Full Length Research Paper
ABSTRACT
The aim of the research was to investigate the antibacterial activities and the phytochemical properties of aqueous and ethanolic extracts of Mango (Mangifera indica) and Neem (Azadirachta indica) leaves, on clinical isolates of Salmonella typhi, Salmonella paratyphi A, B and C, in Lafia, Nigeria. The agar well diffusion technique was used and the analyses were done in triplicates. At the highest aqueous and ethanolic extract concentration of 80 mg/ml, S. typhi, showed inhibition zones of 29.4±0.1mm and 30.0±0.01 mm respectively, while S. paratyphi A, B and C, exhibited inhibition zones of 14.4±0.2, 21.2±0.4, 13.4±0.1 mm and 18.0±0.03, 20.0±0.04, 21.0±0.04 mm, respectively. At aqueous and ethanolic lower extract concentration of 10 mg/ml, S. typhi, exhibited inhibition zones of 8.4±0.01 and 9.0±0.1 mm, respectively, while S. paratyphi A, B and C, showed inhibition zones of 9.5±0.01, 9.1±0.01, 6.2±0.01 mm and 9.0±0.01, 9.0±0.01, 9.0±0.01 mm, respectively of Neem leaf extract. The MIC and MBC, of aqueous and ethanolic extracts against organisms were 2.5 and 5.0 mg/ml, respectively. The qualitative phytochemical results showed the presence of alkaloids, anthraquinones, carbohydrates, cardiac glycosides, flavonoids, tannins, terpenoids and saponins. Conclusively, the aqueous and ethanolic extracts of the studied medicinal plant exhibited bactericidal activities against all tested organisms, and this supports the claim by traditional medical practitioners and vendors of the use of the plants for the cure of typhoid fever. Further studies are recommended on the toxicity and safe dosage regimen of the plants since the infusion of the plant is taken orally by people for cure of typhoid fever.
Key words: Salmonella organisms, typhoid fever, phytochemical constituents, bacteria, medicinal plants.
INTRODUCTION
There is a great concern over the emergence and the spread of bacteria resistant to drug all over the world (Boucher et al., 2009). Therefore, there is the need to find an efficient and safe alternative to microbial resistance to drugs, and the alternative resides in phytomedicine.
Although, various brands of new antibiotics have been manufactured by pharmacological companies every year, the global emergency of multidrug resistant (MDR) bacteria is limiting the potency of these drugs (Hancock, 2005). For example, multidrug resistant Salmonella Typhi, showed resistance to all three first line of drugs: Ampicillin, Chloramphenicol and Trimethioprim-sulfamethoxazole (Ackers et al., 2000). This poses challenges to researchers which prompted studies to explore the phytotherapeutic potential of medicinal plants including Mango (Mangifera indica) and Neem (Azadirachta indica), as a source for alternative medicine to microbial resistant drugs which are cheaper and safer than conventional drugs.
Medicinal plants such as Mango (M. indica) and Neem (A. indica), have been used to treat human ailments including malaria, anemia, diabetes and cancer, for many years because they possess organic compounds including alkaloids, flavonoids, phenols, tannins, saponins, terpenoids, and cardiac glycosides, that are antibacterial and have definite physiological action on the human body (Byarygaba, 2004). For instance, the use of plant extracts for antimicrobial therapy has proven to be a promising remedy in Chinese phytomedicine, Indian Ayurvedic, Arabic and Unani medicines (WHO, 2002 - 2005). In Africa, the use of medicinal plants for the treatment of diseases has been since the introduction of modern medicine (Kabir et al., 2005). In Southern Nigeria, for example, Acalypha wilkesiana, a member of the Euphorbiaceae family, is used to treat malaria, dermatological and gastrointestinal disorders and has antimicrobial properties (Kabir et al., 2005; Oladunmoye, 2006; Erute and Oyibo, 2008). The interest in plants is because they are available, easily accessible, cheap for both the rich and the poor populace, and safer and more cost-effective sources for alternative medicine (Doughari et al., 2007; Osuagwu et al., 2015).
At least 25% of drugs in modern pharmacopoeias are derived from compounds isolated from medicinal plants, such as Mango (M. indica) and Neem (A. indica) (De Silva, 2005). Accordingly, extracts from medicinal plants have a wide range of bioactive and pharmacological activities which essentially include antibacterial, antifungal and anti-inflammatory properties (Okwu, 2005). Infusion and concoctions prepared from different parts of medicinal plants are used in the treatment of various human diseases, including typhoid fever, a systemic disease which is caused by the Salmonella organism, which has been reportedly resistant to both Trimethioprim sulfamexazole and Chloramphenicol (WHO, 2007). Fabricant and Farnsworth (2001) and Bussmann et al. (2006), have reported that plant extracts are traditionally used in most nonindustrialized nations for the treatment of human diseases, including typhoid fever. Gatsin et al. (2007), Agada et al. (2010), Uhuo et al. (2015) and Rachuonyo et al. (2016), have demonstrated the bactericidal effect of plant extracts on S typhi, Salmonella paratyphi A, B and C, in the West Province of Cameroon, Jos Plateau State, Ebonyi State and Kenya, respectively.
Rachuonyo et al. (2016) studied the in-vitro antimicrobial activity of methanolic leaf extracts from four plants (A. secundiflora, Bulbine frutescens, Vernonia lasiopus and Tagetes minuta) against S. typhi in Kenya, using a disc diffusion method. They reported A. secundiflora bactericidal against tested organisms at low concentration of 5.5 mg/ml; MIC of plant extracts on targeted organism varies from 5 to 9 mg/ml; MBC against organism ranged from 7 to 11 mg/ml. Reported qualitative phytochemicals include alkaloids, flavonoids, tannins and saponins. Uhuo et al. (2015), reported herb extracts of Vernonia amygdalina, Allium sativum and Allium cepa, had a strong inhibitory effect against S. typhi, in their study on antibacterial activities of some medicinal plants on S. typhi isolates in Abakiliki, Ebonyi State, Nigeria, using an agar diffusion method. It records the presence of secondary metabolites, including alkaloids, glucose, tannins, glycosides, flavonoids, steroids and phenolic compounds. Gatsing et al. (2007), recorded MIC values of 6 mg/ml against S. typhi and S. paratyphi B, and MBC value of 300 µg/ml against S. typhi and S.paratyphi B, in their study of antibacterial agents from methylene chloride and methanol leaf extract of Crinum purpurascens herb collected from the West Province of Cameroon, using both agar diffusion and broth dilution methods.
