
|
|
|
|
WHO (2005). Preventing chronic diseases: A vital investment. WHO Global Report. Geneva. World Health Organization.
|
|
|
|
|
Yamada T, Hayasaka S, Shibata Y, Ojima T, Saegusa T, Gotoh T, Ishikawa S, Nakamura Y, Kayaba K (2011). Frequency of citrus fruit intake is associated with the incidence of cardiovascular diseases. The Jichi Medical School Cohort Study. J. Epidemiol. 21:169-175.
Crossref
|
|
|
|
|
Zhang LB, Lei C, Gao LX, Li JY, Li J, Hou AJ (2016). Isoprenylated flavonoids with PTPIB inhibition from Macaranga denticulate. Nat. Prod. Bioprospect. 6:25-30.
Crossref
|
|