The mango (M. indica L) plant belongs to the family Anacardiaceae, which consists of about sixty genera and 600 species (Akinpelu and Onakoya, 2006). Mango is one of the tropical fruit bearing trees in the world (Kabuki et al., 2000). Mango is a medicinal herb that is used traditionally in the treatment of diseases including mouth infection in children, diarrhea, dysentery, gastrointestinal tract disorders, typhoid fever, sore throat and scurvy (Campbell et al., 2002; Fowler, 2006). Its ground seeds and leaves have been used to treat diabetes, colic and irritation from scorpions and bee stings (Doughari and Manzara, 2008). The leaves of M. indica have been reported to contain glycoside and mangiferin, which is an antimicrobial agent and mangiferin has been demonstrated to possess antiviral activity against the herpes simplex type 2 virus (Zakaria et al., 2006). The Neem (A. indica) plant, on the other hand, belongs to the family of Melioceae. Neem is a tropical plant that has adapted to a wide range of climatic, topographic and environmental factors and has immense potentials. In Indian traditional Ayurvedic medicine, different parts of Neem tree have been used for the treatment of various ailments. The Neem oil, bark and leaf extracts have been used to control leprosy, intestinal helminthiasis and respiratory disorders.
Mango and Neem plants are medicinal herbs that have been used traditionally in Chinese phytomedicine, Indian Ayurvedic, Arabic, Unani, and African medicines particularly in Nigeria, to treat various human ailments, including typhoid fever, but their antibacterial activity against clinical isolates of Salmonella organisms in Lafia, Nigeria, has not been tested. Therefore, the aim of the research is to study the antibacterial activity and phytochemical properties of mango and Neem leaf, aqueous and ethanolic extracts against clinical isolates of S. typhi, S. paratyphi A, B and C, in Lafia, North Central, Nigeria, as an alternative to medicare for typhoid fever.
MATERIALS AND METHODS
Collection of plant materials
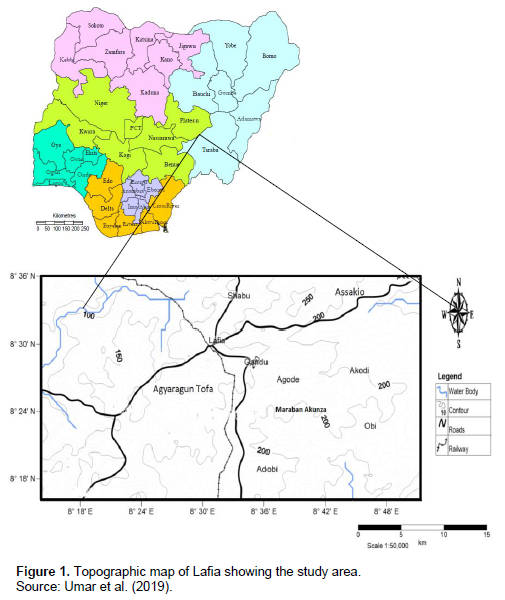
The procedures described by Osuagwu et al. (2015), good agricultural collection practice and Field collection practice (GACP and FCP, 2004), were the sampling techniques adopted for the collection of plant materials. Fresh leaves of mango (M. indica) and Neem (A. indica), were randomly collected from different location sites (Figure 1): Gandu and Akunza, in Lafia local government area, of Nasarawa State, in the morning before sunrise (to avoid degradation of plants biocomponents by ultra violet rays). The leaves were identified by a trained taxonomist and Botanist in the Department of Botany, Federal University of Lafia, Nasarawa State. A voucher specimen was assigned to the samples for record purposes. The leaves were then washed 5 to 6 times with clean water to remove dust and dirt; thereafter, dried in shade at room temperature 27°C till crisp and brittle to touch; separately ground to powder using a laboratory mortar and pestle; separately sieved through a sieve 0.5 to 10 mm to obtain fine particle size (GACP and FCP, 2004). The powdered plant samples were stored in a labeled airtight clean, dried opaque polythene container, until ready for analysis.
Aqueous extraction
One hundred grams each of dried finely powdered plant samples were weighed separately into different glass beakers using analytical weighing balance (aeAdam, Model PW 124, UK). Then, 500 ml of sterile distilled water were added into each beaker. The mixtures were heated on a hot plate (Model SB 160, UK), at 30 to 40°C with intermittent stirring for 20 min. Thereafter, the aqueous extracts were filtered through Whatman filter paper, number one. The filtrates were evaporated separately in a water bath at 65°C, and the crudes were labeled and stored in a refrigerator until ready for analysis (Asowata et al., 2013).
Ethanolic extraction
Using analytical weighing balance (aeAdam, Model PW 124), 100 g of dried finely powdered mango and neem leaves were weighed into separate glass beaker, and 70% ethanol added to each beaker. The mixture was soaked and agitated intermittently for 72 h. After that the contents were filtered through Whatman filter paper number one. Thereafter, the filtrates were evaporated separately in a water bath at 60°C. The yield was weighed and recorded. The dried crude was stored in a refrigerator till ready for analysis (Asowata et al., 2013).
Phytochemical evaluation of the plant extract
Qualitative analysis of phytochemical constituents in plant extracts
Test for alkaloids: The alkaloids content of plant samples was determined by the method described by Aiyelaagbe and Osamudiamen (2009). 0.5 g of the crude extract was mixed with 3 ml of 1% HCL, and boiled for 5 min. The mixture was cooled and filtered. Thereafter, the filtrate was treated with Mayer’s, Wagner’s and Dragendroff’s reagents. The turbidity of the resulting precipitate was an indication of the presence of alkaloids.
Test for reducing sugars: Reducing the sugars content of plant extracts was determined by the method described by Aiyelaagbe and Osamudiamen (2009). One milliliter of plant extract was treated with a mixture of 2 ml of Fehling’s solution (A and B) in a test tube. The setup was gently boiled. The color changes from deep blue to brick red, which indicates the presence of reduced sugars.
Test for glycosides: The method described by Aiyelaagbe and Osamudiamen (2009), was adopted for glycoside determination in plant crude extracts. One millimeter of plant crude extract was mixed with 2 ml of chloroform and 2 ml of acetic acid in a test tube. The setup was cooled in an ice bath, followed by the careful addition of concentrated H2SO4. A color change from violet to blue to green indicates the presence of steroidal nucleus, that is, glycone portion of the glycosides.
Test for flavonoids: The method described by Aiyelaagbe and Osamudiamen (2009) was adopted for the presence of flavonoids in the plant extract. 2 g of plant extract in a glass test tube was detanned using acetone. After that the setup was placed in a hot water bath for traces of acetone to evaporate. Thereafter, boiling water was added to the detanned sample and the mixture was filtered while hot and allowed to cool. Then 5 ml of 20% NaOH solution was added to an equal volume of the filtrate. A yellow solution was evidence of the presence of flavonoids.
Test for tannins/phenol: The method described by Aiyelaagbe and Osamudiamen (2009) was adopted for the presence of tannins in plant crude extract. 0.5 g of plant crude extract was dissolved in 1 ml of distilled water in a glass test tube, and filtered through Whatman filter paper number one. 3 ml of the filtrate in a test tube was added 2 mL of 2% solution of FeCl3. A blue-green or black coloration was an evidence of the presence of tannins.
Test for terpenoids: The method described by Aiyelaagbe and Osamudiamen (2009) was adopted for analysis of terpenoids in plant crude extract. 0.1 g of plant crude extract was dissolved in 2 ml of chloroform in a test tube, then evaporated on a water bath to dryness. Thereafter 2 ml of concentrated H2SO4 was carefully added to the residue, then heated for 2 min. The grayish coloration was evidence of the presence of terpenoids.
Test for anthraquinones: Procedures described by Aiyelaagbe and Osamudiamen (2009), were the method used to determine anthraquinones in plants crude extract. 0.5 g of plant crude extract was dissolved in 5 ml of chloroform in a test tube, and the setup was shaken for 5 min. The resulting mixture was filtered, and 3 ml of the filtrate was shaken with an equal volume of 100% ammonia solution in a test tube. A pink, violet/red color in the ammoniacal layer indicates the presence of free anthraquinones.
Test for saponins: The method described by Aiyelaagbe and Osamudiamen (2009), was used to determine the presence of saponins in plant crude extracts. 0.5 g of the extract in a test tube was shaken with distilled water. The presence of saponins in the sample was indicated by persistence of front during warming.
Quantitative analysis of phytochemicals in plant crude extract
Determination of alkaloids
Harborne (1973) was the method used for the analysis of alkaloids in the crude extract. The test was done in triplicate. 5 g of the sample in a 250 ml beaker and 200 ml of 10% acetic acid in ethanol was added, then covered with a watch glass and allowed to stand for 4 h. The mixture was then filtered, and the filtrate was concentrated in a water bath to one quarter of its original volume. Then concentrated ammonium hydroxide was added to the concentrated filtrate drop wise till the precipitate was completed. The precipitate was then collected on a weighed filter paper and washed in diluted ammonium hydroxide (2M). The residue was dried in an oven at 80°C and the weight was calculated and expressed as a percentage of the weight of the sample.
Assay of flavonoids
Procedures adopted by Boham and Kocipai-Abyazan (1994), were used for the determination. Analysis was done in triplicates. 10 g of the plant sample was extracted repeatedly with 100 ml of 80% aqueous ethanol at room temperature until the residue becomes colorless. After that, the whole mixture was filtered through Whatman filter paper number one. Thereafter, the filtrate was transferred into a weighed crucible and was evaporated to dryness in a water bath. The difference in the constant weight of the crucible gave the value of the flavonoids of the assayed sample and the result was expressed as a percentage of the original sample.
Determination of saponins
The method described by Obadoni and Ochuko (2001), was used for the assay and analysis was carried out in triplicates. 20 g of the plant sample was placed in a 250 ml conical flask and 200 ml of 20% ethanol was added. The setup was heated in a water bath at 55°C for 4 h with continuous stirring. After that, the mixture was filtered and the residue was re-extracted with another 200 ml of 20% ethanol. The combined extracts were reduced to 40 ml in a water bath at 90°C. Then, the concentrate was transferred into a glass separatory funnel and 20 ml of diethyl ether was added and the setup was shaken vigorously. After that, the aqueous layer was recovered while the ether layer was discarded. The purification process was carried out once more. Then, 60 ml normal butanol was added and was washed twice with 10 ml 5% aqueous sodium chloride. After that, the solution was evaporated in a water bath and the residue was dried in an oven at 80°C to a constant weight.
Saponin content was calculated and the result expressed as percentage of the original sample.
Assay of tannins
For the analysis of tannins in the plants crude extract, Van Burden and Robinson (1981), procedures were used for analysis of tannins in plant crude extract. The analysis was done in triplicates. 5 g of plant sample was in a 100 ml plastic bottle and 50 ml distilled water was added and the setup was shaken for 1 h on a mechanical shaker. After that, the mixture was filtered into a 50 ml volumetric flask and diluted to the mark with distilled water. 5 ml of the filtrate were pipetted into a test tube and mixed with 2 ml of 0.1M FeCl3 and 0.1M HCL and 0.008M K4[Fe (CN)6]. A blank sample was also prepared. The absorbance of the sample was read at 395 nm against the blank within 10 min of preparation using the T60 UV-Visible spectrophotometer (PG Instrument, UK). A standard curve was prepared using tannic acid to get the 100 ppm measurement limit.
Assay of total phenol
The analysis was carried out in triplicates. 10 g of powdered plant sample was defatted with 100 ml diethyl ether for 2 h using a Soxhlet apparatus. The free fat sample was then boiled with 50 ml of ether to extract the phenolic component before being filtered. Then 5 ml, of the filtrate was pipetted into a 50 ml volumetric flask and 10 ml of distilled water, 2 ml NH4OH solution and 5 ml amyl alcohol were added and diluted to mark with distilled water. The setup was allowed to react for 30 min for color development. Thereafter, the absorbance of the solution was read at 505 nm using the T60 UV-VIS Spectrophotometer (PG Instrument, UK). A standard curve was prepared using 0, 25, 50, 75, and 100 mg/l solutions of gallic acid in methanol: water (50:50 v/v). The total phenol value was expressed in g gallic acid equivalent per 100 g dry weight (g GAE/100 g dry mass) (Ebrahimzaded et al., 2008).
Determination of cardiac glycosides
The method described by El-Olemy et al. (1994), was used for the analysis of cardiac glycosides of plant extracts. The experiment was done in triplicates with 1 g of fine powdered plant sample used in 250 ml beaker. Ten milliliters of 70% alcohol were added to the setup and allowed to soak for 2 h. After that, the mixture was filtered and 8 ml of the filtrate was diluted to 100 ml with distilled water in a 100 ml standard flask. Then 8 ml of the diluted filtrate was transferred into a 100 ml standard flask and 8 ml of 12.5% lead acetate solution (to precipitate resins, tannins and pigments) was added and the setup was mixed by shaking and was diluted to the mark with distilled water and later filtered. After that, 50 ml of the filtrate was transferred into a 100 ml standard flask and 8 ml of 4.7% disodium hydrogen phosphate solution was added (precipitate excess lead ions) and the content was diluted to the mark with distilled water and the mixture was filtered twice through Whatman filter paper number one (obtain purified filtrate). Then 10 ml of purified filtrate was transferred into a 50 mL beaker and 10 ml of freshly prepared Baljet’s reagent was added into the beaker and the content was mixed and the setup was allowed to stand at room temperature for 1h for color development. A blank sample was treated similarly. The sample was read at 495 nm against a blank using the T60 UV-Vis Spec (PG Instrument). Differences between the intensity of sample and blank, gave the absorbance which is proportional to the concentration of cardiac glycoside in the analyzed sample and the result was expressed in percentage from the relation:
% glycoside = A × 100/17;
Where A = absorbance of sample at 495 nm.
Preparation of media
The media used include Muller-Hinton agar, nutrient broth, nutrient agar, urea broth, triple sugar iron agar, Simmon’s citrate agar, and motility agar. The media was prepared in accordance with the Manufacturer’s instructions. Each batch of prepared media was tested for sterility before being used.
Bacterial stains
Clinical isolates of S. typhi, S. paratyphi A, B and C, were collected from the Department of Microbiology, Dalhatu Araf Specialist Hospital, Lafia, Nasarawa State, Nigeria. All of the bacterial strains were preserved on Bijou agar slants and stored at 4°C until they were ready for analysis.
Bacterial strain confirmation
Bacterial strains were confirmed by biochemical screening including Gram stain, triple sugar iron (TSI), urease, citrate utilization, motility, methyl red and Voges-Proskauer tests, as described by Collins et al. (2004): ISO 6579: 2002 (Sharma, 2009) and the serological test as described by Cheesbrough (2000): ISO 6579: 2002 (Collins et al., 2004; Andrews et al., 2005; Sharma, 2009).
Bacterial strain confirmation by commercial kit
The procedures adopted by Cheesbrough (2000): ISO 6579: 2002 (Andrews et al., 2005), were the methods used to confirm the bacterial strains. Identification of S. typhi, S. paratyphi A, B and C, was performed by slide agglutination tests. Commercial kit was used to confirm serogroup S. typhi, S. paratyphi A, B and C, by their somatic (O) and flagella (H) antigens (A, B, C and D). A single pure colony of individual test organism was picked and placed separately on a ceramic tile and was rocked with the corresponding antisera. The reaction was observed for 2 min. The agglutination reaction confirmed the Salmonella subgroup and was evidenced by a positive organism under test.
Standardization of inoculum
Asowata, et al. (2013), method was used for the standardization of bacterial inoculum. Five colonies of each test organisms were picked aseptically with a wire loop and transferred into separate glass test tubes containing 5 ml of nutrient broth and mixed. The setup was then incubated at 37°C for 24 h. The turbidity that resulted was adjusted to match 0.5 McFarland standard which yielded approximately 1 × 107 ml-1 bacteria.
Preparation of various concentrations of plant extracts
Double dilution procedures were used to obtain various concentrations of plant extracts of 80, 40, 20, 10, 5, and 2.5 mg/ml, for antibacterial activity, MIC and MBC, using sterile distilled water. Thus, 8 g crude plant extract was reconstituted in 100 ml of sterile distilled water to obtain 80 mg/ml solution (that is, 8 g crude plant extract = 8000 mg/100 ml distilled water = 80 mg/ml). A known volume of 80 mg/ml solution was diluted with equal volume of sterile distilled water to obtain 40 mg/ml solution. Double dilution continues until lower concentrations were obtained (Asowata, et al., 2013).
Determination of antibacterial activity
The antibacterial screening of aqueous and ethanolic plant crude extracts were carried out using the agar well diffusion method.
Several dilutions of crude extracts of mango and Neem were separately made in separate glass test tubes as described by (Asowata, et al., 2013). The dilutions were 80, 40, 20, 10, 5 and 2.5 mg/ml. A suspension of S. typhi, S. Paratyphi A, B and C, compared to the 0.5 McFarland standard was each seeded on separate nutrient agar plates and spread with a glass rod and the excess was drained off. A sterile cork borer of 6 mm diameter was used to bore 8 wells on each plate. 0.1 ml of reconstituted extracts were introduced into six labeled well using automatic variable micropipette, and into the remaining 2 wells, one for Ciprofloxacin (250 mg/100 ml), positive control and the other one distilled water, negative control. The setup was allowed to stay on laboratory bench for 1 h for the extracts to diffuse into the agar. Then, the setup was incubated aerobically at 37°C for 24 h. The diameters of inhibition zones were measured with a 120 mm graduated ruler and the results were reported in millimeters (Asowata, et al., 2013).
Determination of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC)
MIC of the plant extract was determined by the method of Asowata, et al., 2013). 0.1 ml of suspension of each standardized S. typhi, S. Paratyphi A, B and C, was inoculated into different series of sterile labeled test tubes of nutrient broth which contained various concentrations (80, 40, 20, 10, 5, and 2.5 mg/ml) of two-fold dilution of plant extract and was incubated at 37°C for 24 h. The minimum inhibition concentration was recorded as the least concentration (highest dilution) that inhibited the growth of tested organisms.
MBC of the plant extract was determined by the method described by Asowata et al. (2013). 1 ml of plant extract was pipetted from tubes which showed no visible growth of MIC, and was sub cultured on freshly prepared nutrient agar plates and incubated at 37°C for 24 h. The MBC was read and recorded as the highest dilution (lowest concentration) of the extract that did not show any colony growth on a new agar plate.
Statistical analysis
Quantitative phytochemical parameters and the minimum inhibitory diameter zone of plant crude extracts analyzed were carried out in triplicates. The data was subjected to statistical analysis to evaluate the differences between the phytochemical constituents and inhibitory diameter zone of the studied plants crude extracts. Data were expressed as mean standard error. Comparison of means was analyzed using one-way analysis of variance (ANOVA) on a statistical programme: Statistical Package for Social Science (SPSS) version 16.0 windows. The difference was significant at P < 0.05.
RESULTS AND DISCUSSION
The qualitative phytochemical constituents’ present in the studied medicinal plants are shown in Table 1. Carbohydrates, tannins, cardiac glycosides, terpenoids, alkaloids, anthraquinones, flavonoids and saponins were the secondary metabolites present in the medicinal plants analyzed. This result agrees with the report by Paulsamy and Jeeshua (2011) who documented that plant secondary metabolites exhibit bioactive and physiological activities. These secondary metabolites are pharmacologically important and could account for their antibacterial activity.
Compared to aqueous solvent, ethanolic leaf extracts had higher quantity of secondary metabolites with a high degree of precipitation (+++) of alkaloids, tannins and terpenoids in the Mango leaves than in the Neem leaves and in the aqueous solvent (Table 1). Moderate degree of precipitation (++) of carbohydrates, cardiac glycosides, flavonoids, tannins and saponins were found in both aqueous and ethanolic extracts of Mango and Neem leaves (Table 1). Lesser/Slightly degree of precipitation (+) of alkaloids, anthraquinones, carbohydrates, cardiac glycosides, flavonoids and saponins, were found in both aqueous and ethanolic extracts of mango and Neem leaves (Table 1).
The ethanolic solvent extracts recorded a higher degree of precipitation than the aqueous solvent. This could be ascribed to the ethanolic solvents higher polarity, which contain a greater variety of plant constituents than aqueous solvent (Paulsamy and Jeeshua, 2011). Also, Doughari and Manzara (2008), reported that different solvents have different capacities for different phytoconstituents. Thus, the differences in the observed activities of various extracts may be due to varying degrees of solubility of the active constituents in the solvent used. However, aqueous solvent could not extract saponins from the Neem leaves in the study. This does not mean saponins are lacking in the plant. Rather, the analytical technique employed was not sensitive enough to extract the substance. In both aqueous and ethanolic leaf extracts of mango and Neem leaves, mango leaf extracts contained higher constituents of phytochemicals than neem leaf (Table 1). The differences in phytochemical constituents in plants could be due to geographical location, genetic constituents and the extraction method employed. However, the obtained secondary metabolite results agreed with the findings reported by Gatsing et al. (2007), Uhuo et al. (2015) and Rachuonye et al. (2016).
Table 2 shows the quantitative phytochemical profile of the studied medicinal herbs. Alkaloids, flavonoids, saponins and tannins contents in mango leaves are higher than the content found in neem leaves. Also, the total phenol content in neem leaves is higher than the content in mango leaves. The quantitative phytochemical constituents ranged between 0.90±0.01% cardiac glycosides in mango leaves and 13.11±0.00% tannins in mango leaves (Table 2). This demonstrates that the studied plants contain compounds which exhibited biological and physiological activities. As well as pharmacological significance that is directly related to secondary metabolites and accounts for their antibacterial activities (Gatsing et al., 2007; Uhuo et al., 2015; Rachuonye et al., 2016). Also, Petti and Scully (2009), documented that plants with a higher amount of phenolic content have the ability to be used to treat inflammatory diseases and can be implicated in wound healing. For instance, in the plants under study, the presence of phytochemical compounds probably justifies the use of the selected plants for the treatment of typhoid fever. The differences in the phytochemical profile of the studied samples could be due to geographical location, genetic constituents, soil condition, variation in the season cycle, the age of plants and extraction method employed. However, results of the study, agree with the findings reported by Gatsing et al. (2007), Petti and Scully (2009), Uhuo et al. (2015) and Rachuonye et al. (2016). Notably, there are significant differences between the phytochemical constituents of the medicinal herbs studied (P < 0.05) (Table 2).
Antibacterial activities of aqueous and ethanolic extracts of mango and neem leaves against S. typhi and S. paratyphi A, B and C, at different extract concentrations are shown in Tables 3 and 4, respectively.
The extracts inhibited the growth of tested organisms at varying degrees of extract concentrations as shown by their diameter (mm) inhibition zones in Tables 3 and 4, respectively. S. typhi showed inhibition zones of 29.4±0.1b mm at the highest aqueous extract concentration of 80 mg/ml, while S. paratyphi A, B and C, exhibited inhibition zones of 14.4±0.02, 21.2±0.04 and 13.4±0.01 mm, respectively (Table 3). Also, S. typhi exhibited inhibition zones of 30.0±0.1 mm at the highest ethanolic extract concentration of 80 mg/ml, while S. paratyphi A, B and C, showed inhibition growth of 18.0±0.03, 20.0±0.04 and 21.0±0.04 mm, respectively (Table 4).
At aqueous lower extract concentration of 10 mg/ml, S. typhi showed inhibition zone of 8.4±0.01 mm, while S. paratyphi A, B and C exhibited inhibition growth of 9.5±0.01, 9.1±0.01 and 6.2±0.01 mm, respectively as exhibited by neem leaf extracts (Table 3). Similarly, ethanolic extract at a lower concentration of 10 mg/ml, S. typhi showed an inhibition zone of 9.0±0.01 mm, while S. paratyphi A, B and C exhibited inhibition zones of 9.0±0.01, 9.0±0.01 and 9.0±0.01 mm, respectively in neem leaf extracts (Table 4).
Essentially, the neem leaves exhibited the highest diameter (mm) inhibition zones compared to mango leaves at all extract concentrations. This could be attributed to the genetic heterogeneity of plant species. In general, this study suggests that the plant extracts possess antibacterial potential for the tested organisms and demonstrates that inhibition zones increase with increasing extract concentrations, indicating that concentration influences the activities against the test organisms. The extracts of these plants could be alternative medicare for typhoid fever. The positive control, Ciprofloxacin (2.5mg/ml), showed an inhibition zone of 31.0±0.3 mm, while the negative control, sterile distilled water, recorded no inhibition zone. However, the zone of inhibition exhibited by the standard drug Ciprofloxacin, is higher than the plants extracts. This could be due to the crude nature of plant extracts which contained other constituents that do not possess antibacterial properties. Also, the ability of plant extracts to diffuse through the gel agar may be hindered because of the large molecules. Even at a higher extract concentration, the inhibition zones are not comparable with the zones of standard drugs.
However, the results of the study are in
agreement with the findings documented by Gatsing et al. (2007), Tambekar and Dahikar (2011), Pankaj et al. (2015), Uhuo et al. (2015) and Rachuonyo et al. (2016). At different extract concentrations, there are significant differences between the mean diameter (mm) inhibition zones of aqueous and ethanolic plant extracts against tested organisms (p <0.05).
The minimum inhibition concentration (MIC, mg/ml) and minimum bactericidal concentration (MBC, mg/ml) of aqueous and ethanolic extracts of studied medicinal plants against tested organisms are shown in Tables 5 and 6 respectively.
At different plant extract concentrations tested organisms were inhibited by both aqueous and ethanolic extracts. Aqueous extracts showed MIC against S. typhi, S. paratyphi A, B and C, at 2.5 mg/ml extract concentration, while at 5 mg/ml concentration, the aqueous extract showed MBC against the tested organisms (Table 5). Similarly, at 2.5 mg/ml ethanolic extract concentration, the extracts exhibited MIC against tested organisms, while at 5 mg/ml ethanolic extract concentration the extracts showed MBC against tested organisms (Table 6).
The lowest MIC of 2.5 mg/ml was exhibited by all tested organisms, and shows that the organisms are more sensitive to the extracts (Tables 5 and 6). This supports the claim by traditional medical practitioners and vendors that the crude extracts of the investigated medicinal plants are a remedy for the cure of typhoid fever. All tested organisms showed a higher MBC than the MIC (Tables 5 and 6). This demonstrates that higher concentrations of extracts were needed to kill the bacteria than to inhibit their growth.
However, the results of the study differ with the findings by Gatsing et al. (2007), Agada et al. (2010) and Rachuonye et al. (2016). This could be due to genetic heterogeneity of plant species, soil factor, variation in season cycle, the age of plants, climatic influences and different geographical locations where plants were collected. Notably, Rao and Rout (2003), reported that there is a relationship between chemical composition of plants and their geographical location.
CONCLUSION
The aqueous and ethanolic extracts of investigated medicinal plants showed activities against all tested organisms at different extract concentrations. The aqueous and ethanolic plant extracts exhibited MIC and MBC against all tested organisms at 2.5 and 5 mg/ml concentrations, respectively. The qualitative and quantitative profile of studied medicinal plants is rich in phytochemical compounds which exhibit biological and physiological activities. This demonstrates that the plants have pharmacological significance that is directly related to the secondary metabolites that account for their antibacterial properties. The diameter (mm) inhibition zones of the medicinal plants studied indicate that they are good candidate for typhoid medicare alternative. However, there are significant differences between the quantitative phytochemical content and diameter (mm) inhibition zones of aqueous and ethanolic extract concentrations against tested organisms (p < 0.05).
Recommendations
Further studies are recommended on the toxicity and safe dosage regimen of the plants since the infusion of the plants are taken orally by local people for the treatment of typhoid fever. Traditional medical practitioners and vendors should be educated about modern and traditional medicine through the use of plants compounds. This will eliminate the challenges to phytomedicine, such as the lack of reproducibility of biological activity of individual herbal extracts after the success of the initial screening process, toxicity, contamination and adulteration, standardization and drug interaction issues. The loss of medical plant species due to risk of extinction as a result of high harvest and destruction of habitats, decrease in wildlife reservoir due to growing human population and excessive conservation of plants should be avoided and should be backed up by legislation.
CONCLUSION
The aqueous and ethanolic extracts of investigated medicinal plants showed activities against all tested organisms at different extract concentrations. The aqueous and ethanolic plant extracts exhibited MIC and MBC against all tested organisms at 2.5 and 5 mg/ml concentrations, respectively. The qualitative and quantitative profile of studied medicinal plants is rich in phytochemical compounds which exhibit biological and physiological activities. This demonstrates that the plants have pharmacological significance that is directly related to the secondary metabolites that account for their antibacterial properties. The diameter (mm) inhibition zones of the medicinal plants studied indicate that they are good candidate for typhoid medicare alternative. However, there are significant differences between the quantitative phytochemical content and diameter (mm) inhibition zones of aqueous and ethanolic extract concentrations against tested organisms (p < 0.05).
Recommendations
Further studies are recommended on the toxicity and safe dosage regimen of the plants since the infusion of the plants are taken orally by local people for the treatment of typhoid fever. Traditional medical practitioners and vendors should be educated about modern and traditional medicine through the use of plants compounds. This will eliminate the challenges to phytomedicine, such as the lack of reproducibility of biological activity of individual herbal extracts after the success of the initial screening process, toxicity, contamination and adulteration, standardization and drug interaction issues. The loss of medical plant species due to risk of extinction as a result of high harvest and destruction of habitats, decrease in wildlife reservoir due to growing human population and excessive conservation of plants should be avoided and should be backed up by legislation.
ACKNOWLEDGEMENTS
The authors are grateful to the authorities of the universities: Federal University of Agriculture Makurdi, Benue State, Nigeria and Federal University of Lafia, Nasarawa State, Nigeria, for making available their laboratories and equipment for the completion of the research.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
REFERENCES
|
Ackers M, Puhr ND, Taure RV, Mintz ED (2000). Laboratory base surveillance of Salmonella serotype typhi infections in the United States. JAMA 283(20):2668-2673. |
|
|
Agada GOA, Gotep JG, Gbise DS, Chollom S (2010). Antibacterial activity of ethanolic extract of Acalypha wilkesiana leaves growing in Jos, Plateau state, Nigeria. Malaysia Journal of Microbiology 6(2):69-74. |
|
|
Akinpelu DA, Onakaya TM (2006). Antimicrobial activities of medicinal plants used in forklore remedies in South-Western Nigeria. African Journal of Biotechnology 5(11):1078-1081. |
|
|
Andrews GP, Hromockyi AE, Coke C, Maurelli AT (2005). Two novel virulence loci, MxiA and MxiB in Shigella flexneri facilitate excretion of invasion plasmid antigens. Infection and Immunity 59(6):19-25. |
|
|
Asowata I, Erhabor JO, Idu M, Odaro T, Obayagbona NO (2013). Preliminary antimicrobial and phytochemical study of the aqueous alcoholic and Chloroform extracts of the leaves of Napoleonaea Vogelli Hook, and planch. (Lecythidiaceae). Journal of Microbiology, Biotechnology and Food Sciences 2(4):2279-2292. |
|
|
Baron JE, Finegold SM (1990). Methods for testing antimicrobial effectiveness. In Bailey Scotts Diag. Microbiol. Mosby C.V. (Ed) Missouri pp. 171-194. |
|
|
Boham BA, Kocipai AC (1994). Flavonoids and Condensed tannis from leaves of Hawaiian Vaccinium Vaticulatum and V. Calycinium. Pacific Science 48(4):458-463. |
|
|
Bussmann RW, Gilbreath GG, Solio J, Lutura M, Lutuluo R, Kunguru K, Wood N, Mathenge SG (2006). Plant use of the Maasai of Sekenani Valley, Maasai Mara Kenya. Journal of Ethnobiology and Ethnomedicine 2(1):1-7. |
|
|
Byarygaba DK (2004). A review on Antimicrobial resistance in Developing countries and responsible risk factors. International Journal of Antimicrobial Agents 24(2):105-10. |
|
|
Campbell RJ, Ledesma N, Campbell CW (2002). Tropical Mangos "How to grow the world's most delicious fruit"; 1st (Ed). Fairchild Tropical Garden, Miami, Florida pp. 222-507. |
|
|
Cheesbrough M (2000). District laboratory practice in tropical countries. E.C.B.S. Cambridge University Press Edition 2:256-267. |
|
|
Collins CH, Lyne PM, Grange JM, Falkinham JO (2004). Collins and Lyne's Microbiological methods, 8th Ed. Arnold Pub., London P 465. |
|
|
De Silva T (2005). Industrial utilization of medicinal plants in developing countries. Industrial sector and Environmental Division UNIDO pp. 1-11. |
|
|
Doughari JH, Elmahmood AM, Manzara S (2007). Studies on the antibacterial activity of root extracts of Carica Papaya L. African Journal of Microbiology Research 1(3):037-041. |
|
|
Doughari JH, Manzara S (2008). In vitro antibacterial activity of crude leaf extract of Mangifera indica Linn. African Journal of Microbiology Research 2(4):67-72. |
|
|
Ebrahimzaded MA, Pourmorad F, Bekhradnia AR (2008). Iron chelating activity, Phenol and Flavonoid content of some medicinal plants from Iran. African Journal of Biotechnology 7(18):3188-3192. |
|
|
El-Olemy MM, Al-Muhtadi FJ, Afifi AFA (1994). Experimental Phytochemistry: A Laboratory manual, King Saud University press, Saudi Arabia pp. 21-27. |
|
|
Erute MO, Oyibo AE (2008). Effects of tree plants extract (Occimum gratissimum, Acalypha wilkesiana and Acalypha macrostachya) on postharvest pathogens of Persia Americana. Journal of Medicinal Plants Research 2(11):311-314. |
|
|
Fabricant DS, Farnsworth NR (2001). The value of plants used in traditional medicine for drug is discovery. Environmental Health perspectives 109(1):69-75. |
|
|
Fauci AS, Touchett NA, Folkers GK (2005). Emerging infectious diseases: a 10-year perspective from the National Institute of Allergy and Infectious Diseases. Emerging Infectious Diseases 11(4):519-525. |
|
|
Fowler DG (2006). Traditional fever remedies: A list of Zambian plants. Accessed April 20,2010. Available from URL: |
|
|
Gatsing D, Djemgou PC, Garba IH, Aliyu R, Tchuendem MH, Tane P, Ngadjui BT, Seguin E, Adoga GI (2007). Dihydronaphtalenone and chromone from Cassia petersiana Bolle and the antisalmonella activity of its crude extract. Research Journal of Phytochemistry 1(1):40-45. |
|
|
Good Agricultural and Field Collection Practices of Medicinal Plants GACP and FCP (2004). WHO, Geneva. The world Health Report. Changing history. Statistical annex. Death by cause, Sex and Mortality Stratum in WHO regions, estimates for 2002. Geneva, Switzerland pp. 120-121. |
|
|
Hancock EW (2005). Mechanisms of action of newer antibiotics for gram-positive pathogens. Lancet Infectious Diseases 5(4):209-218. |
|
|
Harborne JB (1973). Phytochemical methods, London. Chapman and Hall, Ltd. pp. 49-188. |
|
|
International Standards Organization (ISO 6579) (2002). Microbiology of food and animal feeding stuffs-Horizontal method for detection of Salmonella Spp. 6579:2002. |
|
|
Kabir OA, Olukayode O, Chidi EO, Christopher CI, Kehinde AF (2005). Screening of crude extracts of six medicinal plants used in South-West Nigeria orthodox Medicine for antimethicillin resistant Staphylococcus aureus activity. BMC Complementary and Alternative Medicine 5:6. |
|
|
Kabuki T, Hakajima H, Arai M, Ueda S, Kuwabara Y, Dosako S (2000). Characterization of novel antimicrobial compounds from mango (Mangifera indica L). Kernel seeds. Food Chemistry 71(1):61-66. |
|
|
Obadoni BO, Ochuko PO (2001). Phytochemical studies and comparative efficacy of the crude extracts of some Homostatic plants in Edo and Delta States of Nigeria. Global Journal of Pure and Applied Sciences 8(2):203-208. |
|
|
Okwu DE (2005). Phytochemicals, Vitamins and Mineral contents of two Nigerian Medicinal plants. International Journal of Molecular Medicine and Advance Sciences 1(4):375-381. |
|
|
Oladunmoye MK (2006). Comparative evaluation of antimicrobial activities and phytochemical screening of two varieties of Acalypha wilkesiana. Trends in Applied Sciences Research 1:538-541. |
|
|
Osuagwu OS, Oyerinde AA, Ega RIA (2015). Microbial Load Analysis of Moringa oleifera Lam leaves in the Guinea Savanna vegetation zone of Nigeria. NSUK Journal of Science and Technology 5(1):37-42. |
|
|
Pankaj B, Bishnu P, Marasini, Pratibha A, Kashi RG, Sanjiv N, Nabaraj D, Anjana S, Laxman G, Kanti S (2015). Evaluation of antibacterial activity of some traditionally used medicinal plants against Human pathogenic bacteria. BioMed Research Internationalal. |
|
|
Paulsamy S, Jeeshna MV (2011). Preliminary phytochemistry and antimicrobial studies of an endangered medicinal herb. Research Journal of Pharmaceutical, Biological and Chemical Sciences 2(4):447-457. |
|
|
Petti S, Scully C (2009). Polyphenols, oral health and diseases: a review. Journal of Dentistry 37(6):413-423. |
|
|
Rachuonyo HO, Ogola PE, Arika WM, Nyamai DW, Wambani JR (2016). In vitro antimicrobial activity of crude leaf extracts from Aloe Secundiflora, Bulbine frutescens, Vernonia lasiopus and Tagetes Minuta against Salmonella typhi. Journal of Traditional Medicine and Clinical Naturopathy 5:187. |
|
|
Rao YR, Rout PK (2003). Geographical location and harvest time dependent variation in the composition of essential oils of Jasminum sambac. (L) Aiton. Journal of Essential Oil Research 15(6):398-401. |
|
|
Sharma K (2009). Manual of Microbiology, Ane Books. Pvt, Limited. New Delhi, p. 405. |
|
|
Sofowara A (1993). Medicinal plants and Traditional medicine in Africa. Spectrum Books Limited. Ibadan, Nigeria pp. 191-289. |
|
|
Tambekar DH, Dahikar SB (2011). Antibacterial activity of some Indian Ayurvedic preparations against enteric bacterial pathogens. Journal of Advanced Pharmaceutical Technology & Research 2(1):24-29. |
|
|
Trease GE, Evans WC (1989). Pharmacognosy. 11th edition. Brailliar Tiridel Can. Macmillian Publishers. |
|
|
Uhuo CA, Odikamnoro OO, Ikeh IM, Ogiji ED, Ibiam GA, Azi SO, Akpam LJ, Okoh NF (2015). Antibacterial activities of two medicinal herbs on Salmonella typhi isolates in Abakaliki, Ebonyi state, Nigeria: Improvement to herbal medicine. African Journal of Bacteriology Research 7(2):14-18. |
|
|
Umar ND, Igwe O, Idris IG (2019). Evaluation and characterization of ground water of the Maastrichitian Lafia formation central Benue Trough, Nigeria. Journal of Earth System Science 128(6):1-12. |
|
|
Van Burden JP, Robinson WB (1981). Formation of complexes between protein and tannic acid. Journal of Agricultural and Food Chemistry 17(4):772-777. |
|
|
World Health Organization (2002). WHO Traditional Medicine Strategy 2002-2005, World Health Organization, Geneva. Switzerland. |
|
|
World Health Organization (WHO) (2007). Global priority list of antimicrobials - resistant bacteria to guide research, discovery and development of new antibiotics. First revision, 2007. |
|
|
Zakaria ZA, Mat Jais AM, Sulaiman MR, Mohamed Isa SSP, Riffin S (2006). The In vitro antibacterial activity of methanol and ethanol extracts of Carica papaya flowers and Mangifera indica leaves. Journal of Pharmacology and Toxicology 1(3):278-283. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0